Abstract
Under physiological conditions, the opening and closing of the leaflets of an implanted artificial heart valve (AHV) affects the blood components and therefore may cause various complications to the patient such as hemolysis or platelet activation. In this paper, a computational fluid model is presented. The regional distribution of flow shear stress in an AHV is analyzed using computational fluid dynamics and AHV performance is evaluated in terms of the variation of flow velocity and pressure when blood passes the leaflets in the aortic valve. The results suggest that for the design of a mechanical AHV, the maximum opening angle and internal orifice diameter should be increased to improve the fluid structure interaction and decrease the possibility of damage to blood components. Finally, the fluid stress distribution of the AHV leaflet structure was calculated and analyzed under pulsating flow conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Heart valves maintain unidirectional blood flow by the opening and closing of their leaves, which depend on the pressure difference on the two sides of the valve leaflets. If a natural heart valve is damaged by severe deformity or disease, it should be replaced by an artificial heart valve (AHV), either mechanical or biological, to recover biological function of blood flow transport. Currently, over 55 % of implanted AHVs are mechanical valves [1]. Mechanical valves consist of mechanical components with good mechanical behavior and biocompatibility. Among the various types of mechanical valve, bileaflet mechanical valves are the most widely applied clinically.
A fluid–structure interaction analysis computation method has been applied to AHVs [2]. De Hart et al. [3] analyzed the movement variation of leaflets with time using laser Doppler velocimetry and a high-speed camera. Pelliccioni et al. [4] established a two-dimensional (2D) digital model to analyze single- and bi-leaflet valves in aortic position using a discrete lattice Boltzmann method. Zhang et al. [5] analyzed the opening and closing of a bileaflet mechanical heart valve with the finite element method (FEM) by applying a coupling algorithm based on the arbitrary Lagrangian–Eulerian (ALE) method. Zhang et al. [6] analyzed the relationship between a valve and a 2D flow field using ANSYS software. Most artificial mechanical heart valve designs and analyses have adopted 2D models and steady-state flow in the valves, new methods and measures to improve the valve structure would been proposed.
The present study analyzes the impact of the valve structure on blood flow, including hemodynamic aspects. The destruction of blood components and platelet activation by the shear stress generated by the coupled interaction between the valve and flow are discussed. The computation uses the FEM with a coupling algorithm based on the ALE method.
The behavior of blood flow through an AHV is related to the AHV geometry. The fluid velocity is high when the jet passes the valve, which may cause eddies and induce a high shear stress in the blood flow region. Wurzinger et al. [7] found that turbulent stress levels of 100–1000 dyn/cm2 can activate platelets, and that the hemolysis threshold is above 8000 dyn/cm2 [8, 9]. Kroll et al. estimated damage thresholds of blood components by fluid shear stress. Their results are shown in Table 1.
In this paper, a volume method is applied to characterize the blood flow field. The fluid domain is discretized to generate a finite element spatial lattice. In this method, differential equations (e.g., Navier–Stokes equation and k-ε equation) are used to control volume integration, resulting in a set of discrete equations for analyzing the flow field process.
2 Methods
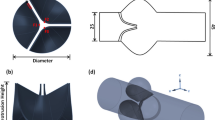
When blood is transported through a natural heart valve (Fig. 1a), the flow is central-field. In contrast, blood flow through a mechanical bileaflet valve is through three channels and is approximately central-field. Makhijan et al. [13] considered the fluid flow through a natural heart valve to be a pulsating laminar flow. Experiments revealed that the flow is laminar in the early acceleration phase [14], and thus a three-dimensional (3D) numerical simulation of the fluid–structure interaction in the initial period can be based on laminar flow.
In the present work, a 25-mm bileaflet mechanical valve is chosen as the experimental model because it is currently the most frequently clinically applied mechanical heart valve [15]. The two leaflets are linked to the valve ring by a pivot and butterfly pit. The leaflets are rotationally opened by the blood stream flowing through the valve. A schematic diagram of the blood flow through the AHV is shown in Fig. 1b.
The interaction of the blood with the leaflets and orifice of the AHV structure was simulated using the FEM. The process is shown schematically in Fig. 2. Meshes were generated using a computational fluid dynamics (CFD) pre-treatment program in integrated computer engineering and manufacturing (ICEM) and Fluent software (Analysis Inc.), combined with the FEM. The valve model was then analyzed.
When the valve is fully opened, blood flows through the orifice along the leaflets. The leaflets can impede the blood flow through the valve in the valval field, increasing the speed of the jet stream. The interaction between the blood flow and the valve causes a high shear stress, which increases the possibility of platelet activation or red blood cell damage [11, 16–18].
2.1 Modeling Design and Simulation
Fluid flow control by an AHV is shown schematically in Fig. 1c, d. The valve leaflets are opened by the blood flow and closed by coupling forces. The force calculation process is as follows. When blood flows through the valve, the leaflets are opened, and when blood refluxes, they are quickly closed. The blood flow is regarded as steady when the valve is fully opened, as it is assumed that the flow conditions, such as velocity, pressure, and temperature, are constant. Adopting the finite volume method, the velocity field can be solved using momentum equations, the pressure field can be solved using continuity and momentum equations, and time integration can be carried out using the implicit momentum equation and the pressure equation.
A 3D geometric model of a mechanical heart valve at the aortic position was constructed as shown in Fig. 3.
3D computational model of physical structure of aorta containing AHV. a Aortic pulsating flow velocity imposed at inflow section [16], b parameter settings, and c boundary conditions
Computer simulation was performed under pulsating blood flow conditions, with the inflow set as 4 L/min [19]. The interaction between the valve and the blood flow was calculated and analyzed for an open valve.
In the valve system, blood flow changes periodically. The valve motion is divided into three phases [16], as shown in Fig. 3a. In phase I, 0 < t ≤ 0.06 s, the valve is closed; in phase II, 0.06 s < t ≤ 0.35 s, the valve is open (includes opening and closing of the valve); in phase III, 0.35 s < t ≤ 0.8 s, the valve is closed. The valve is fully open at time 0.09 s, and the maximum velocity reaches 0.5 m/s at 0.14 s. Under the condition of no reflux in the third phase, the experiment was mainly carried out for the period 0.12–0.30 s (AHV fully opened).
The basic assumptions of the model are as follows: (i) the blood fluid is a viscous, incompressible, isothermal homogeneous Newtonian fluid; (ii) deformation of tissue around the AHV is ignored, and frictionless interfaces between the fluid and solid are assumed; (iii) there is no fluid return at the output end; (iv) the temperature is constant; (v) gravitational force is neglected. According to the above assumptions, laminar steady-state flow is selected on the inflow side in the computation.
A 3D model of the AHV is shown in Fig. 3b and defined in Table 2. The experimental conditions were set as follows. The diameter d of the inlet side close to the heart ventricle is 22.5 mm. The inflow condition is set as u = 0.32 m/s, where u is the inflow velocity. The blood density (ρ) is 1.056 g/cm3. The kinetic viscosity (η) of blood is 3.5 cp at 37 °C [18], and the Reynolds number (Re) is 3186, which is defined by Re = udρ/η. Re is located in the subcritical region (the critical Reynolds number range is generally 2100–4000 in a tube). Vortex turbulence can form in the leaflet wake. For the outlet boundary condition, the initial outlet pressure Pin = 0 Pa. A no-slip boundary condition is imposed for the interaction between the blood flow and the valve. The entrance boundary condition is given by the initial speed; the boundary condition of the pressure on the exit side is set as zero (Pout = 0 Pa) and there is no return of the fluid.
Structural boundary conditions were set as follows. There is no translational motion between the pivot and leaflets. The boundary layers between the flow and the valve are assumed to have non-sliding mesh boundary conditions [22].
Mesh generation: The computational model uses the ALE method, which relies on the re-generation of a continuously deforming mesh capable of maintaining good mesh quality. The mesh roughness is set by the type and size of the grid. The complex fluid blocks are meshed by tetrahedral and triangular grids.
To increase the solution accuracy and reduce calculation time, the flow model was divided mainly into hexahedral, both for the cylindrical fluid runner and transitional cells of the model (Fig. 4a). In the annular regions, the flow field was further subdivided into tetrahedral elements. From grid quality detection, all the elements had maximum skewness less of than 0.8. The smaller the skewness value, the better is the mesh quality, and thus the calculations converge more easily. The checked mesh quality should be acceptable to finite element calculation [23]. Finally, the cell model was translated into polyhedral mesh grids in the computational domain to increase computational efficiency. The mesh consisted of three blocks with a total of 5 × 105 cells. The mesh model is shown in Fig. 4.
The flow field of the AHV at completely open states was analyzed. The interaction force of the flow on the blood components in the completely open condition was evaluated. The transvalvular pressure and the velocity of the flow were investigated, and the transient flow field was analyzed for an entirely opened valve (Fig. 4b). The valve performance was evaluated through flow field analysis to determine the interaction force on blood components.
Ge et al. [24] found that the unsteady Reynolds-averaged Navier–Stokes (URANS) equations are suitable for detached-eddy simulation. Therefore, URANS is adopted for experimental simulation, and a two-order-accuracy in the k-ε equation is applied, where k, the turbulent kinetic energy, equals 5.234 × 10−4 m2/s2; and the turbulent dissipation rate ε equals 8.517 × 10−4 m2/s3. Pulsating flow is selected on the inflow side in the computation. The simulation computation adopts a weak coupling method to analyze the interactions between the AHV and blood flow.
3 Results and Discussion
3.1 Fluid Velocity Field Distribution
Figure 5 shows the flow velocity contours and streamlines expressing the forward flow in the velocity field. The valval field consists of a turbulent region, an eddy zone, and a recirculation zone. The velocity is obviously greater behind the valve, up to a maximum velocity of 0.59 m/s and ranging from twice to three times the initial velocity. The downstream flow is relatively symmetric. In the middle section of the flow stream, a turbulent flow region is generated, which easily forms a zone with high shear stress turbulence as the Reynolds shear stress (RSS) [25]. RSS is an important factor in the formation of blood hemolysis and an irreversible aggregation of platelets. In the wake of the leaflets, vortices appear near the physiologic sinuses. These vortices push blood flow downward to the central region. As the ventricle contracts, the aortic sinus becomes the mainly impacting point of blood flow, which then turns right into the aortic arch [26].
3.2 Wall Shear Stress
Flow shear stress is an important characteristic for the hemodynamics of AHVs. Figure 6 shows the pressure distribution in the fluid field, displaying the absolute value of the Z-direction shear stress in the flow field. The shear stress is expressed as τ = μ(du/dz) [27]. The flow state was analyzed for the heart valve at the maximum opening angle. The maximum RSS occurs in a narrow channel, near the leaflet pivot part and at the edges of the valve.
X–Y section (Z-axis direction, Fig. 4b) wall shear stress (Pa, 1 dyn/cm2 = 0.1 Pa)
Yoganathan et al. [28] found that a shear stress of 100–500 dyn/cm2 greatly enhances the ability of platelets to aggregate, further leading to thrombosis and directly endangering the patient’s life. Additionally, Oosterbaan et al. [29] reported that a change of wall shear stress can influence the growth of surrounding tissue cells. Figure 6 shows that the wall shear stress of valval pivot and wall position is somewhat greater than elsewhere, with a maximum value above 16 Pa (160 dyn/cm2), which may trigger platelet activation [30, 31]. Moreover, a high velocity gradient may be generated between the reflux and the swirl.
3.3 Comparison of Valve Designs Based on Flow Field Calculations
To optimize the parameters of the structure and reduce the maximum transvalvular pressure difference (TPD), various maximum opening angles and annular calibers of the valves were compared.
Figure 7 shows the velocity distribution at maximum opening angles θ = 80°, 85°, and 90°. Results for the TPD at various maximum opening angles, at a location 2.5 times the valve orifice diameter between the front and back of the valve orifice [32], are shown in Fig. 7b. The results reveal that increasing the maximum opening angle from 80° to 90° decreased the average pressure difference ΔP across the valve significantly. The average pressure difference decreases with increasing maximum opening angle.
The TPD and the velocity distribution for orifice internal diameters of 20.4 mm (St. Jude Medical Inc. valve model), 22.5 mm (intermediate diameter), and 23.4 mm (ON-X valve model) were analyzed for a maximum opening angle of 85°.
Figure 8 shows that the differences in the flow velocity field decreased with increasing internal valve diameter. Figure 8b shows the effect of internal valve diameter on the average differential pressure. When the internal diameter increased from 20.4 to 23.4 mm, the average pressure difference ΔP decreased from 109 to 52 Pa.
These results indicate that higher maximum opening angle and internal orifice diameter are beneficial for lower average pressure difference and thus also for lower shear stress in the blood flow, and thus beneficial for decreasing the possibility of damage to blood components.
4 Transient Pulsating Flow
Using the FEM, the drag of the bileaflet AHV structure was calculated and analyzed under pulsating flow conditions. The inflow peak velocity was 0.5 m/s, the leaflet thickness was 0.8 mm, and the maximum opening angle was 85°.
Figure 9a shows the transient velocity distribution in the flow field for three time points: at the highest inflow velocity (0.59 m/s) (I), during the middle period, when the flow velocity distribution of the curved leaflet fluid flow field is uniform (II), and when the valve begins to close (maximum flow velocity is 0.32 m/s) (III).
The flow vorticity of the bileaflet AHV was calculated and analyzed in the transient flow field. The results are shown in Fig. 9b. When the inlet flow velocity reaches the peak of the pulsating flow (0.5 m/s) at 0.15 s, the vorticity distribution is mainly on the leaflets and annulus surface, with the maximum value at the front of the leaflets. This computing result will help to improve blood flow character and reduce the impact of high shear stress on blood components by eddy formation in the valve flow field.
5 Comparison Between Experimental and Computational Data
Table 3 shows a comparison of the experimental results for the maximum opening angle and the related effective open area (EOA) for various bileaflet mechanical heart valves, with EOA = Q/(44.3 × ΔP) (where Q is the blood flow through the valve and ΔP is the average TPD). A higher EOA is beneficial for decreasing the TPD [33]. Table 3 data support the simulation computation results deduced from Fig. 8, even though different bileaflet mechanical heart valve structures may impact the blood flow behavior.
Table 3 shows the in vitro hemodynamic experimental results for various annulus inner diameters for AHVs provided by St. Jude Medical Inc. [39]. These results indicate that increasing the annulus inner size increases the EOA and reduces the average transvalvular pressure. Thus, these data further support the simulation results of Fig. 8.
The experimental results listed in Tables 3 and 4 show that the simulation computation in this work is reliable. The results not only reveal tendencies in hemodynamic behavior, as shown in Figs. 7 and 8, but also indicate that attention should be paid to specific geometric points, such as the peak position in Fig. 6 and the small edge area shown in Fig. 9b (point A).
6 Conclusion
This study investigated a 3D numerical model to analyze the flow field of a bileaflet valve at the maximum opening angle. The formation and distribution of the fluid flow, including the fluid trajectory, velocity, pressure, shear stress, and stress eddy, were analyzed for steady-state flow and pulsating flow conditions. The results show that when the valve is under jet conditions, the flow is close to a subcritical turbulent state and vortices form in the leaflet wake. The sinus can slow the flow velocity, and the leaflets introduce a minor shear force in the flow field. There is a greater shear stress at the pivot position.
The valvular hemodynamic performance at the maximum valve opening angle was analyzed using the FEM under both steady and the pulsating flow conditions. Finally, the maximum opening angle and annulus diameter range were investigated. Some detailed valvular hemodynamic performance characteristics, including the flow pattern and the exact flow pressure and shear stress distribution, were also computed and analyzed.
The flow field of the bileaflet valve has a uniform distribution in the aortic position, which minimizes the resistance of the valve to the flow field. In order to optimize the valve structure, the transprosthetic difference in shear stress should be reduced by rational design of the valve leaflet profile and structure, such as increasing the maximum opening angle and internal orifice diameter, which can lead to a more uniform flow field and decrease the possibility of damage to blood components.
References
Vongpatanasin, W., Hillis, L. D., & Lange, R. A. (1996). Medical progress—Prosthetic heart valves. New England Journal of Medicine, 335, 407–416.
Yeh, H. H., Grecov, D., & Karri, S. (2014). Computational modelling of bileaflet mechanical valves using fluid–structure interaction approach. Journal of Medical and Biological Engineering, 34, 482–486.
De Hart, J., Peters, G. W. M., & Schreurs, P. J. G. (2000). A two-dimensional fluid–structure interaction model of the aortic value. Journal of Biomechanics, 33, 1079–1088.
Pelliccioni, O., Cerrolaza, M., & Surós, R. (2008). A biofluid dynamic computer code using the general lattice Boltzmann equation. Advances in Software Engineering, 39, 593–611.
Jianhai, Z., Dapeng, C., & Shengquan, Z. (1996). ALE finite element analysis of the opening and closing process of the artificial mechanical valve. Applied Mathematics and Mechanics, 17, 403–412.
Zhang, L. R. (2007). A steady numerical analysis of a fluid–solid coupling model in aortic artificial bileaflet heart valves. Journal of Clinical Rehabilitative Tissue Engineering Research, 11(40), 8107–8110.
Wurzinger, L. J., Opitz, R., & Wolf, M. (1984). Shear induced platelet activation—A critical reappraisal. Biorheology, 22, 399–413.
Hellums, J. D. (1994). 1993 Whitaker lecture: Biorheology in thrombosis research. Annals of Biomedical Engineering, 22, 445–455.
Lu, P. C., Lai, H. C., & Liu, J. S. (2001). A reevaluation and discussion on the threshold limit for hemolysis in a turbulent shear flow. Journal of Biomechanics, 34, 1361–1364.
Harrison, P. (2005). Platelet function analysis. Blood Reviews, 19, 111–123.
Kroll, M. H., Hellums, J. D., McIntire, L. V., Schafer, A. I., & Moake, J. L. (1996). Platelets and shear stress. Blood, 88, 1525–1541.
Goto, S., Ikeda, Y., & Saldívar, E. (1998). Distinct mechanisms of platelet aggregation as a consequence of different shearing flow conditions. Journal of Clinical Investigation, 101, 479–485.
Makhijani, V. B., Yang, H. Q., Dionne, P. J., & Thubrikar, M. J. (1997). Three-dimensional coupled fluid–structure simulation of pericardial bioprosthetic aortic valve function. ASAIO Journal, 43, 387–392.
Dasi, L. P., Ge, L., & Simon, H. A. (2007). Vorticity dynamics of a bileaflet mechanical heart valve in an axisymmetric aorta. Physics of Fluids, 19, 067105.
Puvimanasinghe, J. P. A., Takkenberg, J. J. M., & Edwards, M. B. (2004). Comparison of outcomes after aortic valve replacement with a mechanical valve or a bioprosthesis using microsimulation. Heart, 90, 1172–1178.
Woo, Y. R., & Yoganathan, A. P. (1986). In vitro pulsatile flow velocity and shear stress measurements in the vicinity of mechanical mitral heart valve prostheses. Journal of Biomechanics, 19, 39–51.
Hasenkam, J. M., Nygaard, H., Giersiepen, M., Reul, H., & Stodkilde-Jorgensen, H. (1988). Turbulent stress measurements downstream of six mechanical aortic valves in a pulsatile flow model. Journal of Biomechanics, 21, 631–645.
Leverett, L. B., Hellums, J. D., Alfrey, C. P., & Lynch, E. C. (1972). Red blood cell damage by shear stress. Biophysical Journal, 12, 257–273.
Chu, Y. P., Chen, J. L., & Chen, R. K. (2004). Research the fluid dynamics performance of cardiac artificial mechanical valve output flow in vitro. National Medical Journal of China, 84, 896–897.
Tao, Z. L. (2000). Introductory biomechanics. Tianjin: Tianjin Science & Technology Translation & Publishing Corp.
Che, Z. Y., & Wang, Y. S. (1984). St. Jude Medical artificial heart valve. International Journal of Cardiovascular Disease, 3, 12.
Morbiducci, U., Ponzini, R., & Nobili, M. (2009). Blood damage safety of prosthetic heart valves. Shear-induced platelet activation and local flow dynamics: A fluid–structure interaction approach. Journal of Biomechanics, 42, 1952–1960.
Lea, J., & Adesina, A. A. (2007). CFD diagnosis of the cyclotol manufacturing plant: Product quality and process operation. Chemical Product and Process Modeling, 2(1), 1934–2659. doi:10.2202/1934-2659.1027.
Ge, L., Leo, H. L., & Sotiropoulos, F. (2005). Flow in a mechanical bileaflet heart valve at laminar and near-peak systole flow rates: CFD simulations and experiments. Journal of Biomechanical Engineering, 127, 782–797.
Anderson, J. D. (2002). Computational fluid dynamics: The basics with applications. Beijing: Tsinghua University Press.
Kouchoukos, N. T., Blackstone, E. H., & Hanley, F. L. (2012). Kirklin/Barratt-Boyes Cardiac Surgery. Philadelphia, PA: Elsevier Health Sciences.
Zhang, L. R., & Ge, S. R. (2007). A steady numerical analysis of a fluid–solid coupling model in aortic artificial bileaflet heart valves. Journal of Clinical Rehabilitative Tissue Engineering Research, 11, 8107–8110.
Yoganathan, A. P., Woo, Y. R., & Sung, H. W. (1986). Turbulent shear stress measurements in the vicinity of aortic heart valve prostheses. Journal of Biomechanics, 19, 433–442.
Oosterbaan, A. M., Poelma, C., & Bon, E. (2014). Altered wall shear stresses in embryonic chicken outflow tract due to homocysteine exposure. Journal of Medical and Biological Engineering, 34, 56–61.
Wurzinger, L. J., Opitz, R., & Blasberg, P. (1985). Platelet and coagulation parameters following millisecond exposure to laminar shear stress. Thrombosis and Haemostasis, 54, 381–386.
Hellums, J. D., Peterson, D. M., & Stathopoulos, N. A. (1987). Studies on the mechanisms of shear-induced platelet activation. In Cerebral ischemia and hemorheology, Berlin: Springer.
Nobili, M., Morbiducci, U., & Ponzini, R. (2008). Numerical simulation of the dynamics of a bileaflet prosthetic heart valve using a fluid–structure interaction approach. Journal of Biomechanics, 41, 2539–2550.
Xu, S., Han, H. G., & Wang, H. S. (2013). Properties of stentless porcine aortic valve for single-layer suture and implantation. Chinese Journal of Tissue Engineering Research, 17, 7667–7674.
Chen, R. K., Wu, M., & Yang, M. L. (2003). Studies on preparation and implantation of a newly domestic-made solid pyrolytic carbon bileaflet heart valve prosthesis. Chinese Journal of Biomedical Engineering, 22, 453–460.
Chu, Y. P. (2000). Hydrodynamics of the mechanical heart valve prostheses. Hangzhou: Zhejiang University.
Fraund, S., Pethig, K., & Wahlers, T. (1998). ON-X bileaflet valve in aortic position-early experience shows an improved hemodynamic profile. Thoracic and Cardiovascular Surgeon, 46, 293–297.
Palatianos, G. M., Laczkovics, A. M., & Simon, P. (2007). Multicentered European study on safety and effectiveness of the On-X prosthetic heart valve: intermediate follow-up. The Annals of Thoracic Surgery, 83, 40–46.
Feng, Z., Nakamura, T., & Fujimoto, T. (2002). In vitro investigation of opening behavior and hydrodynamics of bileaflet valves in the mitral position. Artificial Organs, 26, 32–39.
St. Jude Medical Home Page. Accessed January 14, 2014, from http://www.sjm.com.
Criscitiello, M. G., & Levine, H. J. (1992). Thromboembolism and prosthetic heart valves. Hospital Practice (Office edition), 27, 69–79, 82–86, 90–96.
Dumont, K., Stijnen, J. M. A., & Vierendeels, J. (2004). Validation of a fluid–structure interaction model of a heart valve using the dynamic mesh method in fluent. Computer Methods in Biomechanics and Biomedical Engineering, 7, 139–146.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, F., Cui, Y.Y., Wu, L.L. et al. Analysis of Flow Field in Mechanical Aortic Bileaflet Heart Valves Using Finite Volume Method. J. Med. Biol. Eng. 36, 110–120 (2016). https://doi.org/10.1007/s40846-016-0106-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40846-016-0106-3