Abstract
This study explores the clinical features and motor impairments, characterized using computerized graphomotor analysis, of individuals with Alzheimer’s disease (AD) and amnestic mild cognitive impairment (aMCI). We recruited 20 participants with AD, 12 with aMCI, and 16 control participants. All participants were asked to perform six graphomotor tasks on a digitizer tablet: formation of straight lines, cursive-connected loops, a single circle, and continuous circles, and two aiming tasks. The results show that slowness and irregularity of movement of AD and aMCI patients were not present in all tasks. Impairment was not found in the straight lines and cursive-connected loops tasks. In the task of drawing a single circle, the AD and aMCI participants had more difficulty drawing perfect circles than did the control group. They also showed greater size variations when drawing continuous circles. In the aiming tasks, performance differed across the three groups. The AD and aMCI participants both performed more slowly than did the controls. When accuracy was specified, the AD and aMCI participants performed the graphomotor tasks requiring wrist and finger coordination more slowly than did the controls. The results of this study suggest that aMCI is characterized by motor dysfunction and cognitive impairment. The degree of motor impairment, particularly in aiming movements with accuracy constraints, may help identify those at risk for AD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder of insidious onset, characterized by memory loss, confusion, and a variety of cognitive disabilities [1]. Its incidence seems to increase with increasing average life expectancy [2]. In its early stages in elderly persons, the symptoms are difficult to distinguish from those of normal aging. In its later stages, AD is sometimes mistaken for other kinds of dementias and mental disease [3]. AD can exist long before symptoms are clinically detectable and very early in the disease it is sometimes difficult to separate cognitive changes due to normal aging from subtle cognitive impairment due to disease. This transitional state between normal aging and the early stages of AD has been designated as mild cognitive impairment (MCI). Subjects with MCI have impaired cognition, especially memory, to a greater extent than would be expected for their age and education, but they do not fulfill the criteria for dementia [4]. One of the subtypes of MCI has been termed amnestic MCI (aMCI) because of the main feature of memory loss. Research suggests that on an annual basis, 12 % of persons diagnosed with aMCI progress to AD compared to only 1–2 % of healthy older adults who progress to aMCI/AD [5].
Researchers have suggested that cognitive and motor function declines with advancing age [6, 7]. Cognitive deficits or “noise” in the sensory-motor system may contribute to reduced levels of motor performance associated with aging or AD [7–9]. Changes in the brain from neurodegenerative dementia (e.g., brain atrophy, neuronal loss, cellular or synaptic dysfunction) cause both cognitive and motor dysfunction or impair the performance of previously learned motor skills [10, 11].
Motor performances, including gait, balance, and finger tapping, are commonly impaired in older persons [12]. Lower levels of motor function were found to be more pronounced in older persons with cognitive impairment compared to those without cognitive impairment [13–16]. Motor impairment and a rapid rate of motor decline in cognitively intact individuals are both predictors of AD. Loss of motor function can precede cognitive impairment by several years [13–17]. This indicates the importance of characterizing motor function to determine who is most at risk for developing AD.
Researchers are therefore interested in whether aMCI patients present motor impairment before being diagnosed with AD. A study showed that motor impairments were not present during the preclinical stages of dementia, but such impairments emerged as a significant feature of mild AD [18]. Individuals with questionable dementia (very mildly demented AD) performed similarly to their healthy peers on measures of gait velocity, finger tapping, reaction time, and movement time. In a study of rapid linear aiming arm movement on the surface of a digitizer, normal control and MCI participants did not differ on any of the three dependent variables (movement time, movement jerk, and percentage of primary submovement) at the baseline [19]. Another study showed different results from those mentioned above [20]. It revealed that patients with MCI and mild AD performed significantly worse than did healthy older adults on tasks assessing both fine (e.g., Peg-Board placement) and complex motor movements (i.e., rapid alternating hand movements, tracking). The motor control of these movements is imperative for using rehabilitation robots to recover or compensate the impaired motor function [21].
Another approach for the measurement and analysis of motor dysfunction is the quantification of kinematic handwriting movements using a digitizer tablet [22]. The task of handwriting has been employed to study fine motor control and executive functions in healthy and unhealthy populations [23, 24]. From the kinematic results of circular and quick handwriting movements performed by the AD, aMCI, and control groups, a greater variability was revealed in the movement velocities of AD and aMCI than in the control norms [23]. It has been shown that handwriting is compromised in demented patients [25]. A correlation exists between the overall severity of handwriting disruption and the severity of general cognitive deficiency [26, 27]. From the kinematic profiles of handwriting movements, AD and MCI patients demonstrated slower, less smooth, less coordinated, and less consistent handwriting movements than did their healthy counterparts [28]. These characteristics of the handwriting process suggest that it might be sensitive to age-related impairments in cognitive functioning and that assessments of handwriting might therefore facilitate the diagnosis of such impairments. In the present study, handwriting and graphomotor function tests are used to address this possibility.
Both ante-mortem and post-mortem data lend support to the notion that AD has a preclinical period when neuropathology accumulates and cognitive function declines, but symptoms are insufficient to warrant a clinical diagnosis of AD. The aim of this study was to test whether computerized graphomotor assessments can be comparably sensitive to traditional tests of cognitive function in identifying persons affected by the earliest stages of AD pathology. We explored subtle changes of fine motor functions of aMCI and AD patients that may not be noticeable with clinical observation or psycho-neurological tests. The objective of this study was to investigate the temporal and kinematic characteristics of graphomotor performance in patients with aMCI or AD. It was hypothesized that as fine motor function of aMCI patients declines, the risk of AD increases. Since minute changes in cognitive function often go unnoticed, motor function decline can be a more accurate predictor of AD risk for aMCI patients. Three types of task were designed to test the fine motor functions of movement speed. The tasks measured speed, accuracy and consistency, and speed and accuracy, respectively. Movement speed changes, from straight lines to loops, were used to measure the slower movement in impaired graphomotor functions. Drawing circles with and without inking outputs was used for testing accuracy and consistency and the deterioration of visual trajectory feedback. In the final task, graphic aiming was used to test both speed and accuracy.
2 Methods
2.1 Subject Recruitment and Confirmed Diagnosis
A total of 48 individuals participated in the present study. We recruited persons with aMCI and those diagnosed with AD from the Veterans Home and EDA Hospital in southern Taiwan. The healthy participants were recruited from the Veterans Home and three day-care centers in the same area. All the participants could walk independently and walked regularly for several minutes every day as a general conditioning exercise. Subjects underwent an evaluation by a neurologist and completed a standardized dementia workup. To be included in the study, participants needed to be at least 65 years of age, be free of significant underlying medical, neurologic, or psychiatric illness, have a Clinical Dementia Rating (CDR) [29] of normal (CDR = 0), aMCI (CDR = 0.5), or AD (CDR > 1), and be willing to participate in the study procedures.
A detailed history and interview with the patient and informant, as well as neurological and physical examinations, were performed as part of the initial visit. The diagnosis of AD was based on the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria [30]. These criteria require a history of cognitive decline and impairment in at least two cognitive domains, one of which must be memory, for a diagnosis of AD. Possible AD cases with atypical onset or progression or other systemic brain diseases capable of producing dementia were excluded from this study.
We recruited aMCI patients, since this disorder is known to be a high-risk factor for developing full-blown dementia. Participants were classified as having aMCI if they met the following conditions: (a) memory complaint usually corroborated by an informant, (b) objective memory impairment for age, (c) essentially preserved general cognitive function, (d) largely intact functional activities, and (e) no presence of dementia [31]. The healthy controls (CN) were confirmed as non-demented based upon the standardized dementia workup. Within the cohort of the longitudinal investigation, the CNs were selected to match the AD and aMCI patients as much as possible with respect to age, education, and sex. All participants were right-handed and had normal or corrected-to-normal vision. Each participant or their legal representative signed an informed consent form approved by the Institutional Review Board prior to the experiment.
2.2 Instruments and Apparatus
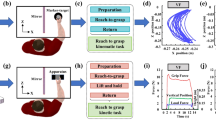
Participants were seated on a chair in front of a table on which a digitizer tablet (487 × 318 × 12 mm, Wacom Intuos 5, Japan) was positioned so that the tablet’s lower edge lined up with the table edge on which the participant was seated. On the digitizer tablet, an A4-sized piece of paper was positioned with the vertical and horizontal edges parallel to the horizontal and vertical edges of the digitizer. The participant’s forearm was positioned perpendicular to the horizontal edge of the digitizer tablet. A normal-looking wireless electronic inking pen with a force-sensitive tip (2048 levels) was used to collect the movement data on the digitizer tablet. The axial pen force and X (horizontal) and Y (vertical) positions of the pen tip were sampled at a frequency of 200 Hz with a spatial resolution of 0.005 mm. The electronic pen had a weight and size typical for regular pens (length = 150 mm, barrel circumference = 35 mm, weight = 11 g).
2.3 Drawing Tasks for Comparisons of Movement Speed and Size Control
The experiment included two separate drawing tasks to test graphomotor control as needed for graphic tasks. The two graphic tasks were: (1) drawing three 5-cm-squared crosses and (2) writing 2.5 cm of connected cursive lllllllls (i.e., connected loops with a progression to the right). They were used to compare the movement speed per stroke across the groups and to investigate movement speed changes due to curvature. Task 2 was also used to measure the participants’ size control by comparing the difference between the first and last loops. The two tasks were performed in the fixed order (task 1 and then task 2) with three trials for each task. The participants were requested to perform three practice trials prior to the measurements. They were instructed to perform the task as quickly and as accurately as possible.
2.3.1 Measures in Movement Speed
The parameters for measuring the speed in straight-line and curvature movement tests were obtained from the temporal and kinematic data of the pen-tip movement. The data of the X and Y positions of the pen tip were smoothed with a 4th-order Butterworth low-pass filter with a cut-off frequency of 15 Hz. In the llllllll task, the local maxima and minima of the X- and Y-coordinates were used to detect the extreme points to segment a loop into two strokes. The definitions of the parameters processed from the registered coordinates are as follows. The velocity was determined by averaging the tangential velocity per stroke. The mean velocity value was calculated as the average of all trials per condition.
2.3.2 Measures in Size Control in Cursive l Loops
In the cursive loops task, the participants were required to copy lllllllls of the same size as that of the sample shown on the table. The sample shown for the participants was 2.5 cm in height and 1.0 cm in width. For measuring the size of an l, the height and width of the two strokes were averaged. The height and width differences between the first and last loops were measured for comparison of size control across the groups.
2.4 Drawing Tasks for Comparisons of Accuracy and Consistency
The experiment included two separate drawing conditions to test accuracy and consistency as needed for graphic tasks. The two graphic conditions were: (1) a single circle with a diameter of 4 cm and (2) 10 continuously overlapping circles with a diameter of 4 cm. For a clear definition of accuracy, two circle conditions were used to compare control in drawing circles. The discrete and continuous circle were performed under two conditions: (A) using the electronic pen with an inking refill and (B) using the electronic pen with a non-inking refill. After three practice trials, the participants were requested to perform three trials in the non-inking and then inking conditions with a fixed order of tasks (task 1 and then task 2). They were instructed to perform the task as accurately and as consistently as possible.
To test participants’ graphomotor skill, the spatial accuracy and consistency for each drawing were determined. To estimate spatial accuracy, the mean squared error (MSE) of the deviation between the drawing trajectory and the target circle was computed. Figure 1 shows an example of a circle drawn by a participant (solid line) and the reference circle (dotted line). The reference circle has the same center location and size as those of the drawn circle. The size was calculated by first estimating the center and then averaging the absolute distance to the center by adding the distance to the center for every sample and averaging it over the samples. The MSE is the squared mean of the deviation in the radius per sample between the drawn circle (see Fig. 1, solid line) and the reference circle (see Fig. 1, dotted line).
In addition to assessing the consistency of the curvature of each circle, the variation of the radius (VR) of each circle was computed. The radius of all sample points for a drawn circle was first averaged. After this averaging procedure, the standard deviation of the radius was calculated across the ten drawn circles, which was normalized by dividing this standard deviation by the mean radius of the ten drawn circles.
2.5 Graphic Aiming Tasks
To examine the capability of participants to use the fingers and wrist, a variation of a Fitts’ task was used [32], requiring the participants to make repetitive aiming movements with the pen to circles with different accuracy constraints. The distance between the targets was always 25 mm. The participants predominantly used the fingers and wrists. The accuracy constraint was varied by using target circles with a diameter of 2.2, 4.4 or 8.8 mm, as shown in Fig. 2. The indices of difficulty of the three accuracy conditions were 4.51, 3.51 and 2.51, respectively. In addition to the variation in task difficulty, two different movements were used. The first movement required back-and-forth movements between the targets positioned to the upper right and lower left, thus requiring the participant to make equivalent movements (i.e., participants were required to flex the fingers and extend the wrist together and vice versa when making the aiming movements). The second movement condition required the participants to move back and forth between targets positioned to the upper left and lower right, thus requiring the participants to make nonequivalent movements (i.e., participants were required to flex the wrist and extend the fingers and to extend the wrist and flex the fingers when making the aiming movements). In every trial, only two target circles in a diagonal direction were presented to the participant. The participants were requested to perform the tasks as quickly and as accuracy as possible in a fixed counterbalanced order (large, medium, small → medium, small, large → small, large, medium).
2.6 Demographic Data and Statistical Analyses
For the statistical comparison of the participants’ demographic characteristics among groups, one-way analysis of variance (ANOVA) for continuous measures was used to compare the three groups of subjects. The relevant pairwise comparisons were made between adjacent groups (e.g., CN vs. aMCI and aMCI vs. AD) using the Bonferroni test with a level of significance set at the 0.05 level. A Chi squared test for goodness-of-fit was used to compare the male/female ratio across the three groups.
Repeated-measures ANOVA was used to test the significance of difference across the groups and tasks (or conditions) and the interaction effect of the group and task. Post hoc tests with Bonferroni correction were utilized to determine the locus of significant effects of the group (i.e., CN, aMCI, and AD). All statistical analyses were conducted using PASW Statistics (Version 18, SPSS Inc., Hong Kong, 2009).
3 Results
3.1 Demographic Characteristics
Table 1 shows the demographic and memory measurements of the participants. There was no significant difference found in the comparisons of age, education, and gender ratios across the three groups. There was significant difference in the age-corrected scaled score of the Wechsler Memory Scale across the adjacent groups (with the exception of Visual Reproduction-II).
3.2 Stroke Movement Speeds in Drawing Straight Lines and Cursive l Loops
Table 2 shows the mean and peak velocities of participants drawing crossed straight lines and cursive l loops. In the comparison of movement speed, there was significant difference between straight lines and cursive l loops but not across the participant groups. The movement speed was significantly reduced in drawing cursive l loops. The results of repeated-measures ANOVA showed no significant interaction between the participant group and the task.
3.3 Size Control in Cursive l Loops
Table 3 shows the sizes of the cursive l loops drawn by participants. A comparison of height and width showed no significant difference across the participant groups and between the first and last loops. The results of repeated-measure ANOVA also showed no significant interaction between the participant group and the loop sequence.
3.4 Accuracy and Consistency in Copying Circles
Table 4 shows the test results of copying circles in tasks with and without inking feedback. A comparison of the circle size showed no significant difference across the participant groups or between the conditions with or without inking feedback. There was no significant interaction between the participant groups and feedback conditions.
A comparison of the MSE showed no significant difference between the two conditions and across the participant groups. The MSE in the task with inking feedback was greater than that in the task without inking feedback. In the post hoc tests with Bonferroni correction, the MSE for aMCI (p = 0.013) and AD (p < 0.001) participants was significantly greater than that of the healthy control participants. There was no significant interaction, showing that the effect of inking feedback was not different across the participant groups.
In the comparison of VR, significant difference was found across the participant groups but not between the two conditions. The post hoc test showed that the CN group drew circles with a smaller VR than did the aMCI (p = 0.05) and AD (p = 0.011) groups. There was no significant interaction between the participant groups and the feedback conditions.
3.5 Graphic Aiming Tasks
Table 5 and Fig. 3 show the mean velocity of participants performing equivalent and nonequivalent movements in accuracy-speed tasks. In equivalent movement, significant difference was found in the movement speed across the participant groups but not across the tasks. However, no significant interaction of group and task was found in tests of equivalent movement. In the post hoc tests with Bonferroni correction, the movement speed of AD (p < 0.001) participants was found to be significantly slower than that of the healthy control participants. The movement speed of aMCI participants was also slower than that of the healthy control participants, but the difference did not reach significance (p = 0.103).
In nonequivalent movement, significant differences were found in the movement speed across the tasks and across the participant groups. In the post hoc tests with Bonferroni correction, the movement speed was significantly different across the task sizes (large task > medium task and medium task > small task, both p values < 0.001). The movement speed of AD participants was found to be significantly slower than that of the healthy control participants (p < 0.001). The movement speed of aMCI participants was also slower than that of the healthy control participants, but the difference did not reach significance (p = 0.099). The occurrence of a significant interaction showed that the effect of the task size was different across the participant groups. For all participants, the differences between medium and small tasks reached statistical significance (p = 0.003 for CN, p = 0.007 for aMCI, and p = 0.042 for AD). Only for CN participants did the difference between large and medium tasks reach statistical significance (p < 0.001 for CN, p = 0.193 for aMCI, and p = 0.075 for AD).
4 Discussion
From the results of this study, the AD and aMCI participants showed slower movement than did the healthy controls in the graphic aiming task. Significant difference was found only in the comparison between the AD group and the healthy controls. The impairment of the graphic aiming task for aMCI patients did not reach statistical significance. This result represents the transitional state between normal aging and the early stages of AD, which has been designated as aMCI. Among all variables in this study, aMCI and AD patients are most strongly differentiated by the graphic aiming tasks. However, the impairment was not found in the straight line and cursive loop handwriting tasks. In addition, with respect to size control in drawing cursive l loops or the task of copying circles, the results also showed no significant difference across the three groups, thus indicating no impairments (such as micrographia) in subjects with aMCI or AD. There were no significant deteriorations in aMCI and AD subjects in graphomotor tasks without an accuracy constraint. These results indicate that movement speed alone cannot differentiate these deficits unless the tasks need the integration of sensorimotor skills.
The results from this study show that all the participants had faster straight-line stroke movement than cursive l loop drawing. However, there was no significant difference across the three groups in both tasks. No significant interaction indicates that the effect of movement type was not different across the participant groups. This suggests that the AD and aMCI participants did not show slowness in either straight or cursive stroke movement. This result is not as consistent with those of previous research where AD and aMCI patients showed slower handwriting movements than their healthy counterparts [25, 28]. In a comparison of the temporal and kinematic handwriting process, significant differences were found between the groups (normal vs. aMCI and aMCI vs. AD) in almost all measures, with the aMCI group assuming a position between the other groups [25]. The difference between handwriting and our graphomotor tasks may explain this inconsistency. Handwriting is actually a very complicated skill. It requires the integration of cognition, visual perception, and fine motor skills. Without controlling the perceptual and cognitive factors, it is difficult to relate these temporal and kinematic results of the handwriting process to meaningful fine motor functions.
This study tried to separate the components of speed and accuracy in handwriting-like movements. Our findings pertaining to movement speed are not consistent with those of previous studies [25, 28], in which researchers found slower movement in subjects with deteriorating memory. However, this study showed significantly lower accuracy and consistency in the AD and aMCI groups. There was no such measurement in previous handwriting research. Handwriting analysis studies require tests for accuracy and legibility. However, these variables are difficult to quantify, especially in elderly subjects with lots of interfering factors. The design in the current study may be much easier than a handwriting task. To reduce cognitive load, this study used straight lines and cursive l loops rather than Chinese handwriting. These pure motor tasks seemed unable to differentiate the difference across the three groups. This suggests that AD and aMCI participants did not show impairments in tasks with less cognitive demand or constraint.
In the accuracy test of circle drawing, this study found that the aMCI and AD participants had more difficulty in drawing correct circles than did the CN group. They also showed greater size variation in drawing consecutive circles. These results were not found in the healthy control participants. This indicates that the impaired fine motor control of patients with aMCI and AD can be revealed in the task of drawing circles which requires coordination between fine motor skills and visuospatial function. This shows a clinical hallmark for the relationship between aMCI and the risk of AD.
In geometric drawing for observing the effect of deprivation of visual trajectory feedback, the accuracy and consistency of size was unexpectedly deteriorated by the visual trajectory feedback in all groups. This shows that the elderly participants did not improve their performance by relying on the visual feedback of the previous trajectory to guide ongoing strokes. It seems that the impaired closed-loop control needs feedback control with more mental demand, such as in tasks that require sensorimotor integration. Sensorimotor integration seems to play a significant role in the disturbances of motor control, which are typically seen in AD, autistic, and schizophrenic patients. In tasks without trajectory feedback information, the open-loop control relies on the preplanned motor program [33]. The need for less sensorimotor integration in the task without visual trajectory feedback may explain the better performance.
In the drawing task without inking feedback, the results showed that the CN and aMCI groups drew larger circles than those drawn with inking feedback. The AD group showed the opposite result. However, there was no significant interaction between the group and visual feedback effects. This subtle change may reflect the AD group’s tendency to decrease the drawing size when the trajectory is not visible. However, this issue needs further study.
In the test of graphic aiming tasks, both the aMCI and AD participants showed difficulty in all tasks of both movement types. In equivalent movement, the movement speed was found to be independent of task difficulty. In all groups, the movement speed did not decrease significantly as the difficulty increased. However, the speed decreased as the difficulty increased in nonequivalent movement. Fitt’s effect seemed to exist only in nonequivalent movement, which required coordination of nonequivalent movements in the wrist and fingers. The equivalent movements are composed of consistent simultaneous flexion/extension movements in the wrist and fingers. The Fitt’s effect did not exist in the equivalent movement probably because it was not sensitive to the difficulty of the task. It might be a subtle but not significant change across the different levels of difficulty. A previous study also found that the equivalent pattern had performance characteristics similar to those observed in the wrist-only and fingers-only pattern. The nonequivalent pattern was more variable and was executed more slowly than the fast-as-possible movement required [34]. Our results point to the role of nonequivalent movements in slowness and reduced target size. The movement enhanced the effect of task size such that there was significant difference across task complexities.
Several limitations must be recognized regarding this study. It is possible that insufficient sensitivity or specificity for the observed parameters was used in the drawing task of this study to differentiate the three groups. Other factors relating to the experimental controls such as time constraints and the number of writing strokes or corrections required may need to be considered during the experiment. The latter relates to the potential explanation that the differences in the kinematic measures among the groups might reflect memory problems. For example, the circle size control would involve the memory of the size of the circles and loops. More studies are needed to address whether motor dysfunctions of aMCI and AD are the resultant effects of central deficits (e.g., cognitive dysfunction) and peripheral disorders (e.g., tremor). Fine motor assessments other than graphomotor tests are suggested as an adjunct in future studies to validate these effects.
5 Conclusion
In summary, as expected, persons with aMCI have impaired graphomotor function, whose degree is similar to but less than that of persons with AD. The results suggest that aMCI is characterized by motor dysfunction and cognitive impairment and that the degree of motor impairment, particularly in aiming movements with accuracy constraint, may help identify those at risk for AD. The psychomotor deteriorations demonstrated in the variables of graphic aiming tasks in this investigation, if replicable with a greater sample size, can be used as supplementary information in identifying individuals with aMCI or AD.
References
Chui, H. C. (1989). Dementia. A review emphasizing clinicopathologic correlation and brain-behavior relationships. Archives of Neurology, 46, 806–814.
Wimo, A., Jonsson, L., & Winblad, B. (2006). An estimate of the worldwide prevalence and direct costs of dementia in 2003. Dementia and Geriatric Cognitive Disorders, 21, 175–181.
Khachaturian, Z. S. (1985). Diagnosis of Alzheimer’s disease. Archives of Neurology, 42, 1097–1105.
Pettersson, A., Olsson, E., & Wahlund, L.-O. (2005). Motor function in subjects with mild cognitive impairment and early Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders, 19, 299–304.
Petersen, R. C., Smith, G. E., Waring, S. C., Ivnik, R. J., Tangalos, E. G., & Kokmen, E. (1999). Mild cognitive impairment: clinical characterization and outcome. Archives of Neurology, 56, 303–308.
Yan, J. H. (2000). Effects of aging on linear and curvilinear aiming arm movements. Experimental Aging Research, 26, 393–407.
Waite, L. M., Broe, G. A., Grayson, D. A., & Creasey, H. (2000). Motor function and disability in the dementias. International Journal of Geriatric Psychiatry, 15, 897–903.
Petersen, R. C., Jack, C. R, Jr., Xu, Y. C., Waring, S. C., O’Brien, P. C., Smith, G. E., et al. (2000). Memory and MRI-based hippocampal volumes in aging and AD. Neurology, 54, 581–587.
Walker, N., Philbin, D. A., & Fisk, A. D. (1997). Age-related differences in movement control: adjusting submovement structure to optimize performance. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 52, P40–P52.
Pennanen, C., Kivipelto, M., Tuomainen, S., Hartikainen, P., Hanninen, T., Laakso, M. P., et al. (2004). Hippocampus and entorhinal cortex in mild cognitive impairment and early AD. Neurobiology of Aging, 25, 303–310.
Dick, M. B., Nielson, K. A., Beth, R. E., Shankle, W. R., & Cotman, C. W. (1995). Acquisition and long-term retention of a fine motor skill in Alzheimer’s disease. Brain and Cognition, 29, 294–306.
Vandervoort, A. A. (2002). Aging of the human neuromuscular system. Muscle and Nerve, 25, 17–25.
Camicioli, R., Howieson, D., Oken, B., Sexton, G., & Kaye, J. (1998). Motor slowing precedes cognitive impairment in the oldest old. Neurology, 50, 1496–1498.
Buracchio, T., Dodge, H. H., Howieson, D., Wasserman, D., & Kaye, J. (2010). The trajectory of gait speed preceding mild cognitive impairment. Archives of Neurology, 67, 980–986.
Boyle, P., Wilson, R., Buchman, A., Aggarwal, N., Tang, Y., Arvanitakis, Z., et al. (2007). Lower extremity motor function and disability in mild cognitive impairment. Experimental Aging Research, 33, 355–371.
Aggarwal, N. T., Wilson, R. S., Beck, T. L., Bienias, J. L., & Bennett, D. A. (2006). Motor dysfunction in mild cognitive impairment and the risk of incident Alzheimer disease. Archives of Neurology, 63, 1763–1769.
Watson, N., Rosano, C., Boudreau, R., Simonsick, E., Ferrucci, L., Sutton-Tyrrell, K., et al. (2010). Executive function, memory and gait speed decline in well-functioning older adults. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 65, 1093–1100.
Goldman, W. P., Baty, J. D., Buckles, V. D., Sahrmann, S., & Morris, J. C. (1999). Motor dysfunction in mildly demented AD individuals without extrapyramidal signs. Neurology, 53, 956–962.
Yan, J. H., & Dick, M. B. (2006). Practice effects on motor control in healthy seniors and patients with mild cognitive impairment and Alzheimer’s disease. Neuropsychology, Development, and Cognition Section B, Aging, Neuropsychology and Cognition, 13, 385–410.
Kluger, A., Gianutsos, J. G., Golomb, J., Ferris, S. H., George, A. E., Franssen, E., & Reisberg, B. (1997). Patterns of motor impairement in normal aging, mild cognitive decline and early Alzheimer’s disease. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 52, P28–P39.
Song, Z., Guo, S., Pang, M., Zhang, S., Xiao, N., Gao, B., & Shi, L. (2014). Implementation of resistance training using an upper-limb exoskeleton rehabilitation device for elbow joint. Journal of Medical and Biological Engineering, 34, 188–196.
Marquardt, C., & Mai, N. (1994). A computational procedure for movement analysis in handwriting. Journal of Neuroscience Methods, 52, 39–45.
Schroter, A., Mergl, R., Burger, K., Hampel, H., Moller, H. J., & Hegerl, U. (2003). Kinematic analysis of handwriting movements in patients with Alzheimer’s disease, mild cognitive impairment, depression and healthy subjects. Dementia and Geriatric Cognitive Disorders, 15, 132–142.
van Gemmert, A. W. A., Teulings, H.-L., & Stelmach, G. E. (1998). The influence of mental and motor load on handwriting movements in Parkinsonian patients. Acta Psychologica, 100, 161–175.
Werner, P., Rosenblum, S., Bar-On, G., Heinik, J., & Korczyn, A. (2006). Handwriting process variables discriminating mild Alzheimer’s disease and mild cognitive impairment. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 61, P228–P236.
Silveri, M. C., Corda, F., & Di Nardo, M. (2007). Central and peripheral aspects of writing disorders in Alzheimer’s disease. Journal of Clinical and Experimental Neuropsychology, 29, 179–186.
Luzzatti, C., Laiacona, M., & Agazzi, D. (2003). Multiple patterns of writing disorders in dementia of the Alzheimer type and their evolution. Neuropsychologia, 41, 759–772.
Yan, J. H., Rountree, S., Massman, P., Doody, R. S., & Li, H. (2008). Alzheimer’s disease and mild cognitive impairment deteriorate fine movement control. Journal of Psychiatric Research, 42, 1203–1212.
Hughes, C. P., Berg, L., Danziger, W. L., Coben, L. A., & Martin, R. L. (1982). A new clinical scale for the staging of dementia. British Journal of Psychiatry, 140, 566–572.
McKhann, G., Drachman, D., Folstein, M., Katzman, R., Price, D., & Stadlan, E. M. (1984). Clinical diagnosis of Alzheimer’s disease Report of the NINCDS-ADRDA Work Group* under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology, 34, 939.
Petersen, R. C. (2004). Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine, 256, 183–194.
Fitts, P. M. (1954). The information capacity of the human motor system in controlling the amplitude of movement. Journal of Experimental Psychology, 47, 381–391.
Velasques, B., Machado, S., Paes, F., Cunha, M., Sanfim, A., Budde, H., et al. (2011). Sensorimotor integration and psychopathology: motor control abnormalities related to psychiatric disorders. The World Journal of Biological Psychiatry, 12, 560–573.
Dounskaia, N., van Gemmert, A. W. A., & Stelmach, G. (2000). Interjoint coordination during handwriting-like movements. Experimental Brain Research, 135, 127–140.
Acknowledgments
The authors would like to thank the Ministry of Science and Technology of the Republic of China for financially supporting this work under Grants NSC 100-2221-E-214-041 and NSC 101-2221-E-214-005.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, NY., Chang, SH. Kinematic Analyses of Graphomotor Functions in Individuals with Alzheimer’s Disease and Amnestic Mild Cognitive Impairment. J. Med. Biol. Eng. 36, 334–343 (2016). https://doi.org/10.1007/s40846-016-0143-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40846-016-0143-y