Abstract
This research aimed at recovering metals retained in the tailings from the flotation of copper (Cu) and cobalt (Co) ores conducted at the New Concentrator in Kipushi (NCK). Metals retention in the tailings (0.73% Cu and 0.37% Co) increased due to the removal of the gravity separation section from the processing circuit together with changes arising in the feed mineralogical characteristics namely the increase in sulfide minerals. The concentrator’s feed was traditionally composed of oxidized minerals of Cu and Co from the Luiswishi deposit (DR Congo). Experiments conducted at the laboratory scale enabled identifying two exploitable routes for recovering metals retained in the tailings: firstly, the sulfuric acid leaching of tailings under reducing conditions in view of preparing a leach liquor (2.43 g/L Cu and 1.10 g/L Co) that can be utilized for cementing Cu, using iron chips and precipitating Co; secondly, the flotation of valuable minerals using xanthates in view of obtaining a rougher concentrate grading 1.43% Cu and 0.75% Co recovered at 56% and 59%, respectively, and later on, the obtaining of a cleaner concentrate assaying 3.97% Cu and 2.4% Co at the recoveries of 35% and 43%, respectively. The final concentrate enables the hydrometallurgical extraction of Cu and Co.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For some time in the past, the production of base metals is conducted worldwide through the reprocessing of mineral wastes such as flotation tailings, electrolysis’s muds, slag from the smelting of sulfide ores, as well as dusts from electric arc furnaces [1,2,3,4,5,6,7]. The interest toward the valuing of process wastes is warranted by the adoption of stringent environmental regulations in all countries in view of improving the management of mineral and hazardous wastes [3, 5,6,7,8,9,10,11,12,13,14]. The aforementioned interest is growing stronger due to the shortage in raw materials experienced in industrialized countries. Moreover, the management of process wastes often implies extras induced by the storage facility maintenance and the environmental monitoring [12, 14]. Recycling of mineral wastes enables avoiding to pay these extras [2, 5, 9, 13,14,15,16,17]. Mineral wastes are presently looked at as low-cost raw materials extensively used in metals production in response to the increasing and sustained demand for raw materials by fast growing economies such as China and India [7, 8, 14, 15, 17,18,19,20].
Another factor favoring the renewal of interest toward mineral wastes is the significant drop in the cut-off grade [14, 15, 18, 21] observed in the majority of mineral deposits mined in the leading base metals producing countries such as the USA and Chile. This drop in the cut-off grade has speeded the scarcity of raw materials, with the rise in metals prices on the world market as the outcome [14, 22].
Concerning the world’s shortage in raw material, Giraud [19] indicated that the technological progress accomplished by the mineral industry, between 1925 and 1970, has enabled decreasing from 2.1 to 0.3% the minimum grade required for the profitable mining of mineral ores. As far as the mining and metallurgical extraction of Cu is concerned, this drop in the minimum grade occurred practically in the same production cost so that a great number of ore deposits (2 to 0.3%), formerly classified as mineral resources, has been progressively converted into mineral reserves. Johnson [22] and Wang et al. [12] indicated that the increased demand for metals used in industries and domestic applications has resulted in the intensive mining of mineral deposits in the last 30 years [10, 14, 16, 18]. Consequently, most of the accessible “rich” ore bodies have been exploited obliging mining companies to increasingly using lower grade primary ores and considering other accessible resources including mineral wastes [7, 13, 14, 18, 23].
The economic value of mineral wastes (slag, tailings, and others) relies on their contents in recoverable metals [6, 8, 10, 13,14,15,16, 18, 21, 23,24,25]. Their reprocessing can be conducted at relatively low operating costs comparatively to freshly mined ores [6, 12, 13, 18, 24, 25]. Indeed, newly mined ores are routinely subjected to crushing and grinding in view of the liberation of valuable minerals prior to metals extraction, an operation that is costly [13, 25]. This is the reason why 55% of the demand for copper, utilized as feed to the European refineries, is not covered using only imported copper as cathodes but also through the recycling of scraps [17]. Moreover, the sustainable functioning of the European mineral industry is highly dependent on imports for many raw materials [8, 16, 17].
In Chile, for instance, copper, molybdenum, precious metals, silica, and iron were formerly recovered through reprocessing of slag (3.5 million tons/year) generated by the smelting of Cu ores [4]. In USA (Arizona), the company Magma Copper was recycling slag (1.8–2.36% Cu) from the smelting of sulfide ores (0.7% Cu) and the conversion of mattes (5–7% Cu) in view of recovering Cu and molybdenum [26].
Even in rich-mineral countries such as the DR Congo, a renewal of interest was noted toward the production of base metals (Cu, Zn, Co, Cd, etc.) using secondary raw materials [27,28,29]. Since 2002, the Big Hill Smelter in Lubumbashi decided producing yearly 4000 tons of Co and 2500 tons of Cu through reprocessing of slag (2.2% Co, 1.3% Cu, and 6–8% Zn) [26,27,28,29]. About 1000 tons/year of Cu were recovered by the Ruashi Mining through reprocessing of ancient reserves of Cu ores (1.79% Cu and 0.49% Co) and tailings [30]. The Anvil Mining project for reprocessing tailings from the Mutoshi washery (1960–1987) was another example illustrating the renewal of interest toward mineral wastes in the DR Congo.
In the region of Katanga (DR Congo), huge piles of mineral wastes are stored for many years around big cities [25]. They consist of mine wastes and tailings generated during the processing of Cu and Co ores. However, their storage is reputed to create pollution that daily threatens wildlife and human health owing to the release of pollutants (airborne and waterborne particles) to the environment [25, 29, 31].
Attempts for valuing metals retained in tailings of our interest date back to 1993 with the achievement of researches focused on the recovery of pyrite and sphalerite [32,33,34]. Indeed, a number of samples were prepared and subjected to experiments and were composed of tailings generated during the selective flotation of Cu–Zn ores conducted between 1935 and 1994 by the “Gécamines.” Later on, interested in the environment safeguarding, a researcher succeeded to float tailings in view of removing sulfide minerals, that is 95% sulfur-bearing matters. This flotation of tailings aimed at inhibiting the formation and spreading of the acid mine drainage through exposure of sulfide minerals to water and air during the storage of tailings [34]. The recovered sulfide minerals were subjected to the oxidizing acid leaching conducted at 98 °C in the presence of ferric ions as catalyst. The same sulfide minerals were also subjected to bioleaching in the presence of thermophilic bacteria at 55 °C and, later on, in that of mesophilic bacteria at 33 °C [34]. The obtained leaching liquors were subjected to solvent extraction of Cu by means of a mixture of a ketoxime and an aldoxime (LIX 984 N) as well as to extraction of Zn using di(2-ethylhexyl) phosphoric acid (D2EHPA), after the removal of ferric ions [34]. The implemented processing route of tailings resulted in the obtaining aqueous solutions suitable for industrial electrolysis of Cu (3 g/L) and Zn (7 g/L). Another researcher, also interested in the valuing of retained metals, succeeded to float tailings by means of potassium amyl xanthate and normal propyl xanthate and recovered 41.88% Zn as a rougher concentrate. The cleaning flotation using sodium ethyl xanthate and a dithiophosphate (AERO 3501) as promoter enabled obtaining a final concentrate (5.52% Zn and 2.51% Cu) suitable for the hydrometallurgical extraction of retained metals [35]. However, it is important stressing on the fact that these researches for valuing tailings were oriented toward the outline of processing schemes or flowsheet that have not yet translated into the industrial extraction of metals of interest.
The present research aims at identifying exploitable routes for recovering metals retained in the tailings from the flotation of Cu and Co ores (oxidized and sulfide) conducted between 2012 and 2015 at the NCK (DR Congo), in those times operated by the “Gécamines.” The “Gécamines” is the biggest state-owned mining company that processed from 1995 until 2015 through flotation Cu–Co oxidized ores from the deposit of Luiswishi [30]. Commercial-grade concentrates (25–30% Cu and 6–7% Co) were produced and exported either to China or Europe for further processing. As the mining depth increased, a great retention of metals in the tailings (0.73% Cu and 0.37% Co) occurred during the flotation of ores. This loss in metals of interest was attributed to the removal from the processing circuit of the gravity separation section that was achieving the tailings scavenging. The loss in metals was also attributed to changes occurring in the concentrator feed characteristics owing to variations in the deposit mineralogy with the mining depth. Indeed, the content of sulfide minerals has progressively increased in the ROM ores with the depth of mining in the deposit of Luiswishi (Haut-Katanga) to an extent that the concentrator feed has achieved 20% sulfide minerals in mixture with 80% of oxidized ones.
Material and Methods
Sampling and Sample Preparation Procedure
The sampling procedure consisted of withdrawals of tailings as pulps (15 L) from the concentrator discharge pipe and their pouring into six 200 L polyethylene barrels. The sample preparation was conducted through the blending of pulps as a mixture that was allowed to settle for 2 or 3 days in view of separating the clarified supernatant water from tailings to be dried during 24 h at 105 °C in a Memmert steam room.
Sample Characterization
It consisted of the granulochemical analysis of samples followed by tests of grindability and chemical analyses.
Granulochemical Analysis of Tailings
A representative sample (500 g) prepared by quartering was sieved using a 38 µm aperture sieve and tap water. The undersize particles (− 38 µm) were collected as pulp in a 15 L container with the oversize particles (+ 38 µm) dried at 105 °C in a Memmert steam room, weighted using a Mettler Toledo SB 1600 analytical balance and assayed for Cu and Co using an Analytikdjena AA 300 spectrophotometer. Additionally, 491 g of tailings was fractionated using a series of sieves (38–300 µm), with each granulometric fraction weighted and spectrophotometrically assayed for Cu and Co using the atomic absorption (Table 1).
It is evident that 32% of the sample consisted of slimes (− 38 µm) grading about 29% Cu and 24% Co. Practically 54% of the tailings consisted of particles with the size greater than 75 µm and grading more than the half of total Cu (≈ 63%) and Co (≈ 70%) in the sample. The remaining metals of interest were distributed in particles with the size comprised between 38 and 75 µm and accounting for about 9% of total Cu and cobalt. These particles accounted practically for 14% of the sample weight. More than 50% of matter consisted of particles with the size greater than 75 µm. Tailings under consideration presented a d80 equal to 138 µm justifying to conduct tests of grindability prior to their flotation beneficiation.
Grindability of Tailings
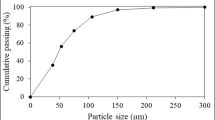
One kg of tailings was added to tap water (1L) and subjected to wet grinding at varying times using a BOECK lab balls mill operated at 30 rpm, with the grinding load kept at 3.338 kg (20 balls with a diameter of 40 mm and 3 balls with a diameter of 62 mm). The pulps given by the wet grinding of tailings were passed over a 75 µm aperture sieve, with the oversize particles recovered, dried at 105 °C, and weighted in view of constructing the grinding curve (Fig. 1).
Using as starting material a sample containing 54% of particles with the size greater than 75 µm, the wet grinding of tailings during 11 min enabled obtaining 75% of passing (Table 2). Consequently, 11 min has been chosen as the optimal grinding time for tailings under consideration.
The proportion of matter with the particle size greater than 75 µm accounted for about 26% of the sample’s weight. It graded about 23% Cu and 25% Co and practically, 47% of the sample was composed of particles passing 38 µm and assaying about 56% Cu and 52% Co. The wet grinding of tailings has enabled increasing the proportion of particles with the size smaller than 38 µm from 32 to 49%.
Tailings Chemical and Mineralogical Characteristics
The chemical analysis of tailings was conducted using the atomic absorption. 1 g of a representative sample was dissolved during 15 min using a leaching liquor consisting of 10 mL of nitric acid (65% w/w) and 2 mL of hydrogen peroxide (100% w/w). The leaching solution was diluted (1:10) in view of the spectrophotometric measurement of copper, cobalt, manganese, zinc using Analytikdjena AA 300 spectrophotometer. As for the remaining characterization analyses of the tailings, they were determined using titrimetric methods and the scanning electron microscopy using a PHILIPS FEI XL30 ESEM-FEG apparatus (Table 3). The tailing sample was prepared as a thin layer by mixing a 2 g aliquot with an epoxy resin of the Araldite type and the whole was air dried. Subsequently, the fabricated thin layer was polished successively using silicon carbide paper with grains of different sizes (P 400, P 600) – 3 M, abrasive discs ESCIL (France) GR. 2400 (800/2400) and GR. 4000 (1200/4000), as well as a (absolute methanol) diamond suspension (1PS – 1MC, ESCIL) with the particle size kept at 1 µm. Each area of interest of the thin layer was surrounded by gluing silver bridges before being carbonized with graphite in a Bolzers MED evaporator. 010. The primary electron’s beam, with the width of 10.1 mm and the voltage of 15 kV, was scanned in raster pattern across areas of interest (mapping) of the thin layer with the aim to determine the special distribution of constituents (Cu, Co, O, S, Si, Ca, Fe, and Al). SEM images were obtained with the magnification of 200 using the scattered electrons (SE) detector (Fig. 2).
Tailings under consideration contained Cu (0.74%) and Co (0.37%) in the majority as oxidized minerals (malachite and heterogenite). They contained also siliceous materials (≈ 64% SiO2) namely quartz together with sulfide minerals of Cu and Co. Iron and calcium were present in tailings as hematite and as dolomite, respectively.
Recovery of Cu and Co Retained in the Flotation Tailings
Tailings were reprocessed while implementing two exploitable processing routes in view of recovering metals of interest:
-
(a)
the sulfuric acid leaching under reducing conditions in view of preparing a leach liquor that can be purified by solvent extraction prior to electrowinning of Cu and precipitation of Co either as hydroxides or carbonates;
-
(b)
the sulphidization with sodium hydrogen sulfide (NaHS) of valuable minerals and their flotation by means of xanthates (potassium amyl xanthate—KAX) in view of producing a Cu–Co concentrate that can either be leached using the sulfuric acid in view of extraction of Cu as cathodes and Co as salts. The cleaner tails can be recirculated in the processing circuit.
Sulfuric Acid Leaching of Tailings
A representative sample of tailings (1 kg), composed of 54% particles with the size greater than 75 µm, was prepared by quartering and subjected to wet grinding with 1 L of tap water during 11 min. A pulp with 25% of particles with the size greater than 75 µm was prepared, with solid materials allowed to settle in a 15 L plastic container in view of siphoning the clarified supernatant water. Later on, solid materials were dried and homogenized. An aliquot (200 g) was stirred at constant speed (600 rpm) in 500 mL beaker containing 500 mL of a sulfuric acid solution (500 g/L). The sulfuric acid leaching of tailings (Fig. 3) was conducted at the room temperature, with the stirring time varied as follows: 30, 60, 90, 120, and 150 min.
After the stirring, the mixture was poured in a glass funnel in view of the vacuum filtration and separation of solid residues that were spectrophotometrically analyzed for Cu and Co by atomic absorption. The leaching process was also conducted at 60 °C, with the reaction time varied as follows: 30, 60, 90, 120, and 150 min. Finally, the leaching of tailings was conducted during 120 min while varying the amount (5, 10 and 15 g) of sodium metabisulfite (Na2S2O5) utilized as the reducing agent of cobaltic Co of heterogenite, the bearing mineral of cobalt.
Tailings Flotation
One liter of tap water was added to tailings subjected or not to wet grinding. The pulp was poured in 5 L cell of a METSO MINERAL INDUSTRY pneumo-mechanic flotation machine. The pulp level was adjusted in the cell using tap water as makeup water and the flotation machine operated at 650 rpm. The pulp conditioning was conducted during 2 min in the presence of sodium silicate (Na2SiO3 as slime depressor) and a mixture (85% tall oil and 15% gas oil as a secondary collector of Cu–Co minerals). Each 5 min’ interval, the froth loaded with minerals of interest was continuously recovered from the top of the pulp under flotation (15 min). The obtained concentrate was fractioned in three pans, dried in a Memmert steam room at 105 °C, and subjected to spectrophotometric analyses of Cu and Co by atomic absorption. Figure 4 depicts the general schema for tailings reprocessing.
The flotation of tailings was conducted in two groups of tests divided into five series. A three-stage processing schema encompassing the roughing (15 min), pre-cleaning (10 min), and cleaning (6 min) was implemented. During the first group of tests, divided into three series, the flotation of tailings was limited to the rougher stage (15 min). The first series of the flotation was performed while varying the feed granulometric composition (54% and 25% of particles with the size greater than 75 µm) together with the dosage rate of NaHS and KAX (Table 4).
Concerning the second series of the first group of tests, the flotation of tailings was also limited to the rougher stage, with the dosage rate of citric acid and NH4SO4 varied (see Table 5). The dosage rate of Na2SiO3 and the mixture was also varied (Table 5) during flotation tests on behalf of the last series also limited to the rougher stage.
As for the second group of tests, the flotation of tailings was conducted in two series limited successively to the pre-cleaner and cleaner stages while varying solely the dosage rates of NaHS and KAX (Table 6).
The flotation of tailings was conducted using industrial-grade chemicals listed below:
-
Slimes dispersant—Sodium silicate 30%;
-
Frother—Dowfroth 250 100%;
-
Sulphidizer—Sodium hydrogen sulfide 36%;
-
Primary collector—Potassium amyl xanthate 10%;
-
Secondary collector—Mixture containing gasoil (85%) and Rinkalore (15%);
-
Regulator of the pulp’s pH—Citric acid 20%;
-
Promoter—Ammonium sulfate 30%.
Results and Discussion
The discussed results are in relationship with the recovery of Cu and Co retained in tailings through the sulfuric acid leaching in the presence or not of sodium metabisulfite. The leaching conditions were determined based on the study of the influence of three main parameters (time, temperature, and reducing agent) on the dissolution process of Cu and Co and were expected to optimize the recovery of retained metals. The same results are also in relationship with the recovery of Cu and Co in the form of a concentrate while studying the influence of the reagent dosage rate on the flotation efficacy.
Recovery of Cu and Co Through the Sulfuric Acid Leaching of Tailings
The sulfuric acid leaching of tailings was conducted while studying the influence of three main parameters on the dissolution of Cu and Co.
Influence of Time on the Leaching of Cu and Co
The variation of time during the leaching of tailings enabled obtaining of results depicted in Fig. 5. At first glance, it can be stated that the extent of metals dissolution increased as time was going on.
This increased dissolution of Cu and Co can be attributed to the prolonged exposition of their bearing minerals to sulfuric acid together with its diffusion inside particles subjected to leaching. However, pursuing the leaching process beyond 120 min (until 150 min) did not significantly vary the extent of dissolution of Cu and Co. This slowed dissolution of Cu and Co may result from to the poor dissolution of sulfide minerals after the depletion of oxidized matters of tailings. Thus, 120 min has been considered as the optimal time leading to the highest dissolution of metals retained in the tailings, with the leach liquor grading 2.18 g/L Cu and 0.92 g/L Co recovered at about 75% and 62%, respectively.
Influence of the Temperature on the Leaching of Cu and Co
Concerning the influence of the temperature on the extent of dissolution of Cu and Co, the leaching process was conducted at 60 °C while varying the time (Fig. 6).
It is evident that the dissolution of metals generally increased a bit faster at 60 °C regardless of the time. This reveals the influence of the temperature on the reaction kinetics of the bearing minerals of Cu and Co. Once more, 120 min has been considered as the time enabling the highest recoveries of metals retained in tailings, with the obtained leach liquor grading 2.30 g/L Cu and 0.87 g/L Cu recovered at about 79% and 56%, respectively.
Influence of the Amount of Sodium Metabisulfite on the Leaching of Cu and Co
When the leaching of tailings was conducted during 120 min, while varying the amount of sodium metabisulfite, it enabled obtaining the results given in Fig. 7.
It is obvious that under reducing conditions the extent of Co dissolution increased faster than that of Cu so that 10 g was considered as the amount of sodium metabisulfite that optimized the dissolution of metals retained in the tailings. Indeed, the leaching of tailings took place such that the recoveries of 70% and 80% were achieved for Cu and Co, respectively, with the obtained leach liquor containing 2.53 g/L Cu and 1.10 g/L Co. Beyond 10 g, the recoveries of metals significantly dropped because their ions were reduced to metals that are not prone to dissolution in sulfuric acid under ordinary conditions and this phenomenon was marked for Cu. The same finding has been made by Thabane [36] when dealing with the dissolution under reducing conditions of valuable metals contained in an oxidized Cu–Co ore sourced from Katanga region in the DR Congo. Thus, the obtained leach liquor can be utilized in view of recovering Cu as cathodes and Co as hydroxides. It is important noticing that its concentration in Cu, though very close to the level achieved (2.5 g/L Cu) by Mbuya et al. [37] during the optimized leaching of similar Cu–Co flotation tailings sourced from Kambove in Katanga region of the DR Congo, remains smaller than the value (3 g/L Cu) given by the leaching of sulfide minerals [34] from flotation tailings of our interest, that is a concentration suitable for an industrial electrowinning [38]. As for cobalt, its concentration in the leach liquor is suitable for an industrial recovery [39] even if it is smaller than the value (2.5 g/L Co) achieved by Mbuya et al. [37] during the leaching of flotation tailings of the similar type using ferric ions as reducing agent.
Reagent Dosage Rate Optimization During the Roughing Flotation of Tailings
The flotation was conducted using as starting matter tailings subjected or not to wet grinding during 11 min. It was either limited at the rougher stage or pursued until the cleaner stage. In both cases, the reagent dosage rates were varied in view of determining their influence on the flotability of Cu and Co.
Flotation of Tailings with Variations in the Dosage Rates of NaHS and KAX
The flotation of tailings, without regrinding (54% of particles with the size greater than 75 µm), while varying the amount of the NaHS and KAX added to the pulp gave the results depicted in Fig. 8a, b.
For the second and third series of tests, the flotation of tailings showed a good selectivity with respect to Cu and Co as can be seen through the variation in their recovery versus their concentrate grade. As for the remaining values of the dosage rates of NaHS and KAX, the flotation of tailings took place without selectivity given that both the recoveries and grades of Cu and Co simultaneously increased in the concentrate. The dosage rate of NaHS and KAX that gave the best metallurgical result was 2500/250–450/45–350/35 g/t given that Cu and Co were recovered at about 40% in the rougher concentrate, with their grades being equal to 1% and 0.56%, respectively. The obtained results revealed that nearly 60% of metals of interest were not recovered. Consequently, the roughing flotation concentrate was prepared with poor variations in the concentration rate, so to say an increase from 0.73 to 1.03% for Cu and from 0.37 to 0.56% for Co, respectively.
Flotation of Tailings with Variations in the Dosage Rates of NaHS and KAX
The flotation of tailings, subjected to wet grinding (25% particles with the size greater than 75 µm), was conducted with variation in the dosage rates of NaHS and KAX. Figure 9a, b depicts the recoveries of metals versus their concentrate grades.
It is with the dosage rates of NaHS and KAX equal to 2500/250 g/t, 450/45 g/t, and 350/35 g/t that the flotation of tailings was successful. Indeed, more than 65% of Cu was recovered in the rougher concentrate, which graded 1.23% Cu. Similarly, 70% of Co was recovered, with the rougher concentrate grading 0.6%. It is under the aforementioned conditions that the flotation of tailings enabled an enrichment rate greater than 65% with respect to the initial content of Cu. Moreover, the concentrate grade in Co was about 1.6 times greater than its level in tailings under consideration.
Flotation of Tailings with Variations in the Dosage Rate of Citric Acid
The flotation of tailings, first subjected to grinding and while varying the dosage rate of citric acid (C6H5O7), gave the results given in Table 7.
It can be stated that the highest recovery of Cu (≈ 56%) was achieved when 800 g/t of citric acid was added to the pulp. A substantial increase in the concentrate grade of Cu (1.77%) was noted given that its value accounted for the double of the feed. However, a setback of 11% was noted in the recovery of Cu contrarily to when the flotation of tailings was conducted using a free-citric acid reagent suite. This improvement in the concentrate grade was attributed to the depressing action of citric acid on carbonate-made gangue minerals identified in tailings (see Table 3), improving the selectivity with respect to Cu.
Similarly, about 62% of Co was recovered in the concentrate when 800 g/t of citric acid was added to the pulp (Table 7). An improvement was noted in the concentrate grade of Co (0.96%) that was about 2.6 times greater than its value in tailings subjected to flotation. However, a decrease of about 8% in the recovery of Co was noted. Thus, 800 g/t was considered as the optimized dosage of citric acid given that it enabled recovering more than the half of metals retained in tailings. The concentrate obtained under these conditions was significantly enriched in metals of interest of which the contents were greater than the double of the feed.
The flotation of tailings was successful when the dosage of citric acid was increased up to 800 g/t. Indeed, when citric acid was added to the pulp to the dosage of 400 g/t, a decrease in efficiency of the flotation of metals was noted owing to the loss in the selectivity with respect to Cu and Co as can be seen through the simultaneous increase in their recoveries and grades in the rougher concentrate. This phenomenon gave evidence of the failure in the regulating action of citric acid on the pulp’s pH. It also confirmed the depression of carbonate-made gangue minerals by citric acid (see Table 3). On the contrary, when the dosage of citric acid was increased up to 1200 g/t, a drop occurred in both the recoveries and grades of metals of interest as the outcome of the modifying reagent excess in the pulp, which resulted in strong depression on carbonate-made minerals (see also the decrease in the mass pull) including malachite (See Fig. 2) as can be seen through a significant drop in the recovery of Cu (Table 7). Indeed, citric acid is acknowledged to release in the aqueous solution both hydrogen and citrate ions. The citrate ion constitutes the stronger conjugate base of citric acid of which the hydrolysis produces citric acid together with the liberation in the pulp of hydroxyl ions as per reactions (1) and (2):
The liberated ions are responsible for the drop in acidity and the increase in hydrophilicity of the minerals surface. Given the decrease in the recoveries of metals of interest, the remaining flotation tests of tailings were conducted using free-citric acid reagent suites.
Flotation of Tailings with Variations in the Dosage Rate of Ammonium Sulfate
After regrinding, tailings were floated using a free-citric acid reagent suite together with optimized dosages of NaHS and KAX, while varying the dosage rate of ammonium sulfate (see Table 8).
It is clear that 400 g/t of ammonium sulfate enabled recovering about 62% Cu in a rougher concentrate which graded 1.78% Cu. About 64% of Co was also recovered, with the concentrate assaying practically 1% Co.
These results can be explained by the fact that, apart from the collecting properties of KAX, the successful recovery of metals of interest has been promoted by the action of ammonium sulfate on the bearing minerals floatability. Indeed, the ammonium ion hydrolysis liberates ammonia in pulp [40] as per reactions (3) and (4):
The liberated ammonia reacts with Cu and Co ions from the bearing minerals (malachite and heterogenite) surfaces rendering positive their charges (See Reactions (5) and (6)). Together with the chemisorption or electrochemical process [40,41,42,43,44], this phenomenon strengthens the valuable minerals hydrophobization via the direct dipole–dipole adsorption of the xanthate anion [40,41,42].
The ammonium ion hydrolysis is involved in the liberation of acidity in the pulp, a phenomenon which enables combating the excess sulphidizer (Reactions (7)–(10)) and prevents hydroxyl ions from competing with xanthate ions (Reaction (11)) during their adsorption on the surface of minerals of interest [31]:
For the remaining dosages of ammonium sulfate (100 and 200 g/t), the flotation of Cu enabled achieving 59% and 62%, respectively, with the concentrate enriched with respect to Cu (1.42% and 1.18%) comparatively to when the flotation was conducted using the dosage of 400 g/t (Table 8). Similarly, Co was recovered at 60% and 65%, with the concentrate grade of Co achieving 0.69% and 0.66%, respectively.
However, the flotation of tailings with the reagent suite comprising either citric acid or ammonium sulfate resulted in improved grades and recoveries of metals of interest offering the possibility to search for a synergism between the dosage rates of modifying reagents. That is the reason why a series of flotation tests was conducted while combining optimized dosages of citric acid and ammonium sulfate (Table 9).
No synergism was induced by the combination of optimized dosages of reagents under consideration. Indeed, the recoveries of metals of interest remained below 55% and 40%, respectively, with the concentrate grading 1.74% Cu and 0.63% Co, respectively. The combination of modifiers dropped the grades and recoveries of metals of interest comparatively to when the flotation of tailings was conducted without modifiers (Fig. 9a, b). The grades and recoveries of metals of interest were smaller than those given by the flotation of tailings in the presence of either citric acid (Table 7) or ammonium sulfate alone (Table 8).
Flotation of Tailings with Variations in Dosage Rates of the Mixture and Sodium Silicate
The flotation of tailings, subjected to regrinding, while varying the dosage rates of the mixture, led to results depicted in Fig. 10a, b.
It is clear that 600 g/t is the optimized dosage of the mixture (85% gasoil and 15% tall oil) that boosts the action of KAX used as the primary collector of the bearing minerals of Cu and Co. Indeed, the optimized dosage of the mixture enabled recovering more than 52% and 57.5% of metals of interest in the rougher concentrate, which graded about 1.8% Cu and 0.84% Co.
When tailings were floated with variation only in the dosage rate of sodium silicate (as limes depressor), the highest achievable recovery of Cu (55%) and Co (44%) remained lower than 60% (Fig. 11a, b).
The highest recoveries of metals of interest were achieved when 150 g/t of sodium silicate were added to the pulp, with an increase in the concentrate content of Cu (2%). This improvement in the concentrate grade corresponded to an increase of about 174% with respect to the tailings content of Cu. As for Co, an increase in the concentrate grade was also noted and accounted for about 122% comparatively to the initial tailings content. However, both the recovery and grade of Cu in the concentrate simultaneously increased during the scavenging stage of the flotation when the pulp was not conditioned with sodium silicate. It can be concluded that the flotation of Cu and Co was taking place without the selectivity owing to the presence of slimes.
Based on the obtained results, the set of reagent dosage’s rates given in Table 10 was selected as optimal because the roughing and scavenging flotations of tailings under consideration were successful. When tailings were floated using optimized dosage rates of the tested reagents, the rougher concentrate was obtained with characteristics described in Fig. 12.
The flotation of 1 kg of tailings gave 307 g of a rougher concentrate assaying 1.34% Cu and 0.70% Co recovered at about 56%. It also enabled obtaining of 693 g as secondary tailings containing 0.47% Cu and 0.23% Co, respectively.
Tailings Flotation Using Free-Citric Acid Reagent Suites
Both the roughing and the cleaning flotation of tailings were conducted in the absence of citric acid (Fig. 13a, b).
The reading of Fig. 13a, b reveals a significant improvement in the recoveries of metals of interest when ammonium sulfate was utilized alone as modifier. The roughing flotation of tailings gave good metallurgical results in the absence of citric acid. Indeed, about 56% Cu and 58% Co were recovered in a roughing concentrate, which assayed 1.43% Cu and 0.67% Co, whereas with a reagent suite comprising citric acid, the roughing flotation of tailings enabled recovering only 53% Cu and 40% Co, respectively. These results reveal a loss in the flotation efficiency during the roughing stage together with a negative synergism brought about by the combination of modifiers (ammonium sulfate and citric acid). Consequently, the roughing and cleaning flotation of tailings were later on conducted using a free-citric acid reagent suite (Table 11).
The reprocessing of tailings through flotation enabled obtaining the results depicted in Fig. 14.
As expected, the removal of citric acid from the reagent suite resulted in an improvement of both the recoveries and grades of metals of interest in the rougher concentrate. The flotation of the rougher concentrate, with optimized dosages of NaHS and KAX, gave a pre-cleaner concentrate grading 1.72% Cu and 0.82% Co recovered at about 55% and 58%, respectively. Consequently, pre-cleaner tails assayed about 8% Cu and 6% Co. Moreover, the pre-cleaner concentrate flotation gave a final concentrate grading practically 4% Cu and 2% Co recovered at about 35% and 43%, respectively. It is clear that both the grades and recoveries of metals in the obtained concentrate are smaller than those given by the flotation of Cu–Co oxidized ores formerly implemented at the NCK [40]. The recovery of valuable metals is smaller than the one achieved (45% Cu and 83% Co) by a previous research conducted on tailings of similar type sourced from the Kambove mine in the DR Congo [45].
Potentially Exploitable Routes for Flotation Tailings Reprocessing
The first route for tailings reprocessing enabled preparing a metalliferous solution. As for the second route, it enabled obtaining a Cu–Co concentrate. Details about the implemented reprocessing routes of the tailings under consideration are given below:
Concerning the acid leaching of tailings, it was conducted while operating under conditions given in Table 12.
The obtained leach liquor graded 2.53 g/L Cu and 1 g/L Co recovered at 80% and 78%, respectively. The leach liquor also contained about 1.4 g/L Fe and 0.3 g/L Mn. It is offering the opportunity for extracting Cu through cementation with iron chips as well as the precipitation of Co as hydroxides.
As for the flotation of tailings, it was conducted while operating under conditions listed in Table 12.
-
(1)
A rougher concentrate grading 1.43% Cu and 0.75% Co recovered at about 56% and 59%, respectively;
-
(2)
A cleaner concentrate containing 3.97% Cu and 2.40% Co recovered at about 35% and 43%, respectively.
The obtaining of the final concentrate offers the opportunity for the hydrometallurgical recovery of Cu and Co.
Conclusion
The obtained results revealed opportunities for valuing Cu and Co retained in tailings either through the sulfuric acid leaching under reducing conditions or using the flotation by means of xanthates. Indeed, the first processing route has enabled preparing a leaching solution that can serve as the starting point for extracting metals of our interest, whereas the second processing route has given a concentrate that can be utilized for extracting metals retained in tailings.
Technically easy to conduct, the leaching of tailings seems to be a cost-saving process implemented using less reagents (sulfuric acid and reducing agent) compared to their flotation. The latter has advantageously resulted in the obtaining of matter ridded of the gangue minerals so that the leaching solution purification can be technically easy to implement contrarily to when tailings are directly subjected to leaching without enrichment. However, the hydrometallurgical extraction of metals of interest requires the leaching solution purification, the process that can be laborious and costly in terms of the reagents’ consumption, regardless of the starting material (tailings or concentrate). It is the reason why a comparison of the operating costs is needed in view of selecting the most cost-saving and suitable route for reprocessing the tailings under consideration.
References
Berry JB, Ferrada JJ, Dole LR, Ally M (2002) Sustainable recovery of by-products in the mining industry, Project conducted by Oak Ridge National Laboratory (ORNL) and Colorado School of Mines engineers and selected for the benefits of mining industry by the U.S. Department of Energy (DOE) and the National Mining Association (NMA), pp 1–16
Das B, Prakash S, Reddy PSR, Misra VN (2007) An overview of utilization of slag and sludge from steel industries. Resour Conserv Recycl 50:40–57
Ghose MK, Sen PK (1999) Recovery of usable ore fines from iron ore tailings and their environmental management—a case study. Land Contam Reclam 7(2):143–149
Sánchez M, Parada F, Parra R, Marquez F, Jara R, Carrasco JC, Palacios J (2004) Management of copper pyrometallurgical slags: giving additional value to copper mining industry. In: VII international conference on molten slags fluxes and salts, the South African Institute of Mining and Metallurgy, pp 543–550. https://pdfs.semanticscholar.org/d1a6/f05a2fae9901b2f755ab77aadec466cd8ffc.pdf.
Sirkeci AA, Gül A, Bulut G, Arslan F, Onal G, Yuce AE (2006) Recovery of Co, Ni, and Cu from the tailings of Divrigi iron ore concentrator. Miner Process Extract Metall Rev 27:131–141
Stanković V, Milošević V, Milićević D, Gorgievski M, Bogdanović G (2018) Reprocessing of the old flotation tailings deposited on the RTB Bor tailings pond—a case study. Chem Indus Chem Eng Q 24(4):333–344. https://doi.org/10.2298/CICEQ170817005S
Zhang R, Hedrich S, Römer F, Goldmann D, Schippers A (2020) Bioleaching of cobalt from Cu/Co-rich sulfidic mine tailings from the polymetallic Rammelsberg mine, Germany. Hydrometallurgy 197:105443. https://doi.org/10.1016/j.hydromet.2020.105443
Bellenfant G, Guezennec A-G, Bodénan F, D’Hugues P, Cassard D (2013) Re-processing of mining waste: combining environmental management and metal recovery? Mine Closure 2013, Sep 2013, Cornwall, United Kingdom, pp 571–582. hal-00849984
Chan BKC, Bouzalakos S, Dudeney AWL (2008) Integrated waste and water management in mining and metallurgical industries. Trans Nonferrous Met Soc China 18:1497–1505
Moors EHM, Mulder KF, Vergragt PJ (2005) Towards cleaner production: barriers and strategies in the base metals producing industry. J Clean Prod 13:657–668
Dutta SK, Upadhyay VP, Sridharan U (2006) Environmental management of industrial hazardous wastes in India. J Environ Sci Eng 40(2):143–150
Wang C, Harbottle D, Liu Q, Xu Z (2014) Current state of fine mineral tailings treatment: a critical review on theory and practice. Miner Eng 58:113–131
Mäkinen J, Salo M, Khoshkhoo M, Sundkvist J-E, Kinnunen P (2020) Bioleaching of cobalt from sulfide mining tailings; a mini-pilot study. Hydrometallurgy 196:105418
Nadeif A, Taha Y, Bouzahzah H, Hakkou R, Benzaazoua M (2019) Desulfurization of the old tailings at the Au-Ag-Cu Tiouit Mine (Anti-Atlas Morocco). Minerals 9(401):1–15. https://doi.org/10.3390/min9070401
Altinkaya P, Liipo J, Kolehmainen E, Haapalainen M, Leikola M, Lundström M (2019) Leaching of trace amounts of metals from flotation tailings in cupric chloride solutions. Min Metall Explor 36:335–342. https://doi.org/10.1007/s42461-018-0015-9
Ayres RU (1997) Metals recycling: economic and environmental implications. Resour Conserv Recycl 21:145–173
Parviainen A, Soto F, Caraballo MA (2020) Revalorization of Haveri Au-Cu mine tailings (SW Finland) for potential reprocessing. J Geochem Explor. https://doi.org/10.1016/j.gexplo.2020.106614
Dong Y, Lin H (2012) Influences of flotation reagents on bioleaching of chalcopyrite by Acidthiobacillus ferrooxidans. Miner Eng 32:27–29
Andrews C, Bocoum B, Tshimena D (2008) Democratic Republic of the Congo, Growth with governance in the mining sector, The World Bank Report No. 43402-ZR, May 2008, Oil/Gas, Mining and Chemicals Department, AFCC2, Africa Region, pp 1-140
Giraud N-P (2003) Economie industrielle des commodités, Centre de Géopolitique de l'Energie et des Matières Premières, Université de Paris-Dauphine, pp.7–154
Mackay I, Videla AR, Brito-Parada PR (2020) The link between particle size and froth stability—implications for reprocessing of flotation tailings. J Clean Prod 241:118436. https://doi.org/10.1016/j.jclepro.2019.118436
Kooroshy J, Christa Meindersma C, Podkolinski R, Rademaker M, Sweijs T, Diederen A, Beerthuizen M, De Goede S (2009) Scarcity of Minerals, A strategic security issue, A report commissioned by TNO, The Hague Centre for Strategic Studies N° 02/ 01/10, The Netherlands, pp 1–144
Babel B, Penz M, Schach E, Boehme S, Rudolph M (2018) Reprocessing of a Southern Chilean Zn tailing by flotation—a case study. Miner 8(295):1–18
Alcalde J, Kelm U, Vergara D (2018) Historical assessment of metal recovery potential from old mine tailings: a study case for porphyry copper tailings, Chile. Miner Eng 127:334–338
Lutandula MS, Banza M (2013) Recovery of cobalt and copper through reprocessing of tailings from flotation of oxidized ores. J Environ Chem Eng 4:1085–1090
U.S. Environmental Protection Agency (US EPA) (1993) Slag reprocessing: Magma Copper Company's San Manuel Facility, pp 1–11. https://p2infohouse.org/ref/18/17052.pdf
Banza AN, Gock E, Kongolo K (2002) Base metals recovery from copper smelter slag by oxidising leaching and solvent extraction. Hydrometallurgy 67:63–69
Ngenda RB, Segers L, Kongolo PK (2009) Base metals recovery from zinc hydrometallurgical plant residues by digestion method. In: Hydrometallurgy conference 2009, Misty Hills, Muldersdrift, Gauteng, The Southern African Institute of Mining and Metallurgy
Lutandula MS, Kashala NG (2013) Zinc oxide production through reprocessing of the electric arc furnace flue dusts. J Environ Chem Eng 1:600–603
Chadwick J, Cattaneo B (2005) Tails of the DRC. Int Min 1(3):23–27
Shengo LM (2013) Etude du recyclage de l’eau résiduaire dans la flottation des minerais oxydés du gisement de Luiswishi, a PhD thesis, in Engineering Sciences, University of Liege
Ahuka MB (1993) Contribution à l'étude de la valorisation de la pyrite contenue dans le rejet de flottation Cu-Zn au concentrateur de Kipushi. a research carried out by the Faculty of Applied Sciences in Lubumbashi, University of Lubumbashi, Republic of Zaïre
Kebonte SA (1993) Possibilité de retraitement des rejets « Katapula » du concentrateur de Kipushi pour blende et pyrite par flottation, a research carried out by the Faculty of Applied Sciences in Lubumbashi. University of Lubumbashi, Republic of Zaïre
Kitobo WS (2009) Dépollution et valorisation des rejets miniers sulfurés du Katanga : « Cas des tailings de l’Ancien Concentrateur de Kipushi », A PhD thesis in Engineering Sciences, Faculty of Applied Sciences, University of Liege, Belgium
Pungwe KM (2016) Amélioration de la qualité du concentré zincifère de la flottation des rejets des concentrateurs de Kipushi en utilisant la méthode de Taguchi, A research project conducted at Faculty of Applied Sciences, Industrial Chemistry Department, University of Lubumbashi
Thabane S. Hydrometallurgical extraction of copper and cobalt from oxidised copper cobalt ore using ammonia solution. A dissertation in part-fulfilment of the requirements for the degree of Master of Science in Engineering, Faculty of Engineering and the Built Environment, University of Witwatersrand, Johannesburg
Mbuya BI, Kime M-B, Tshimombo AMD (2017) Comparative study of approaches based on the Taguchi and ANOVA for optimising the leaching of copper-cobalt flotation tailings. Chem Eng Commun 204:512–521. https://doi.org/10.1080/00986445.2017.1278588
Kathryn CS, Angus MF, Peter MC (2005) Solvent extraction in southern Africa: an update of some recent hydrometallurgical developments. Hydrometallurgy 78:52–78
Crundwell FK, du Preez NB, Knights BDH (2020) Production of cobalt from copper-cobalt ores on the African Copperbelt—an overview. Miner Eng 156:106450
Shengo LM, Kime M-B, Mambwe MP, Nyembo KT (2019) Review of the beneficiation of copper-cobalt-bearing minerals in the Democratic Republic of Congo. J Sustain Min 18(4):226–246
Drzymala J (2007) Mineral processing, foundations of theory and practice of minerallurgy, 1st English edition, Oficyna Wydawnicza PWr, Wroclaw University of Technology, pp. 344, 357, 361, 362
Davidson MS (2009) An investigation of copper recovery from a sulphide oxide ore with a mixed collector system. A thesis submitted to the Department of Mining Engineering in conformity with the requirements for the degree of Master of Science in Engineering, Queen's University, Kingston, Ontario, Canada, 2009, pp 1–32
Kongolo K, Kipoka M, Minanga K, Mpoyo M (2003) Improving the efficiency of oxide copper–cobalt ores flotation by combination of sulphidisers. Miner Eng 16:1023–1026
Bulatovic SM (2007) Handbook of flotation reagents, chemistry, theory and practice: flotation of sulfide ores. Elsevier Science & Technology Books, pp. 177–178, 105–109
Lutandula MS, Maloba B (2013) Recovery of cobalt and copper through reprocessing of tailings from flotation of oxidised ores. J Environ Chem Eng 1:1085–1090
Acknowledgements
We gratefully thank the managing staff of the New Concentrator in Kipushi for their involvement in the achievement of the present research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author states that there is no conflict of interest.
Additional information
The contributing editor for this article was D. Panias.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shengo, L.M. Potentially Exploitable Reprocessing Routes for Recovering Copper and Cobalt Retained in Flotation Tailings. J. Sustain. Metall. 7, 60–77 (2021). https://doi.org/10.1007/s40831-020-00325-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-020-00325-z