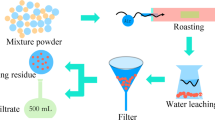
Abstract
Serious concerns have been raised from the solid waste processing field about minimizing the environmental footprint of electroplating sludge (ES), not only because of its hazardousness but also due to the rising demand for recycling non-renewable metal resources. A low-temperature alkaline matte smelting process is proposed in the present study. Na2CO3 was used as a flux reagent for smelting ES at 1250 °C, which is 100–250 °C lower than those previously reported. XRD and SEM–EDS revealed that the presence of Na species in the matte and slag phases was conducive to reducing the smelting temperature. The relationship between the processing parameters, i.e., Na2CO3, coal powder, and sulfur dosages, and the partitioning of Cu, Ni, and Cr were investigated for optimal metal recovery. A copper–nickel matte (Cu + Ni = 46.9 wt%) was produced. Cu and Ni recovery achieved 95% and 90%, respectively, while over 99% Cr was concentrated in the slag. The TCLP test indicated that heavy metal leaching toxicities of the slag were below the Chinese national identification standards (GB5085.3-2007) due to the formation of stable spinel phases and embedment of heavy metals in the glassy slag matrix.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The electroplating process is widely applied in various manufacturing industries, such as automobiles, electronics, metal finishing, etc., but leads to massive electroplating sludge (ES) disposal [1]. ES produced in China amounts to several million tons annually and is characterized by complex composition, strong migration ability, and severe ecological toxicity [2], which is a high-risk threat to the ecological environment [3]. Therefore, serious concerns from both legislation and industry are raised to minimize the environmental footprint of ES, not only because of its hazardousness but also due to the rising demand for sustainable use of non-renewable metal resources [4].
Generally, 3R rules, namely, remediation, reduction, and recycling, should be considered when processing solid wastes. The solidification/stabilization method is the most widely applied technique for safe ES disposal due to its simplicity and low cost. Using a stabilization agent and/or a high-temperature calcination method [5], heavy metals can be stabilized in the matrix of fire clay brick, ceramics, or backfill materials [6,7,8]. However, the metal values in ES can no longer be extracted from the stabilized products, except for the volatile elements or compounds [9]. Hydrometallurgical methods were also proposed due to their ability to extract metals from ES with high recovery [10]. Typically, ES was leached by sulfuric acid, hydrochloric acid, or ammonium hydroxide solution, with subsequent precipitation, solvent extraction, or electrowinning steps for further separation/purification of various metals [1, 11, 12]. Value-added materials also can be synthesized from the leaching solution to enhance the economic feasibility of the processes [13,14,15]. Nevertheless, secondary pollution of leaching residues and wastewater remains challenging [2].
Recently, high-temperature smelting processes attracted great interest in extracting valuable metals from ES [16]. Tian et al. [17] reported a carbon thermal reduction and low-carbon reduction refining process for Cu ES, by which 96% Cu and 85% Ni were enriched in an anode copper plate, while 90.77% Pb, 95.14% Sn, and 99.92% Zn were recovered in the flue dust. This technique showed a high potential for industrial application because not only metal values were efficiently recovered, but also the final slag can be safely used as a construction material [18]. However, Cr is a refractory element and its content in ES usually ranges from 1.5 to 15 wt% [1], resulting in high smelting temperatures of ES. Zhou et al. [19] reported that smelting an ES with a Cr content of 6 wt% at 1500 °C using starch as the reductive agent. Zhang et al. [20] also found that an ES containing 4 wt% Cr should be smelted at a temperature above 1350 °C. Moreover, these high-temperature reductive smelting methods resulted in the formation of metal alloys to which Cu, Ni, Fe, and Cr were simultaneously reported, leading to difficulties in further purification [21, 22]. Therefore, developing a smelting technique for ES at a typical nonferrous metal smelting temperature (1000–1300 °C) would save energy and benefit further metal purification, finally leading to lower carbon emissions and sustainability of the industry.
The alkaline smelting process, in which alkaline fluxes such as Na2CO3, NaOH, etc. are used as flux [23], is a low-temperature smelting method for various raw materials [24]. It was reported that the smelting temperatures of Sb concentrate and waste lead paste were reduced from 1200 to 1000 °C [25] and 1350 to 880 °C [26], respectively, using alkaline smelting techniques. Meanwhile, the reaction between alkaline flux and sulfur led to the formation of a low melting point Na2S in the matte, which facilitated phase separation during smelting [27]. Dang et al. [28] pointed out that Na2CO3 can also react with Cr2O3 to form the low melting point compound Na2CrO4, as shown by Eq. (1).
Therefore, the alkaline smelting method showed a high potential to fill the gap between the current ES recycling technique and the need to extract metal values based on the 3R rules. However, the relationship between the element partitioning and smelting parameters needs to be extrapolated considering a possible industrial application. Concerns about the toxicity of the alkaline smelting products to the ecological system should also be addressed. In this work, alkaline smelting of ES was studied using Na2CO3 as the alkaline flux. The effect of Na2CO3 dosage, coal dosage, sulfur dosage, and FeO/SiO2 and CaO/SiO2 weight ratios on the partitioning of typical heavy metals, i.e., Cu, Ni and Cr, in the products was investigated. The phase and toxicity of the smelting slag were analyzed. This work suggests a low-temperature smelting route for processing ES, which will facilitate the development of harmless processing techniques for recycling abundant heavy metal-containing sludges.
Experimental
Raw Material
The raw material in this work was ES provided by Zhejiang Taitong Renewable Resources Utilization Co., Ltd., China. The ES was dried, ground, and sieved at 200 mesh to ensure homogeneity. The composition of the ES was analyzed by an X-ray fluorescence spectrometer (XRF), and metals of interest, i.e., Cu, Ni and Cr, were analyzed by inductively coupled plasma-atomic emission spectroscopy (ICP-AES). The results are listed in Table 1, showing that the mass contents of Cu, Ni, and Cr were 4.0%, 7.3%, and 3.9%, respectively. XRD analysis indicated that the ES was mainly amorphous, with only characteristic peaks of CaSO4, CaCO3, and SiO2, as shown in Fig. S1 (see Supplementary File), because it was a chemical deposition from electroplating wastewater.
Alkaline Smelting Experiment
According to the composition of raw ES, the FeOx–CaO–SiO2 slag system, which is frequently used in nonferrous metal smelting processes, was chosen in this work. The slag composition can be adjusted to obtain a melting point between 1200 and 1250 °C by adding an appropriate amount of flux according to the FeOx–CaO–SiO2 ternary [29]. Therefore, 1250 °C was used as the smelting temperature in the study, which is also a typical smelting temperature in copper pyrometallurgical processes [30]. A schematic diagram of the alkaline smelting procedure is illustrated in Fig. S2. The ES (50 g) was mixed with certain amounts of Fe2O3 (99.0%, Tianjin Kemiou Chemical Reagent Co., Ltd.) and SiO2 (99.0%, Hunan Huihong Reagent Co., Ltd.) to adjust the slag composition according to the required w(FeO)/w(SiO2) (weight ratio of FeO to SiO2) and w(CaO)/w(SiO2) (weight ratio of CaO to SiO2). FeO was used here for presentation convenience, although both Fe(III) and Fe(II) existed in the slag. Na2CO3 (99.9%, Sinopharm Chemical Reagent Co., Ltd.), coal powder (55.4 wt% carbon, 27.3 wt% volatiles and 16.8 wt% ash), and sulfur (99.0%, Hunan Huihong Reagent Co., Ltd.) were also added according to the set weight percent of the ES. The mixture was mixed in a mortar for 30 min before being transferred to a 500-mL fire clay crucible. After the furnace (SRJX-8-13, Changshi Furnace) temperature was raised to 650 °C, the crucible was transferred into the furnace, and the temperature was raised at 20 °C/min to 1250 °C. After smelting for 2 h, the crucible was lifted out of the furnace and cooled in the air atmosphere. Then, the crucible was broken, and the products, namely, matte and slag, were separated and weighed. Due to the low volatilities of Cu, Ni, and Cr compounds, their distribution in the flue dust during reduction was neglectable [17], thus, dust was not considered as a smelting product in this work. Finally, the Cu, Ni, and Cr contents in the products were analyzed.
The distribution ratio was calculated by Eqs. (2)–(3)
where DMeM and DESl denote the mass distribution ratios of metals (Me) in the matte and slag, mMeM and mESl denote the weight ratios of metals in the matte and slag, Mm and MSl denote the weights of matte and slag produced in the smelting process, and MMe denotes the total mass of metal (Me) in the ES.
Characterization
Composition Analysis
X-ray fluorescence spectrometer (XRF, XRF-1800, Shimadzu, Japan) was used to quantify element contents in the solid samples. The sample was scanned with a scanning rate of 20°/min by a tube current of 140 mA with an Rh target.
Inductively coupled plasma-atomic emission spectra (ICP-AES, ICAP 7400 Radial, Thermo Fisher Scientific, USA) were used to quantify the Cu, Ni, and Cr contents in the samples. When the standard solution with a concentration of 1 ppm or 10 ppm was measured, the RSD of ten consecutive measurements was less than 1.0%. First, standard curves were built based on Cu, Ni, and Cr standard solutions with various contents (National Nonferrous Metals and Electronic Materials Analysis and Testing Center, China). Second, the solid products, i.e., matte and slag, were digested in sulfuric (AR, Sinopharm Chemical Reagent Co., Ltd.) and phosphoric acid (AR, Sinopharm Chemical Reagent Co., Ltd.) mixtures, and then, the metal contents in the acid mixtures were analyzed.
Phase Analysis
X-ray diffraction pattern (XRD, Empyrean 2, PANalytical, Netherlands) was used to analyze the crystal phases of the samples with Cu Kα radiation (λ = 1.5406 Å). 2θ Ranging from 10° to 80° was scanned with a scanning rate of 10°/min, and the spectra were analyzed by Jade 6 using standard PDF cards (JCPDF cards) to obtain phase information.
Emission scanning electron microscopy (SEM, JSM-7900F, JEOL, Japan) was used to observe the morphology of the smelting products. The SEM was equipped with an energy-dispersive spectrometer (EDS, EDX-GENESIS60S, EDAX, USA) to analyze the micro-areas observed by SEM, and the working distance was 12 mm. The EDS data obtained in this study can be regarded as semi-quantitative only, with an uncertainty > ± 1 wt%. The samples were mounted with epoxy resin and polished. After preliminary examination by optical microscopy, the samples were sputtered with carbon to improve the conductivity under an electron beam.
Toxicity Evaluation
The toxicities of the samples were evaluated by a standard toxicity characteristic leaching procedure (TCLP) according to the Chinese National Identification standards for hazardous wastes (GB5085.3-2007), as described in the Supplementary File.
Results and Discussion
Thermodynamic Analysis and Preliminary Investigation
Superimposed predominance diagrams of Cu–S–O, Ni–S–O and Cr–S–O at 1250 °C were depicted using the Predom module in FactSage 8.0 software [31], and the results are shown in Fig. 1. It suggests that Cu2O and NiO in the ES can be reduced to Cu(l) and Ni(s) (Area (2)) at \(\log P_{{{\text{O}}_{2} }} < - 8\;{\text{atm}}\), while Cr2O3 remains unchanged. In this regard, Cu and Ni will report to metal and Cr reports to slag in reductive smelting, which would realize their separation. However, due to its high melting point (1453 °C), Ni is in solid-state at 1250 °C, which accounts for the high smelting temperature for ES with high Ni contents [19]. On the other hand, Cu2O and NiO can be converted to Cu2S (l) and Ni3S2 (l) (Area (15)) by raising \(\log P_{{{\text{SO}}_{2} }}\) at \(\log P_{{{\text{O}}_{2} }} < - 8\;{\text{atm}}\), which would facilitate the enrichment of Ni and Cu in liquid matte at 1250 °C. Meanwhile, Cr remains to be solid Cr2O3. Therefore, the predominance diagram suggested that a matte smelting route would facilitate the enrichment of Ni and Cu and the separation of Cr at a typical matte smelting temperature.
According to the thermodynamic analysis, the ES was mixed with reduction and sulfurization agents, i.e., coal and sulfur powder, and preliminary matte smelting experiments were investigated with or without alkaline flux (Na2CO3). The conditions were w(FeO)/w(SiO2) = 1.5, w(CaO)/w(SiO2) = 0.8, the dosage of coal powder and sulfur powder = 10 wt% of the ES, the dosage of Na2CO3 = 38.2 wt% of the ES, smelting temperature = 1250 °C, and smelting time = 2 h. As shown in Fig. 2, neither homogeneous phases nor clear boundaries between different phases were identified in the sample without Na2CO3. The upper layer was dark gray glassy slag with white solid particles embedded, while the lower layer was heterogeneous mixed phases of slag and gray silver matte. Pores were also observed on this layer. The sample indicated that homogeneous molten phases were not produced without Na2CO3, leading to difficulties in not only matte making but also products separation. On the other hand, a clear boundary between the matte and slag can be observed in the sample with the addition of Na2CO3. The formation of homogeneous light–dark glassy slag and gray silver matte indicated that metal values were effectively enriched in the matte phase that can be separated from the slag phase, suggesting that alkaline matte smelting was feasible to recover Cu and Ni in the ES at 1250 °C.
Effect of Na2CO3 Dosage
The effect of Na2CO3 dosage on the metal content of the smelting products was investigated under the following conditions: w(FeO)/w(SiO2) = 1.5 and w(CaO)/w(SiO2) = 0.8. The dosages of coal powder and sulfur powder were 4% and 8% of the ES weight, respectively. The smelting temperature and time of all the experiments were set at 1250 °C and 2 h otherwise mentioned.
As shown in Fig. 3A, B, the Cu and Ni contents of the slag decreased with increasing Na2CO3 dosage, while those of the matte increased. At a Na2CO3 dosage of 38.2%, the contents of Cu, Ni, and Cr of the slag (denoted as mCuSl, mNiSl and mCrSl) were 0.2 wt%, 0.7 wt%, and 4.2 wt%, respectively, while those of the matte (denoted as mCuM, mNiM and mCrM) were 19.0 wt%, 27.9 wt%, and 0.1 wt%, respectively. In accordance with the content, the matte distribution ratio of Cu and Ni (denoted as DCuM and DNiM) reached 95.1% and 90.1%, and that of Cr in the slag (denoted as DCrSl) amounted to 99.3%, as shown in Fig. 3C. This result indicated that the increase of Na2CO3 dosage was conducive to recovering Cu and Ni but had little effect on Cr distribution. Although a further increase in Na2CO3 dosage may lead to even higher recovery ratios, considering that mCuSl and mNiSl were already below 1 wt% at a Na2CO3 dosage of 38.2%, great improvement in metal recovery was not expected by a further increase of Na2CO3 dosage.
Effect of Coal Dosage
Coal powder was used as an agent to produce a reductive atmosphere. The effect of coal powder was investigated by varying the coal dosage from 2 to 13% of the ES weight, and fixing other conditions: w(FeO)/w(SiO2) = 1.5 and w(CaO)/w(SiO2) = 0.8, and the dosages of Na2CO3 and sulfur powder were 38.2% and 10% of the ES weight.
According to Fig. 4A, mCuSl, mNiSl, and mCrSl all increased with increasing coal dosage. With a coal dosage of 2%, mCuSl, mNiSl, and mCrSl were 0.2 wt%, 0.4 wt%, and 2.2 wt%, which rose to 1.5 wt%, 1.0 wt%, and 5.7 wt%, respectively, with a coal dosage of 13%. Meanwhile, mCuM, mNiM, and mCrM decreased by 3.8 wt%, 1.3 wt%, and 1.7 wt%, respectively (Fig. 4B). Figure 4C shows the effect of coal dosage on the element distribution. This result indicated that Cu and Ni were mainly reported to the matte, while Cr was reported to the slag. DCuM, DNiM, and DCrM were 94.9%, 92.3%, and 4.8%, respectively, with a coal dosage of 2 wt%. Increasing the coal dosage to 4 wt% resulted in a decrease of DCrM to 1.3%, while DCuM and DNiM remained almost unchanged. A further increase in coal dosage led to decreases of DCuM and DNiM; in particular, DCuM sharply decreased to 77.5% with a 13% coal dosage. Although coal powder was necessary for reducing metal oxides, its negative effect on metal recovery at high coal dosages could be attributed to two reasons: first, a strong reductive atmosphere may lead to the formation of metal; second, a higher coal dosage may lead to more ash containing refractory oxides, as proved by composition analysis (Table 2), the content of aluminum oxides raised with the increase of coal dosage, which may raise the viscosity and melting point of the slag. Therefore, a 4% coal dosage was chosen to be the optimal condition.
Effect of Sulfur Dosage
The effect of sulfur dosage was studied by changing from 4 to 12% of the ES weight, while fixing other conditions as w(FeO)/w(SiO2) = 1.5 and w(CaO)/w(SiO2) = 0.8, and the dosages of Na2CO3 and coal powder were 38.2% and 4% of the ES weight.
As shown in Fig. 5A, B, mCuSl, mNiSl, and mCrSl decreased from 0.4 wt%, 1.4 wt%, and 4.7 wt% to 0.3 wt%, 0.7 wt%, and 3.4 wt% by raising the sulfur dosage from 4 to 10%. This can be explained by the fact that a higher sulfur dosage facilitated the formation of Cu2S and Ni3S2, which reduced their content in the slag. Meanwhile, the Fe content of the matte (denoted as mFeM) also increased, as implied by the drop of mCuM and mNiM. Consistently, Sridhar et al. [32] studied Cu loss in smelting slag and pointed out that mCuSl decreased with increasing mFeM. However, a further increase in sulfur dosage to 12 wt% led to higher mCuSl, mNiSl, and mCrSl, which could be attributed to the movement of slag composition to the CaO–SiO2 side with excessive Fe reported to the matte phase, as the increase in [SiO4]4− content would raise the viscosity of the slag, leading to bad separation of the smelting products. Figure 5C shows the element distributions at various sulfur dosages. This result suggested that DCuM and DNiM increased with increasing sulfur dosage before 10%; however, sulfur dosages higher than 8% showed little effect on improving Cu recovery; therefore, an 8% sulfur dosage would be optimal for Cu and Ni recovery.
Effect of w(FeO)/w(SiO2)
The effect of w(FeO)/w(SiO2) ranging from 1.1 to 1.9 was studied using the conditions of w(CaO)/w(SiO2) = 0.8, and the dosages of Na2CO3, coal powder, and sulfur were 38.2%, 4%, and 8% of the ES weight, respectively.
Figure 6A, B indicates that mCuSl and mNiSl decreased before w(FeO)/w(SiO2) = 1.5 and then slightly increased, while mCrSl showed a downward trend. The possible reason for this could be that the increase of w(FeO)/w(SiO2) within a certain range is beneficial for the sedimentation of metal droplets [33], but excessive FeOx resulted in the movement of slag composition toward the FeOx apex, where the formation of solid spinel with high melting points is favored at a prevailing partial pressure of matte smelting, i.e., approximately 10−8 atm. Figure 6C suggests a consistent trend in which the optimal Cu and Ni recoveries were achieved at w(FeO)/w(SiO2) = 1.5, DCuM and DNiM were 95.1% and 90.1%, respectively, while DCrSl reached 99.3%.
Effect of w(CaO)/w(SiO2)
The effect of w(CaO)/w(SiO2) ranging from 0.6 to 0.9 was studied using the conditions of w(FeO)/w(SiO2) = 1.5, and the dosages of Na2CO3, coal powder, and sulfur were 38.2%, 4%, and 8% of the ES weight, respectively.
According to Fig. 7A, B, the effect of w(CaO)/w(SiO2) was similar to that of w(FeO)/w(SiO2). The optimal w(CaO)/w(SiO2) was found to be 0.8, by which the lowest mCuSl and mNiSl were obtained. Consistently, Fig. 7C suggests that DCuM, DNiM, and DCrSl were the highest in this condition. Nikolic et al. [34] and Shishin et al. [35] suggested that an increase in CaO content in the FeOx–SiO2–CaO slag system raised the activity coefficient of Cu in the slag, which was conducive to reducing its distribution in the slag.
Composition and Phase Analysis of the Smelting Products
The composition of some smelting products is analyzed by XRF and is listed in Table 2, and the conditions of obtaining these products are listed in Table S1. It can be informed that the Cu + Ni content of the matte ranged from 33.5 to 52.9 wt%, as described in the previous sections. It is noted that w(FeO)/w(SiO2) and w(CaO)/w(SiO2) in the slag were lower than the set values, which can be ascribed to the deportment of Fe to the matte phase and the reaction between the slag and crucible.
SEM–EDS and XRD analyses were performed to obtain phase information of the smelting products, namely, matte and slag, as shown in Figs. 8 and 9. As shown in Fig. 8A, there were two distinguishable contrasts in the slag. EDS mapping figures revealed that Fe and Cr were concentrated in the brighter area, Na distributed evenly, while Cu and Ni were almost negligible in the whole sample. Based on the EDS analysis of the respective points (Table 3), the brighter phase was mainly spinel phases of FeCr2O4, NiCr2O4, FeAl2O4, Fe3O4, or their composites, which is consistent with the XRD analysis (Fig. 9). The darker one was the glassy slag.
It is also worth mentioning that Cr was mainly detected in the spinel phases rather than the glassy slag phase. According to the phase diagram of the slag at 1250 °C (Fig. S3), liquid slag equilibrated with spinel during smelting. As the sample was not quenched but cooled in air after the experiment, the occurrence of Cr-containing spinel can be either a solid phase at the smelting temperature or formed during the cooling. The glassy slag had a higher Na content than the other phases, which could account for the lower smelting temperature of the alkaline smelting technique. On the other hand, SEM–EDS analysis of the matte revealed the existence of Cu2−xFeS and Ni3−xFexS2, in accordance with the XRD results. EDS mapping revealed that the distribution of Na was highly coincident with that of Cu, suggesting that Na2S indeed combined with Cu2S during the matte making. In fact, EDS analysis for point 4 (Table 3) also revealed a Na content of 9.4 wt% in the Cu2−xFexS matte, which is in accordance with the EDS mapping analysis.
Discussion
The liquidus temperature of slag is a determining factor in regard to the smelting temperature. According to the FeOx–CaO–SiO2 ternary [29], the liquidus is approximately 1200–1250 °C at w(FeO)/w(SiO2) = 1.5, w(CaO)/w(SiO2) = 0.8 and \(\log P_{{{\text{O}}_{2} }} = - 8\;{\text{atm}}\). In this regard, the ES can theoretically be smelted at 1250 °C. However, the presence of Cr in the ES hindered the smelting process due to its effect on raising the liquidus temperature. Sukhomlinov et al. [36] pointed out that the solubility of Cr in a FeOx–SiO2–CaO (0–10 wt%) system was only 0.3 wt% in a reducing condition (\({\mathrm{P}{\text{O}}}_{2}\)=10−10 atm). Excessive Cr over the solubility in the slag system promoted the formation of a high melting point spinel phase, which led to an increase in the liquidus temperature by 50–60 °C, as reported by Ilyushechkin et al. [37].
The addition of alkaline flux, i.e., Na2CO3, facilitated the smelting of ES at a typical nonferrous metal smelting temperature (1250 °C), which is 100–250 °C lower than those reported in previous works [17, 19]. As shown in Fig. 2, slag and matte were distinguishably separated compared with the products without Na2CO3 addition. Figure 8 further shows that Cu and Ni sulfides were enriched in the matte phase, while Cr was deported to the slag phase. This could be attributed to two reasons:
Firstly, the introduction of alkaline flux reduced the melting temperature of the slag. FactSage 8.0 was used to simulate the effect of Na2O addition on the isotherms of pseudo FeO–SiO2–CaO with 5.8 wt% Cr2O3 (equals to 4 wt% Cr) at 1250 °C and \(\log P_{{{\text{O}}_{2} }} = - 8\;{\text{atm}}\). The boundary conditions for the calculation are listed in the Supplementary File. Although the system was simplified for calculation convenience and may not reflect the real melting point of the experimental slag system, the liquidus at the set slag composition [red dots in Fig. 10 (color figure online)] was reduced by over 40 °C with the addition of 13.5% Na2O (equal to 10 wt% Na). The main reason for the extremely high liquidus of the slag was due to the high temperature required to fully melt chromium compounds, e.g., FeCr2O4, etc. However, the proportion of these solid compounds in the liquid slag may be influenced by alkaline flux.
As shown in Fig. 11, the effect of Na2O on the proportion of equilibrium phases, i.e., liquid slag and solid spinel, of the product was calculated at 1250 °C and \(\log P_{{{\text{O}}_{2} }} = - 8\;{\text{atm}}\) (Fig. 11; Table S2). The results indicated that solid spinel proportion decreased from 31.5 to 10.9% as Na2O/(FeO + SiO2 + CaO + Cr2O3) increased from 0 to 15 wt%, which was the main reason why alkaline smelting of ES can be conducted at a lower temperature.
Secondly, although Mäkinen and Taskinen [38] reported the liquidus of copper–nickel mattes could be below 600 °C, the addition of Na2CO3 led to the formation of Na2S, which could further reduce the melting point of Cu–Ni–Fe sulfides, as has been reported in copper dross smelting processes [27, 39].
The element distribution results indicated that 95.1% Cu and 90.1% Ni of the ES were recovered in the matte after the alkaline smelting process. Although the data cannot be directly compared with other studies due to the different raw materials and final products, Cu and Ni recovery were close to those of the carbon thermal reduction–reduction refining process (Cu recovery: 96.4%, Ni recovery: 85%) for ES with a high Cu content (24 wt%) and low Cr content (0.47 wt%) [17], suggesting that the alkaline smelting technique can effectively recover Cu and Ni in the ES with 10 times higher Cr content using a relatively lower smelting temperature, i.e., 1250 °C.
The cost–benefit analysis is listed in Table 4. To simplify the calculation, the labor cost of the operation certainly depends on the scale of the operation was omitted. In addition, the prices of the raw material used in the calculations retrieved from a raw material wholesale sales platform operated by Alibaba Group (https://www.1688.com). The reagent and electricity cost of the present technique for the ES would be approximately 1600 RMB/tES, which is far below the landfill charge for hazardous solid waste (2700 RMB/t) [40]. Moreover, the recovered Cu and Ni can also be profitable products. In this regard, the alkaline smelting technique for ES is economically feasible.
On the other hand, over 99% Cr was reported to the slag after the alkaline smelting process. Typically, Cr was extracted from chromium ores by a soda roasting process in which Cr2O3 was transferred into soluble Na2CrO4 for a higher leaching ratio. In this regard, alkaline smelting slag may also pollute the environment by releasing soluble Cr, Cu, Ni, or other heavy metals. To test its toxicity, the TCLP process [42] was applied to the slag produced in the optimal smelting condition, and the results are listed in Table 5. The concentrations of Cu, Ni, Cr, Pb, and Zn in the leachate were 0.17 mg/L, 0.15 mg/L, 0.25 mg/L, 0.06 mg/L, and 0.17 mg/L, respectively, which are below the standard of extraction toxicity of hazardous waste in China [42] and in the US [43, 44]. As indicated by SEM–EDS analysis (Fig. 8), Cr, Cu, Ni, and Zn mainly existed in the form of (Fe, Ni, Cu, Zn)Cr2O4 spinel which was reported to be a stable phase resistant to leaching [6]. Moreover, the diffusion of leaching solution to the spinel phases was further prohibited by the encapsulation of spinel in the glassy slag matrix [45].
Conclusions
Alkaline matte smelting was proposed for recovering Cu and Ni in the ES, and the effect of various smelting parameters on the distribution of metal values was investigated in this work. The main conclusions are as follows:
-
(1)
The alkaline matte smelting technique effectively enriched Cu and Ni in the matte phase and Cr oxide in the slag phase. Matte and slag were well separated with a clear boundary at a typical nonferrous metal smelting temperature, i.e., 1250 °C, which is 100–250 °C lower than the temperatures previously reported. Cost benefit analysis indicated that the processing cost for the ES was 1600.2 RMB/t, showing a high potential for industrial application.
-
(2)
The optimal process condition was obtained as w(FeO)/w(SiO2) = 1.5, w(CaO)/w(SiO2) = 0.8, Na2CO3, coal powder, and sulfur dosages were 38.2%, 4%, and 8% of the ES weight, in which DCuM, DNiM, and DCrSl reached 95.1%, 90.1%, and 99.3%, respectively.
-
(3)
The TCLP test indicated that the Cu, Ni, Cr, Pb, and Zn leaching toxicities of the slag were much lower than the Chinese national identification standards for hazardous wastes (GB5085.3-2007) and the US Federal Regulations ((2022) 261.24). The formation of stable spinel phases containing Cu, Ni, Cr, and Zn, plus their embedment in the glassy slag matrix, could be accounted for the low toxicity.
References
Yan K, Liu Z, Li Z, Yue R, Guo F, Xu Z (2019) Selective separation of chromium from sulphuric acid leaching solutions of mixed electroplating sludge using phosphate precipitation. Hydrometallurgy 186:42–49. https://doi.org/10.1016/j.hydromet.2019.03.013
Zhang B, Wang Y, Tang X, Wang S, Wei C, Wang R, Zhang W (2019) Oxidation of high iron content electroplating sludge in supercritical water: stabilization of zinc and chromium. Environ Sci Pollut Res Int 26(15):15001–15010. https://doi.org/10.1007/s11356-019-04897-6
Chen H, Yuan H, Mao L, Hashmi MZ, Xu F, Tang X (2020) Stabilization/solidification of chromium-bearing electroplating sludge with alkali-activated slag binders. Chemosphere 240:124885. https://doi.org/10.1016/j.chemosphere.2019.124885
Hu S, Hu J, Sun Y, Zhu Q, Wu L, Liu B, Xiao K, Liang S, Yang J, Hou H (2021) Simultaneous heavy metal removal and sludge deep dewatering with Fe(II) assisted electrooxidation technology. J Hazard Mater 405:124072. https://doi.org/10.1016/j.jhazmat.2020.124072
Cai XW, Wei XG, Du CM (2020) Thermal plasma treatment and co-processing of sludge for utilization of energy and material. Energy Fuels 34(7):7775–7805. https://doi.org/10.1021/acs.energyfuels.0c00572
Mao L, Tang R, Wang Y, Guo Y, Su P, Zhang W (2018) Stabilization of electroplating sludge with iron sludge by thermal treatment via incorporating heavy metals into spinel phase. J Clean Prod 187:616–624. https://doi.org/10.1016/j.jclepro.2018.03.235
Zhang Y, Shi P, Chen L, Tang Q (2018) Utilization of electroplating sludge as subgrade backfill materials: mechanical and environmental risk evaluation. Adv Civ Eng 2018:4891418. https://doi.org/10.1155/2018/4891418
Dai Z, Zhou H, Zhang W, Hu L, Huang Q, Mao L (2019) The improvement in properties and environmental safety of fired clay bricks containing hazardous waste electroplating sludge: the role of Na2SiO3. J Clean Prod 228:1455–1463. https://doi.org/10.1016/j.jclepro.2019.04.274
Yue Y, Zhang J, Sun F, Wu S, Pan Y, Zhou J, Qian G (2019) Heavy metal leaching and distribution in glass products from the co-melting treatment of electroplating sludge and MSWI fly ash. J Environ Manag 232:226–235. https://doi.org/10.1016/j.jenvman.2018.11.053
Wu P, Zhang LJ, Liu YD, Xie XX, Zhou J, Jia HH, Wei P (2019) Enhancing Cu–Zn–Cr–Ni co-extraction from electroplating sludge in acid leaching process by optimizing Fe3+ addition and redox potential. Environ Eng Sci 36(9):1244–1257. https://doi.org/10.1089/ees.2019.0127
Zhang L, Zhou W, Liu Y, Jia H, Zhou J, Wei P, Zhou H (2020) Bioleaching of dewatered electroplating sludge for the extraction of base metals using an adapted microbial consortium: process optimization and kinetics. Hydrometallurgy 191:105227. https://doi.org/10.1016/j.hydromet.2019.105227
Huyen PT, Dang TD, Mai TT, Huyen N, Roy S (2016) Electrochemical copper recovery from galvanic sludge. Hydrometallurgy 164:295–303. https://doi.org/10.1016/j.hydromet.2016.06.028
Peng G, Deng S, Liu F, Li T, Yu G (2020) Superhigh adsorption of nickel from electroplating wastewater by raw and calcined electroplating sludge waste. J Clean Prod 246:118948. https://doi.org/10.1016/j.jclepro.2019.118948
Weng C, Sun X, Han B, Ye X, Zhong Z, Li W, Liu W, Deng H, Lin Z (2020) Targeted conversion of Ni in electroplating sludge to nickel ferrite nanomaterial with stable lithium storage performance. J Hazard Mater 393:122296. https://doi.org/10.1016/j.jhazmat.2020.122296
Ye X, Lin Z, Liang S, Huang X, Qiu X, Qiu Y, Liu X, Xie D, Deng H, Xiong X, Lin Z (2019) Upcycling of electroplating sludge into ultrafine Sn@C nanorods with highly stable lithium storage performance. Nano Lett 19(3):1860–1866. https://doi.org/10.1021/acs.nanolett.8b04944
Shen H, Liu B, Shi Z, Zhao S, Zhang J, Zhang S (2021) Reduction for heavy metals in pickling sludge with aluminum nitride in secondary aluminum dross by pyrometallurgy, followed by glass ceramics manufacture. J Hazard Mater 418:126331. https://doi.org/10.1016/j.jhazmat.2021.126331
Tian L, Chen L, Gong A, Wu X, Cao C, Liu D, Chen Z-Q, Xu Z-F, Liu Y (2020) Separation and extraction of valuable metals from electroplating sludge by carbothermal reduction and low-carbon reduction refining. JOM 72(2):782–789. https://doi.org/10.1007/s11837-019-03880-3
Zhou C, Ge S, Yu H, Zhang T, Cheng H, Sun Q, Xiao R (2018) Environmental risk assessment of pyrometallurgical residues derived from electroplating and pickling sludges. J Clean Prod 177:699–707. https://doi.org/10.1016/j.jclepro.2017.12.285
Zhou L, Yan X, Wu M, Li Y, Wang L, Zhu L, Bing N, Qiao Y, Wang L (2016) Research on reduction and recovery metals from electroplating sludge by potato starch. J Shanghai Polytech Univ 33(1):27–30. https://doi.org/10.19570/j.cnki.jsspu.2016.01.005
Zhang D, Reng G, Yang T, Liu W, Chen L, Liu R (2019) A harmless method for disposaling electroplating sludge. Chinese Patent 201911340666.0
Yue T, Niu Z, Hu Y, Han H, Lyu D, Sun W (2019) Cr(III) and Fe(II) recovery from the polymetallic leach solution of electroplating sludge by Cr(III)–Fe(III) coprecipitation on maghemite. Hydrometallurgy 184:132–139. https://doi.org/10.1016/j.hydromet.2018.11.013
Ma Y, Zhou X, Tang J, Liu X, Gan H, Yang J (2021) One-step selective recovery and cyclic utilization of valuable metals from spent lithium-ion batteries via low-temperature chlorination pyrolysis. Resour Conserv Recycl 175:105840. https://doi.org/10.1016/j.resconrec.2021.105840
Chen B, He J, Sun X, Zhao J, Jiang H, Zhang L (2020) Separating and recycling metal mixture of pyrolyzed waste printed circuit boards by a combined method. Waste Manag 107:113–120. https://doi.org/10.1016/j.wasman.2020.04.006
Guo X-Y, Liu J-X, Tian Q-H, Li D (2013) Principle and method of low temperature alkaline smelting in non-ferrous metallurgy complicated resources. Nonferr Met Sci Eng 4(2):8–13. https://doi.org/10.13264/j.cnki.ysjskx.2013.02.015
Ye L, Tang C, Liu H, Chen Y (2019) Efficient bath-smelting reduction of antimony oxide in FeO–SiO2–CaO–Na2O quaternary slag with low melting point. JOM 71(11):3903–3908. https://doi.org/10.1007/s11837-018-3262-9
Yu-Jie H, Chao-Bo T, Mo-Tang T, Yong-Ming C (2015) Reductive smelting of spent lead–acid battery colloid sludge in a molten Na2CO3 salt. Int J Miner Metall Mater 22:798–803. https://doi.org/10.1007/s12613-015-1136-5
Chen P, Xiao H, Chen J, Chen L, Zhang D, Liu W, Yang T (2020) Oxygen-rich side-blown bath smelting of copper dross: a process. J Sustain Metall 6(2):344–354. https://doi.org/10.1007/s40831-020-00278-3
Dang X, Liu A, Lyu J (2018) Metal phase transformation and equilibrium distribution in sodium roasting process of the electroplating sludge. Chin J Environ Eng 12(10):2944–2951. https://doi.org/10.12030/j.cjee.201803085
Hidayat T, Shishin D, Decterov SA, Jak E (2017) Critical thermodynamic re-evaluation and re-optimization of the CaO–FeO–Fe2O3–SiO2 system. CALPHAD 56:58–71. https://doi.org/10.1016/j.calphad.2016.11.009
Sridhar R, Toguri JM, Simeonov S (1997) Thermodynamic considerations in copper pyrometallurgy. JOM 49(4):48–52. https://doi.org/10.1007/bf02914876
Bale CW, Bélisle E, Chartrand P, Decterov SA, Eriksson G, Gheribi AE, Hack K, Jung IH, Kang YB, Melançon J, Pelton AD, Petersen S, Robelin C, Sangster J, Spencer P, Van Ende MA (2016) FactSage thermochemical software and databases, 2010–2016. CALPHAD 54:35–53. https://doi.org/10.1016/j.calphad.2016.05.002
Sridhar R, Toguri JM, Simeonov S (1997) Copper losses and thermodynamic considerations in copper smelting. Metall Mater Trans B 28(2):191–200. https://doi.org/10.1007/s11663-997-0084-5
Chen L, Yang T, Bin S, Liu W, Zhang D, Bin W, Zhang L (2014) An efficient reactor for high-lead slag reduction process: oxygen-rich side blow furnace. JOM 66(9):1664–1669. https://doi.org/10.1007/s11837-014-1057-1
Nikolic S, Hayes PC, Jak E (2008) Phase equilibria in ferrous calcium silicate slags: Part IV. Liquidus temperatures and solubility of copper in “Cu2O”–FeO–Fe2O3–CaO–SiO2 slags at 1250°C and 1300°C at an oxygen partial pressure of 10–6 atm. Metall Mater Trans B 39(2):210–217. https://doi.org/10.1007/s11663-008-9129-7
Shishin D, Shevchenko M, Jak E (2021) Experimental study and thermodynamic calculations in the CaO–Cu2O–FeO–Fe2O3–SiO2 system for applications in novel copper-based processes. J Sustain Metall 7:300–313. https://doi.org/10.1007/s40831-021-00341-7
Sukhomlinov D, Avarmaa K, Virtanen O, Taskinen P, Jokilaakso A (2020) Slag–copper equilibria of selected trace elements in black copper smelting. Part I. Properties of the slag and chromium solubility. Miner Process Extr Metall Rev 41(1):32–40. https://doi.org/10.1080/08827508.2019.1575212
Ilyushechkin A, Hayes PC, Jak E (2014) Effects of Al2O3, CaO and Cr2O3 on liquidus temperatures of Fe–Mg–Si–O slags. Can Metall Q 54(2):185–197. https://doi.org/10.1179/1879139514y.0000000177
Mäkinen T, Taskinen P (2013) State of the art in nickel smelting: direct Outokumpu nickel technology. Miner Process Extr Metall 117(2):86–94. https://doi.org/10.1179/174328508X290867
Bates C, Dimartini C (1986) Sodium treatment of copper dross. JOM 38(8):43–45. https://doi.org/10.1007/bf03257788
Chen L, Chen P, Wang Z, Liu W, Zhang D, Yang T (2021) Selective reduction of lead sulfate containing slag using the fluidized bed reactor. J Clean Prod 296:126512. https://doi.org/10.1016/j.jclepro.2021.126512
Chen W, Mei C (1993) A handbook for extractive metallurgy of nonferrous metals—modern equipments. Metallurgical Industry Press, Beijing
State Environmental Protection Administration of China (2007) Identification standards for hazardous wastes—identification for extraction toxicity. http://openstd.samr.gov.cn/bzgk/gb/newGbInfo?hcno=186B648DFF1A74025E1AA0117DFE3917. Accessed 2 Mar 2022
Code of Federal Regulations (2022) 261.24 Toxicity characteristic. https://www.ecfr.gov/current/title-40/chapter-I/subchapter-I/part-261/subpart-C/section-261.24. Accessed 4 Mar 2022
United States Environmental Protection Agency (2022) SW-846 test method 1311: toxicity characteristic leaching procedure. https://www.epa.gov/hw-sw846/sw-846-test-method-1311-toxicity-characteristic-leaching-procedure. Accessed 4 Mar 2022
Ivan K, Slavica Z, Vesna L, Miodrag S, Tatjana G (2018) Use of sintering to immobilize toxic metals present in galvanic sludge into a stabile glass–ceramic structure. Sci Sinter 50(2):139–147. https://doi.org/10.2298/SOS1802139K
Acknowledgements
The financial supports by National Key Research and Development Program of China (2018YFC1901604) and Natural Science Foundation of Hunan Province (No. 2018JJ3662) are gratefully acknowledged. The valuable comments by the reviewers are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
The contributing editor for this article was Sharif Jahanshahi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xiao, H., Chen, P., Chen, L. et al. Recovery of Cu and Ni in Electroplating Sludge by a Low-Temperature Alkaline Smelting Technique. J. Sustain. Metall. 8, 1026–1040 (2022). https://doi.org/10.1007/s40831-022-00545-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-022-00545-5