Abstract
The second law of thermodynamics (2LT) helps to quantify the limits as well as the resource efficiency of the circular economy (CE) in the transformation of resources, which include materials, energy, or water, into products and residues, some of which will be irreversibly lost. Furthermore, material and energy losses will also occur, as well as the residues and emissions that are generated have an environmental impact. Identifying the limits of circularity of large-scale CE systems, i.e., flowsheets, is necessary to understand the viability of the CE. With this deeper understanding, the full social, environmental, and economic sustainability can be explored. Exergy dissipation, a measure of resource consumption, material recoveries, and environmental impact indicators together provide a quantitative basis for designing a resource-efficient CE system. Unique and very large simulation models, linking up to 223 detailed modeled unit operations, over 860 flows and 30 elements, and all associated compounds, apply this thermoeconomic (exergy-based) methodology showing (i) the resource efficiency limits, in terms of material losses and exergy dissipation of the CdTe photovoltaic (PV) module CE system (i.e., from ore to metal production, PV module production, and end-of-life recycling of the original metal into the system again) and (ii) the analysis of the zinc processing subsystem of the CdTe PV system, for which the material recovery, resource consumption, and environmental impacts of different processing routes were evaluated, and the most resource-efficient alternative to minimize the residue production during zinc production was selected. This study also quantifies the key role that metallurgy plays in enabling sustainability. Therefore, it highlights the criticality of the metallurgical infrastructure to the CE, above and beyond simply focusing on the criticality of the elements.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Living on a finite planet, where the world population is expected to grow from the current 7.5 billion (2017) to 9.8 billion in 2050 [1], has also increased the concern about the resource efficiency and the environmental impact of our society, e.g., the climate change. The International Resource Panel has estimated an increase of 119.5% on the resource extraction in 2050 with respect to the resources extracted in 2015, if the existing trends are considered [2]. Furthermore, the Intergovernmental Panel on Climate Change (IPCC) predicted the global mean temperature to increase between 2.6 and 4.8 °C at the end of the twenty-first century in a business as usual scenario [3]. Therefore, moving towards a more resource-efficient and environmentally friendly society is required to guarantee the material and environment foundations of life as well as a fair quality of life to the current and future generations.
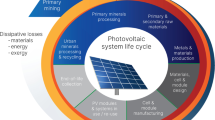
The transition to a renewable energy scenario and having a more circular economy (CE) are becoming more critical to designing a globally sustainable future. This transition is intimately linked to a metal/material infrastructure as it provides economic solutions for material production, processing, and recycling. As the complexity of the current products and commodities is increasing from a material point of view, a complex metal/material infrastructure is required to recycle these complex mixtures back into high-purity materials and metals, alloys of precise specification, as well as energy. Some authors have already reported possible bottlenecks on the material supply because of the expected demand for raw materials for the renewable energy transition [4,5,6,7,8]. Therefore, as Reuter et al. state, this complex metallurgical infrastructure, and particularly its unavoidable losses and residues produced, cannot be ignored when the CE is discussed and evaluated. This is, in fact, an inconvenient truth [9]. For instance, the energy production through renewable energy such as cadmium telluride (CdTe) photovoltaic (PV) panels, which accounts for around 5% of the worldwide PV energy production, requires cadmium, which is a by-product of zinc smelters. It also requires tellurium, produced mainly as a by-product of copper or lead metallurgy. For this reason, the metallurgical infrastructure to provide these elements must consider the base metals such as copper, zinc, and lead, as well as the refining stages for cadmium and tellurium from the residues produced during the metallurgy of these base metals. Since cadmium is toxic and tellurium is a technologically relevant metal that can also produce some hazardous compounds [10, 11], special care is required during the recycling of these panels at their end-of-life (EoL), especially when considering these to be a part of a circular society and as they are included in the scope of the Directive 2012/19/EU on waste electrical and electronic equipment (WEEE) [12]. This directive sets a minimum annual collection rate of 65% with respect to the total electrical and electronic equipment placed on the market. 85% of the collected fraction of WEEE (large equipment) must be recovered, while 80% of the recovered panels must be recycled or prepared for re-use. Therefore, to understand the CE of PV systems, a recycling process must be evaluated within the material/metal infrastructure required to produce the modules. This interconnected complex infrastructure is central to the CE of the CdTe PV module as depicted in Fig. 1.
Representation of the CE showing how materials are mined and processed to produce metals that will be used to produce commodities (depicted in blue). This complexly linked material processing infrastructure is connected to the dynamic energy grid as materials and energy enable renewable energy production as well as the recycling of its end-of-life infrastructure (gray arrows). The CE losses are depicted as residues and exergy dissipation throughout the cycle (orange). These losses reduce the secondary resources remaining “in the loop,” replenished through primary resources (Color figure online)
If an ideal representation of CE were considered, the resource depletion problem would be solved by simply returning the residues and end-of-life products to the metallurgical infrastructure and recovering everything repeatedly. However, the second law of thermodynamics (2LT) suggests that these processes are non-ideal; every real process generates entropy. This entropy creation means that resources, which include materials, minerals, metals, energy (incl. fuels), and water, must be consumed or irreversibly lost, implying that wastes will be inevitably produced and an environmental impact generated [13]. This implies that the residues and material losses of the society can only be reduced, but never be eliminated within the confines of our present time-constrained economically driven society. Moreover, this reduction of the residue generation and material losses in CE can be only achieved through the consumption of resources and the generation of an associated environmental impact. This means that the CE is not a perfectly closed circle as so easily depicted; it has limits as shown in Fig. 1. Therefore, the target of process engineers, metallurgists, product designers, policy makers, and the society in general must be to find these limits, in order to push them to sensible and economically achievable boundaries while guarantying that technological solutions are aligned with the United Nation’s Sustainable Development Goals (SDGs) [14]. This sustainable development will be achieved only if the social, environmental, and economic sustainability are fulfilled simultaneously [15,16,17]. These three pillars often have conflicting goals, and thus they must be evaluated through different perspectives always considering the SDGs, public opinion, effects on the society’s welfare, legislation, environmental concerns, or economic resilience on an existing market [16, 18]. Therefore, a rigorous approach is required that underpins the thermoeconomic analysis of large CE systems. The resulting evaluation of the resource efficiency and environmental impacts of CE maps the large CE system in terms of rigorous system simulation models. For instance, in the case of the CE of the mentioned CdTe PV modules, one of the key processing infrastructures is primary zinc metallurgy, through which cadmium is produced as a by-product. 90% of the global primary zinc production occurs through hydrometallurgy, which also generates iron-rich precipitates, i.e., jarosite, goethite, or hematite (a plant with an annual zinc production of 200 kt generates 88.3 kt of jarosite [19]). These residues are landfilled in ponds, which occupy a considerable land area and may generate environmental impacts if they are not safely operated. However, these iron precipitates entrain important technological metals such as indium or germanium, which are also landfilled and lost, but may create an incentive to process these if the valuable contained elements have an economic value. Therefore, the zinc industry has always been investigated for possibilities of dealing with the residues by, for example, (i) a new way of producing primary zinc such as direct zinc smelting [20] in a proactive manner while producing slag as a manner to capture the iron in a higher density form than the water-rich precipitates of iron or (ii) treatment of these iron residues once they are produced through the roast-leach-electrowinning process (reactive approach) [19, 21].
In order to understand very large-scale circular economy systems, a simulation-based methodology is applied to quantify, evaluate, and improve the resource efficiency in terms of exergy dissipation (thermoeconomics) and environmental impacts of the metal/material infrastructure central to the CE. This discussion is conducted in two steps:
Firstly, the resource efficiency limits of the complete CE system of CdTe PV modules are quantified by the creation of a very large-scale simulation model composed of 223 detailed modeled unit operations, over 860 flows, 30 elements, and all associated compounds. The simulation of this system provides the simulation-based indicators that are used to evaluate the exergy dissipation along the life cycle of the CdTe PV modules, as well as its material losses. This CE example illustrates two of the main thoughts of this paper:
A comprehensive metallurgical processing infrastructure is necessary to move towards a CE since the production and recycling of most metals are integrated with the metallurgies of other metals
A complete CE is not possible because of the inevitable losses, residues generated, and the additional resource consumption that is required for processing residues.
Secondly, the optimization of the primary zinc production, a subsystem of the CdTe PV module CE, was evaluated following the methodology presented in this paper. Ten scenarios to decrease or eliminate the generation of iron precipitates during the roast-leach-electrowinning (RLE) process for zinc production are considered and their resource efficiency and environmental impacts are quantified. Subsequently, the impacts of every scenario in the three dimensions of sustainability are discussed by using proxies for societal, economic, and environmental impacts obtained from the simulation-based indicators that were previously calculated, including their synergies and possible trade-offs.
Simulation-Based Methodology for the Resource Efficiency Evaluation of Large-Scale CE Systems
Large-scale CE systems are complex. The CE of the CdTe PV module requires base-metal metallurgies such as zinc, copper, and lead as well as the PV module manufacturing, EoL collection, and recycling stages. Therefore, simulation models capable of predicting the performance and resilience of these CE systems must be used to facilitate decision-making. A simulation-based methodology aiming to do this is presented in this section.
Step 1: Digital Twin of the CE System Using a Metallurgical Process Simulation Tool
Through the simulation of the system, every element, compound, mineral, alloy, residue or end-of-life product (along with their thermochemical properties), and process engineering knowledge (including metallurgical, energy, minerals processing) can be combined into a digital platform to obtain a digital twin of the system [22, 23]. This requires detailed process knowledge of the metallurgical and physical separation as well as product design steps in the CE system. Reuter and Verhoef [24] developed a dynamic simulation model to investigate the effect of the removal of lead from solders on the metallurgies of copper, silver, tin, or nickel among others, at that time excluding exergy analysis. HSC Sim is the simulation platform used in this paper that permits the exergy analysis. It is a module of the HSC Chemistry 9.9 software that allows the creation of flowsheets composed of unit operations, whose models are built by the user, and the streams connecting them, which contain all their thermochemical properties [25]. Furthermore, the simulation of the system provides the mass, energy, and exergy balances that are used to obtain the indicators required to evaluate the resource efficiency and environmental impacts of the system.
Step 2: Material Recovery and Losses and Residue Production
Through the mass and energy balances provided by the simulation of the CE system, the recovery rates of all the elements (through their various compounds) can be evaluated, as well as where from the system and in which form they are recovered [26]. Furthermore, the unavoidable material losses can be identified and tracked along the flowsheet. These indicators provide an idea of how much material or metals can be recovered and also what materials can be lost from the system. By using this approach, Reuter et al. have studied different processing and recycling routes for a cellphone with the objective of evaluating the material losses so that the circularity limits of the life cycle of the cellphone can be identified and pushed towards a more circular scenario [27].
Step 3: Resource Consumption—Thermoeconomics
The CE system requires resources (materials, energy, and water) to operate. Ideally, these resources would be entirely transformed into useful products. However, the ubiquitous 2LT dictates that some of the resources will be transformed into residues/emissions or they can be irreversibly lost. Exergy, which is a measure of the thermodynamic quality of a system, can be used to evaluate this resource consumption. As it is based on the 2LT, every process performed in the system will create entropy, i.e., its thermodynamic quality will be degraded. This means that the resources of the system will be downgraded or irreversibly destroyed. Therefore, the irreversibility of a process or the entire system, i.e., its exergy dissipation, can be used to evaluate its resource consumption. This exergy dissipation also implies that every product of the system will have an exergy cost, i.e., the exergy destroyed to produce it. To calculate these exergy costs in large systems, thermoeconomics, a methodology combining thermodynamics and economics, was developed [28]. Thermoeconomics has been largely used for the assessment and optimization of energy systems [29, 30]. However, it has been also applied to industrial symbiosis cases [31, 32]; however it lacks the metallurgical detail as reported in [9].
The evaluation of resource consumption through the 2LT permits the evaluation of all the resources with the same indicator (exergy flow units, kW), which solves the problem of integrating different resources such as energy resources (Joule), water, or raw materials, which are measured in mass units. For these reasons, the 2LT is applied as a good basis to evaluate the resource consumption of complex systems [32,33,34,35,36,37]. It has been largely used for this purpose in energy systems [29, 38,39,40,41], while other authors have already applied it to evaluate resource consumption in metallurgical processes [42,43,44,45]. Thermoeconomics has been implemented in HSC Sim (2019) by Abadías Llamas et al. to perform this task [45]. Chemical and physical exergy are considered for the exergy calculation of raw materials and water, while the exergy value of heat flows is calculated considering the Carnot efficiency [35, 46].
Step 4: Emissions and Their Associated Environmental Impact
During the operation of metallurgical processes, different emissions may appear, e.g., emissions to air or water. The emissions of the system are well characterized in terms of composition and mass flow through the mass balances performed by simulation of the process. Therefore, the environmental impact of the CE system can be evaluated from the simulated data by linking the results of the simulation platform to a life cycle assessment (LCA) software through the dedicated LCA tool of HSC Sim and GaBi [47]. Reuter et al. [23] have implemented and applied this methodology to evaluate the environmental impact of primary copper production, e-waste recycling, and nickel pig iron using the HSC Sim process simulation tool [25]. The value of the simulation-based approach is that the destination of all compounds, cations, ions, solutions, etc., can be predicted and subsequently evaluated. Since the processes are becoming more complex, environmental databases lack sufficient granularity to still provide meaningful assessments of these very large CE systems.
Step 5: Optimization of the CE System and Effect on Sustainable Development—Social, Environmental, and Economic Impacts
Once the flows of the CE have been quantified in kWh/h (kW) for different possible flow sheets and the different components in each stream, the optimization of a subsystem or the entire CE can be performed. Several scenarios can be assessed by using these simulation-based indicators to find the most resource-efficient and environmentally friendly alternative, which, moreover, assures a sustainable development. The simulation of CE and the indicators explained previously provide an appropriate picture to start finding and discussing all the factors, and associated trade-offs, affecting the social, environmental, and economic sustainability of our society, thus maximizing the resource efficiency of the CE system.
A first quantification of the factors affecting these three pillars can be performed by normalizing the indicators provided by the simulation. For instance, the CO2 emissions, which affect the environmental sustainability, can be normalized with respect to the lowest emissions value of all the evaluated alternatives (best-case scenario), or even to the emission limit, to evaluate the deviation of the emission values for every scenario. The same can be done for resource consumption, land use, or capital expenditure (CAPEX) and operational expenditure (OPEX). If the normalized values are calculated in a scale from 0 to 100%, where 0 is the scenario with the most negative impact and 100 is the best-case scenario (the positive one), a good comparative overview of all the evaluated factors affecting society, environment, and economics can be obtained. Thus, a first conclusion about the effect of the different solutions in sustainability can be obtained.
The Non-ideality of the CdTe PV Module Life Cycle
Figure 2 shows that base-metal metallurgies are central to the metal/material infrastructure needed in a CE society such as copper, lead, and zinc studied in the production of CdTe PV modules. Additionally, the manufacturing of the modules as well as an efficient EoL collection and recycling stages are required for the correct performance of the CE of the CdTe PV modules. As previously discussed, these large systems have unavoidable resource losses, even if they are optimized to improve their circularity, e.g., by adding residue-treatment processes. This affects negatively the resource efficiency of the CE. Therefore, these resource efficiency limits must be determined so that the system can be optimized, and our society can migrate towards a more informed CE in a most sustainable manner. For this reason, the methodology presented in this paper was used to evaluate the material recovery and losses that appear during the CE of the CdTe PV modules, as well as the exergy dissipation of the system to account for its resource consumption.
Exergetic harmonization of the energy and resource system. Production route of CdTe PV modules from raw materials to metals and product, and from end-of-life product to metals. Cu, Pb, and Zn metallurgy must be integrated to produce the Te and Cd in a resource-efficient way, while slag is a feed to construction materials (Color figure online)
Simulation of the Circular Route of the CdTe PV Module
A unique simulation model including 223 interconnected unit operations, 869 flows, and 30 different elements and their compounds was created in HSC Sim (HSC 9.9, [25]) to simulate the system shown in Fig. 2. In this model (flowsheets describing it are shown in “Appendix”), special attention was paid to the metallurgy of the base metals required for the production of the CdTe films, their production, and recycling, creating a unique detailed simulation model:
Zinc simulation model: the electrolytic (roasting, leaching, and electrowinning—RLE) and direct smelting routes for zinc sulfide concentrates were simulated, also based on the best available techniques [19, 48]. During the purification of the Zn solution, several residues are produced. One of them is a cadmium cementate, which can be treated to produce the cadmium that is going to be used to manufacture the PV modules. The details of this model are discussed in the next example and give the reader an indication of the detail of the other models included in this example, i.e., for copper and lead.
Copper simulation model: the primary and secondary production of copper was simulated based on the best available techniques and industrial data [49, 50]. The main blocks considered in this model are (i) the primary route for copper sulfide concentrates through flash smelting, converting, and refining and (ii) secondary route or black copper smelting. The configuration of the simulated black copper smelting uses a reduction stage followed by oxidation and refining. However, as shown in Fig. 3, other flowsheets were linked to the copper one to treat the residues produced during the copper production (see “Appendix”). The most relevant in this case is the precious metals’ recovery, where the anode slimes from the copper anode electrorefining are treated to recover gold, silver, or the tellurium that will be used to produce the CdTe film.
Lead simulation model: the direct smelting and bullion refining route was chosen and simulated to treat lead sulfide minerals [51, 52]. In this flowsheet, tellurium is also produced through the treatment of a caustic slag produced during the bullion refining.
Valuable technology element and residue treatment: The considered system has several common flowsheets to treat the residues produced on the three base-metal simulation models. For instance, as these metals are produced from sulfides, a common sulfur capture plant was created and connected to eliminate the sulfur of the off-gases and to produce sulfuric acid. Additionally, a slag fumer used to clean the slags with high zinc content has been included. Here, the slags from the direct lead smelting and secondary copper are reduced to fume the zinc. This is necessary to clean the slags so that they can be further used as, e.g., construction material or as a supplementary cementitious material. Fumes are treated in various reactors in the system.
Manufacturing: Once the cadmium and tellurium have been produced through the abovementioned metallurgical infrastructure, the PV cell is manufactured. This manufacturing process was simulated based on the current technologies for the production of CdTe thin-film PVs, that is to say, through the production of the CdTe and CdS compounds and vapor transport deposition [53, 54]. During the use phase of the PV modules, resource consumption and emissions have not been considered, i.e., the cleaning and maintenance of the modules during the use phase are not considered in the simulation as well as the production of the electrical infrastructure required to connect the modules to the electricity grid.
Recycling: At end-of-life, the modules are recycled in a recycling infrastructure. Collection efficiency of 95% has been assumed; thus, 95% of the produced PV modules enter the recycling process, and the rest goes to an undefined loss. During recycling the frame of the PV panel is dismantled before being shredded. Subsequently, the shredded module (glass and films) is leached with a sulfuric acid and hydrogen peroxide solution to dissolve the cadmium and tellurium of the thin films [55]. After a solid–liquid separation to split the glass and the metals, the cadmium and tellurium are precipitated from the solution [56, 57], forming a sludge or, when dewatered, an unrefined semiconductor material [58].
In summary, Fig. 3 shows the different in the tab at the bottom (from left to right) of all the linked and integrated flowsheets, viz. (i) primary copper flowsheet (Fig. 17), (ii) gas cleaning (Fig. 18), (iii) copper reduction furnace (Fig. 19), (iv) copper/cobalt–nickel solvent extraction (Fig. 20), (v) cobalt/nickel solvent extraction (Fig. 21), (vi) precious metals’ recovery (Fig. 22), (vii) tellurium production (Fig. 23), (viii) electricity and heat production (Fig. 24), (ix) sulfur capture plant (Fig. 25), (x) oxygen production plant (Fig. 26), (xi) copper electrolyte cleaning (Fig. 27), (xii) secondary copper flowsheet (Fig. 28), (xiii) zinc roasting + leaching (R + NL) (Fig. 29), (xiv) jarosite precipitation and cadmium production (Fig. 30), (xv) direct zinc smelting (Fig. 31), (xvi) lead production (Fig. 32), (xvii) slag fuming (Fig. 33), (xviii) PV cell manufacturing, use, and collection (Fig. 34), and (xix) PV cell recycling (Fig. 35). Note, while there are hydrometallurgical processes to refine the semiconductor material (CdTe), the unrefined material in the sludges is recycled back to the existing metallurgical process to produce pure cadmium and tellurium. These sludges are introduced into the reduction stage of lead smelting, where the cadmium reports mainly to the off-gas and the tellurium reports to the bullion, after which it is recovered through refining processes. The recovery of cadmium and tellurium through lead smelting by introducing the unrefined semiconductor material must be cognizant of the fact that a high concentration of tellurium in lead affects the production and refining of lead. This highlights that the quantity and quality of material fed to any smelter must be controlled to ensure maximal resource efficiency. A flexible and economically viable infrastructure is thus required to make the CE work.
Material Recovery and Losses
The metallurgical infrastructure required to produce the CdTe PV modules is capable of recovering to a large extent (over 90%) metals such as silver, gold, zinc, copper, or cadmium as depicted in Fig. 4 [27]. Moreover, other metals such as selenium or nickel could be produced in pure form if further treatment is performed, e.g., processing of the nickel sulfate produced during the electrolyte purification to produce pure nickel.
Metal recovery flower showing the element recovery to slags, i.e., Al, Fe, Mg, or Si, is generally not economically recoverable, whereas Ni or Se is recovered as by-products through further treatment. Sulfur is recovered as sulfuric acid, while the high recovery of Hg is efficiently captured during the off-gas cleaning process as calomel (Color figure online)
Elements such as aluminum, iron, magnesium, or silicon form oxides that report to the slags; thus, they cannot be recovered economically in pure form. Furthermore, some elements, such as zinc and, to some extent, cobalt or lead, are also entrained in the slag, which is the main source of material losses of this system. These slags, if cleaned, can be used as a construction material.
This reflects the necessity to have a metallurgical processing infrastructure to process materials. It is of great importance for policy to recognize that without the critical metallurgical processing infrastructure, there is no basis for recovering critical elements in a CE system; therefore, the CE system breaks down.
Resource Consumption of the CdTe CE System
An exergy analysis of this CE system was performed by using the dedicated tool of the simulation module of the HSC software, HSC Sim [25]. All the processes composing the CE system were grouped in subsystems and their exergy dissipation was represented through the Sankey diagram shown in Fig. 5. As depicted, every subsystem requires entering resources, which some of them will be transformed into products, as final or intermediate products, and others will be irreversibly lost, represented by the irreversibility. These losses include, for instance, metals entrained in the slags or sulfur that cannot be totally recovered through the sulfur capture plant. Therefore, even if the system is optimized to recover as much material as possible and minimize the residue production, a complete CE will not be achieved as the Sankey diagram of this CdTe PV module example shows.
A unique Sankey diagram representing the exergy dissipation along the circular system of the CdTe PV cell. For every subgroup, e.g., “Primary Copper,” “Sulfur Capture,” or “Cadmium Processing,” the resources required, the products produced, and the resources lost are depicted in MW. A large quantity of resources exchanged between the different infrastructures of Zn, Pb, and Cu is required to maximize the resource efficiency, while the Cd and Te are recovered in the Cu/Pb infrastructure (Color figure online)
As shown in Fig. 5, primary metallurgy accounts for large resource consumption. Sulfide minerals have high chemical exergy, e.g., 1534.4 kJ/mol of chalcopyrite vs. 134.3 kJ/mol of metallic copper. However, this thermodynamic quality is downgraded during the extractive metallurgy of these minerals. Some of this exergy is used to supply heat to the process, e.g., the smelting of chalcopyrite is exothermic, while the rest is lost. However, the recycling of these metals is also resource consuming as the “Secondary Copper” process shows. This exergy dissipation happens because the thermodynamic quality of metallic copper is low. Therefore, energy carriers must be used to counter this and perform the recycling process. Additionally, the more impurities in the copper feed, the more complex the secondary copper flowsheet, increasing its exergy consumption.
Cadmium and tellurium are produced as by-products of base metals such as copper, zinc, and lead, and they are concentrated along their metal extraction and refining processes. This makes the production processes of cadmium and tellurium not very resource consuming as shown in Fig. 5, where the “Tellurium Processing” and “Cadmium Processing” subgroups have an irreversibility of 0.4 MW and 1 MW, respectively, compared to 211 MW for primary copper production. The same occurs for the precious metal production, which demonstrates that producing gold, silver, or platinum-group metals (PGMs) as by-products of copper, lead, or zinc is not as resource consuming as extracting them directly from gold or silver ores. However, in order to produce all these minor but technologically and economically important metals, a base metals’ infrastructure is required. Therefore, if the metallurgy of a base metal collapses, for instance a production location must shut down because of changes in market, or a base metal is banned, e.g. banning lead because it is a hazardous element, the dream of having a CE or a more renewable energy supply could be jeopardized [59]. This is due to the fact the metallurgical/metal infrastructure required for the good performance of a resource-efficient society would not be able to provide and recycle the materials our society needs, or it could do it, but at the cost of consuming more resources and causing a larger environmental impact, producing a rebound effect. Therefore, any decision or optimization procedure of the CE system must be evaluated through a systemic approach that is capable of predicting their effects on the performance of the entire system, as well as on its surroundings, i.e., society, economy, and environment.
Furthermore, the idea that CE is the panache that transforms the residues or end-of-life products into valuable products without any waste production or cost is erroneous, since the required metallurgical infrastructure to “closing” the loop has limits. Even if the system is optimized to recover as much material as possible and minimize the residue production as this CdTe PV module example, a complete CE will not be achieved, a rather inconvenient truth. It will need additional resource consumption, as some of these resources will be inevitably lost as irreversibilities or residues of the system as depicted in Fig. 6. For instance, the sulfuric acid plant is required to remove the sulfur dioxide from the off-gases and the secondary copper production is required to recycle copper as well as metals such as silver, gold, or platinum. These two processes or systems are key to a CE; however, they have an inevitable irreversibility as shown in Fig. 5. Note also the large exergy content of solutions, that, if not well managed will create a large dissipation of exergy.
The CdTe PV module entering resources are depicted as fuel in blue, which are partially transformed into products, while some of them are lost as residues (represented in purple) and others are irreversibly lost (orange fraction). The surface area in orange suggests that there is large dissipation of exergy, showing the rather poor performance of the system, i.e., there is a limit to achieving circularity as also revealed in Fig. 5 (Color figure online)
Optimal Processing Routes in the CE System: Zinc Production
The inevitable resource losses happening in CE cannot be eliminated as demonstrated for the CdTe PV module life cycle. However, they can be reduced through the optimization of the system. One of the subsystems composing the CdTe PV module CE system is the production of zinc. 90% of the primary extraction of zinc is based on the roast-leach-electrowinning (RLE) process, which is shown in Fig. 7. In this technology, zinc sulfide concentrates are roasted to eliminate the sulfur, transforming the feed material into an oxide form that will be leached through the “Neutral Leach—NL” and “Weak Acid Leach—WAL” stages. The zinc sulfate solution produced during the leaching is purified to remove elements such as Cu, Cd, or Co, before reporting to the electrowinning stage, where super high-grade zinc cathodes are produced through electrowinning [19, 48]. The tab at the bottom of Fig. 7 shows all the interconnected zinc processing flowsheets, which include (i) zinc concentrate splitting between RLE and DZS, (ii) RLE flowsheet, (iii) zinc ferrite leaching and jarosite precipitation, (iv) electrolyte storage and distribution, (v) DZS flowsheet, and (vi) material recoveries of the system.
Zinc ferrites (ZnFe2O4) are formed during the roasting of the concentrate, requiring another leaching stage (or stages depending on the plant configuration) under more acidic conditions to leach the ferrites. This leaching stage dissolves both zinc and iron. The dissolved iron must be eliminated from the solution by precipitation as either jarosite, goethite, or hematite. Two routes of zinc ferrite leaching, as well as iron precipitation, are depicted in Fig. 8. These are the conventional route, i.e., ferrite leaching and jarosite precipitation, where lead and silver can be recovered as solid residue prior to the iron removal, and the conversion process, where the leaching of ferrites and iron precipitations happen in the same operation. Lead and silver cannot be precipitated and separated prior iron precipitation in the conversion process; thus, these are lost in the jarosite residue. These iron residues have to be stabilized and ponded well to avoid environmental risks. Furthermore, the iron precipitate includes technologically valuable metals such as indium or germanium, which are lost if the residue is landfilled [19].
Zinc ferrite leaching and jarosite precipitation flowsheet (third tab of the simulation pane shown in Fig. 7) for which two routes have been simulated: (i) ferrite treatment through hot acid leaching, solution pre-neutralization, and jarosite precipitation, and (ii) conversion process. The solution after iron removal is recirculated back to the neutral leach stage, while the jarosite residues are landfilled in a pond or, depending on the studied scenario, used as feed for a direct zinc smelting flowsheet. The lead–silver residue is recovered a processed in a lead processing (Color figure online)
Several options to avoid producing an iron precipitate exist. The ferrites may be treated in a Waelz kiln process or in a lead smelter with a zinc fumer, where the remaining zinc was recovered as zinc oxide fume that is recirculated to the leaching plant. The iron reports to the slag of this process in a stable, high density, and dry form. However, this process can produce a slag with a high base-metal content, e.g., 0.2–0.4% of Pb, which does not meet the elution criteria for environmental disposal [19].
The direct zinc smelting process (DZS) depicted in Fig. 9 is another option to reduce the generation of the iron precipitate during the RLE process [20]. In this processing route, zinc oxide dust is produced through a pyrometallurgical flowsheet where two main processes are performed, viz. (i) concentrate smelting and (ii) zinc fuming through slag reduction to clean the slag. In the first stage, the concentrate is smelted to remove the sulfur as sulfur dioxide through the off-gases and generate a slag. During this stage, elements such as Zn, Pb, Ag, In, or Ge are fumed and collected as oxide dust. In the second stage, the slag is cleaned and zinc is fumed, along with Cd, Sb, or Ge to produce a clean iron-rich slag containing 2–3% of Zn. This step is included to further clean the slag so that in better complies with the quality constraints of construction material. This, however, requires an additional consumption of resources (mainly reductants). Additionally, reducing the zinc content in the final slag to significantly below 1% would imply the reduction of iron, which will then collect various other elements due to the strong reducing conditions. This “dirty” iron alloy can then be used somewhere else in the depicted non-ferrous flowsheets depicted in “Appendix” section, e.g., as a reducing agent for Fe3+ to Fe2+ to control magnetite formation in the slag. If the DZS is integrated with an RLE plant, the iron residue produced during the RLE can be fed to the DZS smelting stage to control its slag chemistry, while the zinc oxide-rich dust can be leached in the neutral and weak acid leach stages of the RLE. This would free land occupied by residue dumps and recover co-precipitated elements during jarosite process such as indium and germanium. This option would extend the life of the current RLE plants [20] as jarosite dumping is minimized. Recent industrial trails [60] have shown that > 90% of zinc is already recovered during the smelting stage. However, the resource consumption, material recovery and losses, environmental impact, and residue production of this circular action must be carefully evaluated so that the resource efficiency of the system can be assessed objectively. Therefore, the methodology presented in this paper was applied to this case to find the most resource-efficient option to reduce or eliminate the generation of iron-rich precipitates during the primary production of zinc.
Direct zinc smelting flowsheet (fifth tab of the simulation pane in Fig. 7) where the zinc concentrate is smelted to produce a fume containing volatile metals under smelting conditions such as Ag, e.g., as AgO(g) [20], Pb, Zn, As, or In, and a slag which will be cleaned in a second stage under reducing conditions. During the reduction stage, volatile metals are fumed, while non-volatile and reducible metals such as Cu or residual Ag and As can form a speiss/alloy phase. A clean slag (less than 3% Zn) is produced and the fumes are processed in a neutral leaching stage of the RLE [20] (Color figure online)
Simulation of the RLE-DZS System
An integrated RLE-DZS system has been modeled with the simulation platform HSC Sim. This sub-model of the PV model is composed of 58 unit operations, 222 streams, and over 20 elements (and all compounds), whose main flowsheets are depicted in Figs. 7, 8, and 9. The input of the process is an average zinc concentrate with a flow rate of 60 t/h, which is composed of 51% Zn, 5% Fe, 3% Pb and minor elements such as silver, indium, germanium, or arsenic in their average concentration in zinc concentrates, while its water content is 5%. The zinc concentrate is split between the RLE and the DZS flowsheets and the ratio is varied between 100% RLE–0% DZS to 0% RLE–100% DZS in six different scenarios to evaluate how it affects the resource efficiency of this preventive alternative. The jarosite (sodium and potassium jarosites) produced is fed to the DZS to dilute the zinc oxide in the smelting slag, while the dust collected in the DZS flowsheet is recirculated back to the RLE to be leached. Furthermore, two 100% DZS cases with different operation parameters are considered. The fuming of the zinc ferrites and the pyrometallurgical treatment of jarosite have been also considered as they are not preventive solutions to avoid the generation of jarosite, but reactive, i.e., the jarosite is generated and then treated.
Material Losses, Metal Recoveries, and Residue Production of the Evaluated Scenarios
In the 100% RLE scenario considered, metals such as indium, germanium, or antimony report to the jarosite residue, and thus they are lost. Additionally, as the jarosite is not treated, total recovery of lead, silver, or zinc is lower since these metals contained in the jarosite are landfilled. Usually, the total recovery of these three elements in a 100% RLE scenario is over 90% (99% in the case of zinc), as shown in Fig. 10. Furthermore, metals as cobalt, nickel, or cadmium are recovered during the purification of the electrolyte, as well as the calcium as gypsum, which is filtered and washed to ensure its safe disposal.
Metal recovery flowers showing the recovery rates of different elements considered in the simulation model, both in pure or in a by-product form. Three different configurations of the integrated RLE-DZS are depicted: (i) 100% of the concentrate going to the RLE, (ii) concentrate splits equally between RLE and DZS, where the jarosite residue is partially fed to the DZS flowsheet to control the slag chemistry, and (iii) 100% DZS, where zinc dust is produced and leached in neutral and weak acid leach stages of the RLE and iron reports mainly to slag (Color figure online)
If the concentrate is split equally between RLE and DZS and the jarosite residue is fed to the DZS process, the recoveries of zinc, lead, or silver increase since they are collected through the smelting and reduction dust. Additionally, as the dust is leached again in the RLE plant, it increases the concentration of the germanium and indium into the jarosite residue fraction that is not treated in the DZS; thus, it is more suitable for the recovery of these technological metals. However, during DZS, elements such as cobalt or nickel go to the slag and they are lost since they are too diluted in the slag. Therefore, the cobalt and nickel introduced to the DZS flowsheet will likely be lost, reducing the recovery rates of the entire system for these metals. These elements follow the same trend when a 100% DZS scenario is studied, as shown in Fig. 10. Metals such as indium, silver, and germanium can be recovered while nickel and cobalt may be lost in the clean slag of the DZS flowsheet. The performance of cadmium is independent on the processing route since it is recovered through the fumes in the DZS. As the cadmium is leached with zinc in the neutral leaching stage, it is recovered along the solution purification.
The integration of the DZS and RLE transforms the problematic hydrometallurgical iron precipitate into a clean disposable slag that may be used as construction material. Through this integration, the clean slag becomes a valuable product for the plant, while freeing landfill space around zinc smelters, which would help to improve the society’s perception of zinc metallurgy.
Resource Consumption Evaluated Through the Exergy Dissipation of the Ten Scenarios
The integrated RLE-DZS system simulation model for zinc production provides also the exergy values of all the streams composing the flowsheet. With the exergy values of the flows and the thermoeconomics tool of HSC Sim, the resource consumption of 10 scenarios has been evaluated and depicted in Fig. 11.
Resource consumption depicted as exergy dissipation for 10 different cases for the jarosite residue treatment produced during RLE. “100 RLE – 0 DZS” is the reference case. a The exergy dissipation from a 100% RLE scenario to 100% DZS. b The resource consumption associated to the treatment of jarosite and the fuming of ferrites, as well as two 100% DZS scenarios with different operation parameters from the DZS cases represented in a. Resource consumption allocated to the hydrometallurgical route, i.e., RLE, is represented as a solid blue color. Exergy dissipation allocated to the pyrometallurgical flowsheet, i.e., DZS and pyrometallurgical treatment of RLE residues, is depicted as striped-red (Color figure online)
The base case, i.e., the current operation of the zinc smelters (100% RLE), and the five scenarios varying the fraction of concentrate introduced to the DZS flowsheet are depicted in Fig. 11a. The DZS operation parameters of these five DZS scenarios are defined according to Hoang et al. [20] with an oxygen enrichment of 60%, which reports zinc fuming rates of 62% in the smelting stage. However, improved zinc fuming rates have been recently estimated during the smelting stage of the DZS on a commercial scale in one plant located in China, where 93% of the zinc is fumed during this stage [60]. Therefore, a 100% DZS scenario considering these fuming rates has been also evaluated (“0 RLE–100 DZS (Smelt.)” scenario), as well as a scenario where no oxygen enrichment was considered (“0 RLE–100 DZS (No O2 Enr.)”). The other two scenarios, whose resource consumption is depicted in Fig. 11b, correspond to (i) roasting, neutral leaching (R + NL), and ferrite fuming to extract the zinc of the ferrites as an iron-free zinc dust and (ii) jarosite treatment through smelting and reduction stages for material recovery and the conversion of the residue into a slag that, if the composition complies the specifications, can be used as building material [61].
The lowest resource consumption, by a very narrow margin, appears during the base case (“100 RLE–0 DZS”) since the electrolyte of the RLE flowsheet can be reused, while the reducing agents and energy carriers required to perform the smelting and reduction stages of the DZS are irreversibly lost, representing a large exergy dissipation. However, the total resource consumption of a 100% DZS scenario with an oxygen enrichment of 60% and the zinc fuming rates of the smelting stage reported by Wood et al. [60] (93% of Zn fumed in smelting), i.e., the “0 RLE–100 DZS (Smelt.)” scenario, is just slightly higher. The resources required to operate the DZS flowsheet are compensated with the lower resources consumed in the RLE since the roasting, hot acid leaching, and iron precipitation stages are eliminated. The high oxygen enrichment DZS has the additional advantage that exergy dissipation can be balanced between hydro- and pyrometallurgical operation, and the RLE route is much more limited in this regard.
Furthermore, if the zinc fuming rates of the DZS plant are the expected by Hoang et al. [20] (63% of Zn fumed during smelting), the resource consumption will follow an increasing linear tendency when the quantity of concentrate treated during DZS increases, as shown in Fig. 11a for the cases 2–6. The reason is that the zinc concentration in the smelting slag must be diluted by recirculating clean slag and feeding iron residues to the smelter, which increases the quantity of material circulating along the DZS flowsheet; thus, the energy requirements are larger. However, if more zinc is fumed in the smelting stage as happens in case 9, the zinc content in the smelting slag will be below 25%; thus, no slag recirculation will be required, which implies that the material quantity entering the furnace is lower, and hence the energy carrier and reductant required decrease. Furthermore, the use of oxygen-enriched (Enr.) air in DZS reduces the resource consumption as the quantity of nitrogen introduced to the furnace decreases, as the “0 RLE–100 DZS (No O2 Enr.)” shows.
When the jarosite precipitation process is avoided through the zinc fuming of the ferrites (“100 R + NL + Ferrites Fuming” scenario), i.e., the zinc ferrites are treated in a fumer instead of leaching them in the hot acid leach, its resource consumption is comparable to treating 80% of the concentrate in a DZS flowsheet. The reason for this high resource consumption is that the zinc ferrites would be fumed together with the lead–silver residue precipitated, increasing the amount of material to fume. This action would also increase the lead circulation load since the lead–silver residue where lead reports is not produced. Therefore, the lower resource consumption of the hydrometallurgical route for the treatment of the ferrites, i.e., the base case, is one of the reasons why zinc ferrite fuming fell from favor after the new hydrometallurgical methods to leach the ferrites and precipitate the iron were successfully applied, since the pyrometallurgical process required to fume the zinc of the ferrites is removed.
Another good option would be the pyrometallurgical treatment of the jarosite residue produced during the RLE (i.e., case 7), as depicted in Fig. 11. This option would convert the jarosite residue into a cleaned slag, which could be reusable depending on its characteristics and composition, and elements such as indium, germanium, or antimony would be fumed, as well as the co-precipitated zinc and lead. Obviously, these extra jarosite smelting and reduction stages would require extra resource consumption; however, it is not as high as the other options evaluated. This is because the jarosite smelting stage would not need a slag recirculation to control the zinc oxide content in the smelting slag since the zinc content in the jarosite is low enough to avoid problems associated to a high viscous slag. Therefore, the quantity of energy carrier and reductant required to perform the smelting would decrease since the amount of material to be treated would be lower in comparison to the one treated during DZS or ferrite fuming.
Emissions and Environmental Impact Associated with the Primary Production of Zinc
The environmental impacts of the studied RLE-DZS system have been obtained through the connection between the simulation platform and the LCA software GaBi. Several indicators can be obtained through the LCA; however, the global warming potential (GWP), measured in kg of CO2 equivalent, will be discussed in this case. The GWP follows the same trend that the resource consumption explained before, as depicted in Fig. 12. The CO2 emissions associated with the RLE flowsheet come mainly from the production of electricity required during the zinc electrowinning. It is the same for all the cases since the amount of zinc cathodes produced is the same (30 t/h). However, the CO2 emissions increase with the use of the DZS technology due to the use of fuels as energy carriers and reductants. Despite the need of a roasting stage during the RLE, the lower temperatures required and the lower quantity of input material in comparison with the smelting stage of the DZS (due to the requirement of controlling the slag chemistry) make the consumption of fuel of the RLE considerably lower; thus, the CO2 emissions of the 100% RLE case because of the concentrate roasting are small.
Global Warming Potential (GWP) represented as kg of CO2 equivalent, for the 10 studied scenarios. The solid-yellow part represents the CO2 emissions associated to the RLE process, which come mostly from the production of electricity required to electrowinning the zinc from the solution (electricity mix from China chosen for this study as representative zinc producer). The striped-red area represents the emissions related to the production of the metallurgical coke required for the pyrometallurgical operations, while the plaid-green part shows the CO2 emissions produced in the pyrometallurgical flowsheet, both during DZS or pyrometallurgical treatment of RLE residues (Color figure online)
The pyrometallurgical treatment of jarosite appears as the best alternative to the RLE in terms of GWP, since the emissions associated to the direct fuming of the zinc ferrites are considerable because of the fuel and reductant requirements for the fuming process. However, improved zinc fuming rates during DZS and oxygen enrichment make the difference to the pyrometallurgical treatment of jarosite smaller.
Selection of the most resource-efficient alternative and its effect of the CE on sustainable development—social, environmental, and economic impacts
Although the 100% DZS with high zinc fuming rates (scenario 9) is the best option from the point of view of resource consumption, elements such as cobalt or nickel would not be recovered as they would be mainly captured by the slag, and thus they would be lost. Additionally, CO2 emissions would increase in comparison with the base case. For these reasons, a 100% RLE followed by a pyrometallurgical treatment of the jarosite residue would be the most suitable option to avoid the generation of a wet hydrometallurgical residue. An existing RLE plant and the infrastructure required for the DZS could create this integrated system, with the difference of, instead of splitting the concentrate and feeding it to the DZS, only the produced jarosite residue would be fed to the pyrometallurgical flowsheet; thus, the concentrate would be entirely fed to the RLE flowsheet (scenario “100 RLE + jarosite treatment”).
In terms of material recovery, elements co-precipitated with the jarosite residue, e.g., indium, germanium, or antimony, could be recovered through the fumes generated during the pyrometallurgical treatment of the jarosite, if these were not recovered during the typical RLE operations. Additionally, the copper, cobalt, nickel, cadmium, and lead–silver residues would be still produced; thus, the recovery of these elements through further treatment would be possible. Therefore, the material recovery would improve with respect to the base scenario, while in addition the jarosite residue is converted into a clean slag with the potential of being commercialized as a construction material.
Obviously, the resource consumption of the integrated system would increase in comparison to the base case and the high-fuming DZS. The reason is simple, the 2LT dictates that every extra process performed will require extra resources to be used or destroyed. However, the extra resources required to conduct this pyrometallurgical treatment are not much larger than the options shown in Fig. 11. Furthermore, the same occurs with the GWP of the integrated system. An increase in the CO2 emissions is expected when the system is integrated because of the fuel and reductant required for the thermal treatment; however, the CO2 emissions of this scenario are the lowest of all the considered options.
The proposed alternatives must fit into the CE society depicted in Fig. 1 in a way that sustainable development is guaranteed when they are applied. Sustainable development is based on keeping the balance between social, environmental, and economic sustainability. For this reason, the effects of the CE solutions for the reduction of iron-rich precipitate during hydrometallurgical zinc production on these three pillars are discussed, with a few examples on how the impact of some factors affecting them can be quantified.
Effects on Society
As mentioned before, the hydrometallurgical production of zinc generates around 0.5 to 1 t of jarosite per ton of zinc produced, which must be landfilled. Therefore, the problems related to this residue production could affect this social welfare due to large landfill areas required for the disposal of this residue. This fact affects especially the communities living next to the places where the jarosite pond is situated, generating a “not in my backyard NIMBY” effect as it happens with other projects such as landfill sites or incineration plants [62, 63]. Therefore, the social acceptance for the jarosite volume increase in ponds may be jeopardized. Reducing pond volume and creating high-density and dry slag from jarosite, which is suitable for safe disposal, or even as a building material if the slag is clean enough, is a positive development for the society, helping the zinc industry to get the “social license to operate” over the years [64]. This can be confirmed by linking the simulation-based indicators obtained to, for example, land use reduction. As a proxy, the iron removal as slag is studied and normalized with respect to the best scenario, i.e., 100% of the iron removed through a slag (represents the highest density and cleanest of products); the deviations of each of the 10 scenarios evaluated with respect to the ideal case to freeing up land can be obtained, as represented in Fig. 13. In summary, this means, 0% is the worst scenario, i.e., all the iron is disposed off as jarosite as it happens during a 100% RLE case; and 100% the best one, where all the iron is removed as slag, which may find an application as a building material. As shown in Fig. 13, the more concentrate is treated in the DZS flowsheet, the more land will be released. This relationship is not linear since the quantity of iron removed as slag depends on several factors such as the concentrate feed ratio or the quantity of jarosite the DZS flowsheet can treat (the lower concentrate feed to DZS, the lower jarosite can be treated since the DZS facilities would be smaller). In the same way, the ferrite fuming and jarosite treatment cases do not require a large land use since the iron is transformed into a clean slag through these two alternatives.
Normalized value of iron removed as slag (highest density and land freeing) with respect to the best-case scenario, i.e., 100% iron removed as slag during 100% DZS operation. This indicator can be used to discuss how the land is freed up, being 100% the best case. Green represents the pyrometallurgical treatment of jarosite, while yellow depicts the iron removed as slag during DZS, whose values are the maximum (Color figure online)
Effects on the Environment
Besides the social benefits of reducing the land use discussed in the previous section, clearing up land also avoids potential environmental issues, e.g., pond failures or metal emissions to the environment. Therefore, it entails a synergy between society and the environment. However, the resource consumption, mainly energy resources as coal or pet coke, and the GWP associated with this new configuration for the zinc smelters increases. Therefore, this supposes a trade-off that the environment must accept if the production of jarosite wants to be eliminated. For this reason, a quantitative evaluation of factors affecting the environmental sustainability of the system can be also done in the same way that the land use indicator used to start discussing the social impacts. In this case, the resource consumption increases in every alternative evaluated. Therefore, by normalizing the resource consumption with respect to the base scenario, i.e., dividing the exergy dissipation of the base case by the exergy dissipation of every case, an indicator of how close the alternative is to the minimum exergy dissipation, on a scale from 0 to 100%, can be obtained as represented in Fig. 14. As the results show, the 100% DZS with high zinc fuming rate alternative (represented in yellow) accounts for the lowest resource consumption within the alternatives to the RLE. The largest resource consumption, as discussed before, happens when the DZS is operated without oxygen enrichment, while the pyrometallurgical treatment of jarosite accounts for the second-lowest resource consumption within the alternatives.
Normalized value of resource consumption, measured by exergy dissipation, with respect to the minimum resource consumption value. The closer to 100%, the lower resource consumption. Red represents the base case, green the pyrometallurgical treatment of the jarosite, and yellow the DZS with high zinc fuming rates (Color figure online)
Another indicator of the effects of the system on the environment is the CO2 emissions. As done with the exergy indicator, the CO2 emissions of the system can be normalized with respect to the 100% RLE scenario, which accounts for the lowest CO2 emissions of all the cases, as explained before. The results of Fig. 15 show that the alternative that is closer to the minimum of CO2 emissions is the pyrometallurgical treatment of the jarosite with a deviation of 9% on its relative value with respect to the minimum CO2 emission (best case represented as 100%) as depicted by the green bar.
Normalized value CO2 emissions with respect to the minimum emission value, which happens in the base case. The closer to 100%, the lower CO2 emission of the alternative; red represents the base case, green the pyrometallurgical treatment of the jarosite, and yellow the DZS with high zinc fuming rates (Color figure online)
Effects on Economics
The pyrometallurgical treatment of the jarosite residues would imply an investment by the RLE plants. Even if the smelting and reduction stages can be performed in one reactor, if it permits a flexible operation in oxidizing and reducing conditions, e.g., top submerged lance technology, the investment on the furnace and the peripheral equipment required, i.e., off-gas treatment, dust filters or slag granulation, would be considerable. Additionally, larger operational costs are expected since the larger resource consumption is required, whose normalized value with respect to the minimum has been depicted in Fig. 14 and discussed previously. Therefore, these economic requirements for the operation of the CE action imply that a compromise between cost/benefit must be achieved to guarantee the economic sustainability of the selected CE action.
The economic benefits of this circular option include that the products of the RLE plant would increase, e.g., germanium and indium-rich dust or a potentially marketable slag if it is cleaned enough. Furthermore, the problems related to the jarosite landfilling and its cost would be avoided, which can also suppose an economic saving and a more circular and environmentally friendly image for the smelter. This is very important for RLE plants located in countries where the restrictions on residue landfilling are strict or the space available for residue deposition is small, since they could keep operating if the legislation for residue production or landfilling becomes more restrictive, e.g., the Directive 2018/851 on waste of the European Union, which suggest the state members restrictions or economic penalties for the landfill of waste [65]. This must be confirmed through a CAPEX/OPEX analysis so that all the factors affecting the economics of the solution are considered, e.g., metal prices, downstream refining costs, or avoided landfilled costs. A CAPEX/OPEX analysis is outside the objective of the paper.
Sustainability of the DZS and Pyrometallurgical Treatment of Jarosite Solutions
The factors affecting society, environment, and economics, which are quantified by normalization as shown in Figs. 13, 14, and 15 can now be gathered together to evaluate the sustainability of the options selected to deal with the reduction of residues during zinc production. There may be factors affecting several pillars at the same time, e.g., diminishing water use by industries may affect positively the society in regions where water resources are scarce as well as it prevents the risk that aquatic ecosystems are affected by industrial practices, i.e., having a beneficial impact for the environment too. If the impacts on the three dimensions of sustainability of the two best options from a resource efficiency and environmental impact point of view are evaluated, i.e., DZS operated with oxygen enrichment and achieving high fuming rates and the pyrometallurgical treatment of jarosite, positive impacts are obtained. In terms of social impacts, both options minimize the generation of jarosite as shown in Fig. 14, and thus the growth of jarosite ponds is avoided. However, the pyrometallurgical treatment of jarosite has a more positive impact on the environment than the DZS because of the higher CO2 emissions, at the cost of expending more resources. However, the impact of the CO2 emissions is more negative than the extra resources that must be consumed; thus, the best option that guarantees a better sustainable development is the pyrometallurgical treatment of jarosite. Obviously, this is a case to show how the indicators provided by the simulation can be used to start quantifying and discussing the sustainability of the different CE solutions, and thus more factors affecting to every pillar of sustainability must be evaluated to perform a rigorous evaluation, e.g., water use, CAPEX/OPEX, and more environmental impact indicators.
Conclusions
A CE society needs a capable and agile metallurgical infrastructure to “close” the material loop; in short, the metallurgical infrastructure is the key driver [66]. While CE thinking is key to resolving the fact that our society lives on a finite planet, it has been clearly quantified in this paper that the ubiquitous material/metallurgical system associated with a CE has material and energy losses, consumes resources, and produces secondary and tertiary residues; thus, the CE has clear limits. These limits must be quantified to inform society and policy so that the ideals of the CE can be realistically achieved in a sustainable manner.
This paper shows how to quantify these limits by developing a detailed large-scale flowsheet of the CE system. The mapping of the system with a process simulation platform is a good option since process engineering and the resources consumed, produced, lost, or exchanged between all the actors of the CE system, including all their thermochemical data, can be defined. This platform predicts and shows all material recovery and losses, residue generation, resource consumption, and environmental impact. On this basis, alternatives to move the system towards a more CE can be evaluated and selected to guide policy to improve resource efficiency in the best possible way to achieve circularity, i.e., guarantying a sustainable development.
The paper shows firstly how very large CE systems can be evaluated to find their resource efficiency limits and then how these limits can be optimized by using the simulation-based methodology explained in this paper:
Resource efficiency quantification of the CdTe CE system: The zinc, lead, and copper base-metal infrastructures are required since elements such as cadmium and tellurium are produced as by-products of this metallurgical infrastructure and applied in the CdTe infrastructure. Manufacturing and recycling infrastructures are also required to produce the panels from these metals and recycling them to produce cadmium and tellurium at the end-of-life of the solar panels. This complex infrastructure for the CE of the CdTe PV modules was simulated using the systemic approach explained in this paper. This example shows uniquely the exergy dissipation, thus the non-ideality, of this complex CE system linking energy and resources between 223 unit operations, furnaces, manufacturing, etc. through 869 flows and around 30 different elements and all their compounds. It is the largest such model existing to show how to simulate a complete CE system for a product. It is clear that from all the resources required only a part is transformed into products as shown in Fig. 6, the losses are significant. Much of the resources are transformed into residues, which generate an environmental impact that could be calculated in the same way it was done for the zinc production case, while some of them are irreversibly lost. The CdTe example shows that the effect of the 2LT on the CE systems can be studied to find the limits of new products and renewable energy infrastructure. A unique Sankey diagram shows the true exergy dissipation of the system summarized by the losses depicted in Fig. 5.
Resource efficiency optimization in zinc production: Through the simulation of the problem, a good basis to evaluate and optimize the material losses, residue production, environmental impact, and resource consumption of the current RLE for the zinc production was obtained. Obviously, the reduction of the volume of residue, e.g., as a clean slag plays an important role. The results clearly show which routes would be best to clear up land as efficiently as possible, i.e., diminishing the ponding volume. To arrive at this result, several options to understand CE scenario were simulated, and the best option was selected from resource efficiency and an environmental impact point of view. The selected alternative was the integration of the RLE flowsheet with the pyrometallurgical treatment of the iron-rich precipitate through smelting and reduction stages to recover the valuable co-precipitated metals and convert the residue into a benign slag, which could be marketable for construction applications. It can be concluded from this analysis that the integration of a pyrometallurgical treatment with the RLE flowsheets of the current zinc production plants ensures social sustainability since it affects society positively due to the low land use required to landfill residues. However, it must be considered that an increase in resource consumption and CO2 emission would appear. Therefore, a trade-off between land freeing and environmental sustainability will happen.
In summary, this paper demonstrates that the 2LT can be used to quantify the limits of our CE society. Mapping the CE system as reflected in Fig. 16 through system simulation platforms provides a rigorous quantification of its material losses, residues produced, the environmental impact associated, and resource consumption. This is key to understanding the economics and efficiency as well as limits of the CE system. The complex interactions shown in Fig. 16 can only be done if its Smart Materials Grid is evaluated in the manner shown in this paper while also linking the simulation models to the water and energy systems. The complex models in this paper show that this can be done to the depth of evaluating the total exergy dissipation while also being able to understand all the other usual impacts based on the shown rigorous simulation platform.
A resource-efficient society must connect the water, energy, material, and mobility infrastructures/grids for maximizing the resource-efficient performance of circular society. A smart and adaptive metal and material production, processing, and recycling infrastructure are central to “fueling” the materials needs of the renewable energy infrastructure of society while minimizing residues production [9] (Color figure online)
Central to making all of this work is a smart and adaptive metallurgical processing infrastructure as shown by all the flowsheets in this paper. Showing how to create a digital twin of a very large CE system to evaluate this infrastructure is a key contribution of this paper. Protecting this metallurgical and therefore critical processing infrastructure is thus a key to realizing a circular society, the key message of this paper [59]; just discussing the criticality of elements is not good enough. We need to understand how sustainable metallurgy and its infrastructure drives the CE system to its maximum resource efficiency and therefore to its lowest exergy dissipation. This paper shows that this can be done, therefore linking resources and their mineral compositions to product design and their composition and their subsequent recycling, therefore true geometallurgy enabling sustainable metallurgy!
References
United Nations, Department of Economic and Social Affairs, Population Division (2017) World population prospects the 2017 revision, key findings and advance tables. Working paper no. ESA/P/WP/248. (Online). https://population.un.org/wpp/Publications/Files/WPP2017_KeyFindings.pdf. Accessed 15 July 2019
Ekins P et al (2017) Resource efficiency: potential and economic implications. A report of the International Resource Panel (UNEP). (Online). http://www.resourcepanel.org/sites/default/files/documents/document/media/resource_efficiency_report_march_2017_web_res.pdf. Accessed 16 Jun 2019
IPCC (2014) Climate change 2014: synthesis report. Contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change. IPCC, Geneva. (Online). https://www.ipcc.ch/site/assets/uploads/2018/05/SYR_AR5_FINAL_full_wcover.pdf. Accessed 15 July 2019
Valero A, Valero A, Calvo G, Ortego A (2018) Material bottlenecks in the future development of green technologies. Renew Sustain Energy Rev 93:178–200
Ali SH et al (2017) Mineral supply for sustainable development requires resource governance. Nature 543(7645):367–372
Månberger A, Stenqvist B (2018) Global metal flows in the renewable energy transition: exploring the effects of substitutes, technological mix and development. Energy Policy 119(April):226–241
Grandell L, Lehtilä A, Kivinen M, Koljonen T, Kihlman S, Lauri LS (2017) Role of critical metals in the future markets of clean energy technologies. Renew Energy 95:53–62
Moreau V, Dos Reis P, Vuille F (2019) Enough metals? Resource constraints to supply a fully renewable energy system. Resources 8(1):29
Reuter MA, van Schaik A, Gutzmer J, Bartie N, Abadías Llamas A (2019) Challenges of the circular economy—a metallurgical and product design perspective. Annu Rev Mater Res 49:10.1–10.22
Schulte-Schrepping K-H, Piscator M (2005) Cadmium and cadmium compounds. In: Ullmann’s Encyclopedia of Industrial Chemistry, vol 100C, Wiley, Hoboken
Knockaert G (2005) Tellurium and tellurium compounds. In: Ullmann’s encyclopedia of industrial chemistry. Wiley, Hoboken
European Parliament and Council (2012) Directive 2012/19/EU on waste electrical and electronic equipment (WEEE). Official Journal of the European Union. (Online). https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2012:197:0038:0071:en:PDF. Accessed 11 July 2019
Reuter MA, Hudson C, van Schaik A, Heiskanen K, Meskers C, Hagelüken C (2013) Metal recycling: opportunities, limits, infrastructure. United Nations Environmental Protection (UNEP) Report. (Online). http://www.resourcepanel.org/reports/metal-recycling. Accessed 26 July 2018
United Nations (2015) Transforming our world: the 2030 agenda for sustainable development. Working Paper No. A/RES/70/1. (Online). https://sustainabledevelopment.un.org/content/documents/21252030%20Agenda%20for%20Sustainable%20Development%20web.pdf. Accessed 15 July 2019
World Commission on Environment and Development (1987) Our Common Future”. Oxford University Press, Oxford
Kates RW, Parris TM, Leiserowitz A (2005) What is sustainable development? Goals, indicators, values, and practice. Environment 47(3):8–21
Purvis B, Mao Y, Robinson D (2019) Three pillars of sustainability: in search of conceptual origins. Sustain Sci 14(3):681–695
Barbier EB (1987) The concept of sustainable economic development. Environ Conserv 14(2):101–110
Sinclair RJ (2005) The extractive metallurgy of zinc, vol 13. The Australasian Institute of Mining and Metallurgy, Melbourne
Hoang J, Reuter MA, Matusewicz R, Hughes S, Piret N (2009) Top submerged lance direct zinc smelting. Miner Eng 22(9–10):742–751
Creedy S, Glinin A, Matusewicz R, Hughes S, Reuter MA (2013) Outotec® Ausmelt technology for treating zinc residues. World Metall Erzmetall 66(4):230–235
Reuter MA (2016) Digitalizing the circular economy: circular economy engineering defined by the metallurgical internet of things. Metall Mater Trans B 47(6):3194–3220
Reuter MA, van Schaik A, Gediga J (2015) Simulation-based design for resource efficiency of metal production and recycling systems: Cases—copper production and recycling, e-waste (LED lamps) and nickel pig iron. Int J Life Cycle Assess 20(5):671–693
Reuter MA, Verhoef EV (2004) A dynamic model for the assessment of the replacement of lead in solders. J Electron Mater 33(12):1567–1580
Outotec, “HSC Chemistry 9,” 2019. (Online). https://www.outotec.com/. Accessed 28 Feb 2019
van Schaik A, Reuter MA (2016) Recycling indices visualizing the performance of the circular economy. World Metall Erzmetall 69(4):201–216
Reuter MA, van Schaik A, Ballester M (2018) Limits of circular economy: fairphone modular design pushing the limits. World Metall Erzmetall 71(2):68–79
Valero A, Lozano M, Munoz M (1986) A general theory of exergy saving I. On the exergetic cost. ASME. Adv Energy Syst Div 2:1–8
Tsatsaronis G (1993) Thermoeconomic analysis and optimization of energy systems. Prog Energy Combust Sci 19(3):227–257
Lozano MA, Valero A (1993) Theory of the exergetic cost. Energy 18(9):939–960
Valero AA, Usón S, Torres C, Valero AA, Agudelo A, Costa J (2013) Thermoeconomic tools for the analysis of eco-industrial parks. Energy 62:62–72
Usón S, Valero A, Agudelo A (2012) Thermoeconomics and industrial symbiosis. effect of by-product integration in cost assessment. Energy 45(1):43–51
Valero A, Usón S, Torres C, Valero A (2010) Application of thermoeconomics to industrial ecology. Entropy 12(3):591–612
Connelly L, Koshland CP (2002) Exergy and industrial ecology. Part 2: a non-dimensional analysis of means to reduce resource depletion. Exergy Int J 1(4):234–255
Szargut J, Morris D, Steward F (1988) Exergy analysis of thermal, chemical and metallurgical processes. Hemisphere Publishing Corporation, New York
Dewulf JP, Van Langenhove HR (2002) Quantitative assessment of solid waste treatment systems in the industrial ecology perspective by exergy analysis. Environ Sci Technol 36(5):1130–1135
Ayres RU (1998) Eco-thermodynamics: economics and the second law. Ecol Econ 26(2):189–209
Dewulf J, Van Langenhove H, Van De Velde B (2005) Exergy-based efficiency and renewability assessment of biofuel production. Environ Sci Technol 39(10):3878–3882
Valero A, Lozano MA, Serra L, Torres C (1994) Application of the exergetic cost theory to the CGAM problem. Energy 19(3):365–381
Wall G (1988) Exergy flows in industrial processes. Energy 13(2):197–208
Hernandez AG, Cullen JM (2016) Unlocking plant-level resource efficiency options: a unified exergy measure. Procedia CIRP 48:122–127
Castro MBG, Remmerswaal JAM, Brezet JC, Reuter MA (2007) Exergy losses during recycling and the resource efficiency of product systems. Resour Conserv Recycl 52(2):219–233
Ignatenko O, van Schaik A, Reuter MA (2007) Exergy as a tool for evaluation of the resource efficiency of recycling systems. Miner Eng 20(9):862–874
Ayres RU, Ayres LW, Masini A (2006) An application of exergy accounting to five basic metal industries. Sustainable metals management: securing our future—steps towards a closed loop economy. Springer, New York, pp 141–194
Abadías Llamas A et al (2019) Simulation-based exergy, thermo-economic and environmental footprint analysis of primary copper production. Miner Eng 131:51–65
Szargut J (1989) Chemical exergies of the elements. Appl Energy 32(4):269–286
Thinkstep (2019) GaBi LCA software. (Online). https://www.thinkstep.com/. Accessed 28 Feb 2019
Graf GG (2005) Zinc. Ullmann’s encyclopedia of industrial chemistry. Wiley, Hoboken, p 23
Schlesinger ME, King MJ, Sole KC, Davenport WG (2011) Extractive metallurgy of copper. Elsevier, Amsterdam
Lossin A (2005) Copper. Ullmann’s encyclopedia of industrial chemistry. Wiley, Hoboken, p 67
Sinclair RJ (2009) The extractive metallurgy of lead, vol 15. The Australasian Institute of Mining and Metallurgy, Melbourne
Sutherland CA, Milner EF, Kerby RC, Teindl H, Melin A, Bolt HM (2006) Lead. Ullmann’s Encyclopedia of Industrial Chemistry. Wiley, Hoboken, p 48
Bonnet D (2012) CdTe thin-film PV modules. Practical handbook of photovoltaics. Elsevier, Amsterdam, pp 283–322
Fthenakis VM (2004) Life cycle impact analysis of cadmium in CdTe PV production. Renew Sustain Energy Rev 8(4):303–334
Wang W, Fthenakis V (2004) Leaching of cadmium, tellurium and copper from cadmium telluride photovoltaic modules
Marwede M, Reller A (2012) Future recycling flows of tellurium from cadmium telluride photovoltaic waste. Resour Conserv Recycl 69:35–49
Krueger L (2010) Overview of first solar’s module collection and recycling program. Berlin
Sinha P, Cossette M, Ménard J-F (2012) End-of-life CdTe PV recycling with semiconductor refining. 27th European photovoltaic solar energy conference and exhibition, pp 4653–4656
Blanpain B, Reuter MA, Malfliet A (2019) Lead metallurgy is fundamental to the circular economy. SOCRATES Policy Brief. (Online). https://kuleuven.sim2.be/wp-content/uploads/2019/02/SOCRATES-Policy-Brief-2019-Lead.pdf. Accessed 16 June 2019
Wood J, Coveney J, Helin G, Xu L, Xincheng S (2015) The Outotec® direct zinc smelting process. Proc Pb-Zn 2015(2):537–548
Rämä M, Nurmi S, Jokilaakso A, Klemettinen L, Taskinen P, Salminen J (2018) Thermal processing of jarosite leach residue for a safe disposable slag and valuable metals recovery. Metals (Basel) 8(10):744
Lee GF, Jones-Lee A, Martin F (1994) Landfill NIMBY and systems engineering: a paradigm for urban planning. INCOSE Int Symp 4(1):924–931
Hsu S-H (2006) NIMBY opposition and solid waste incinerator siting in democratizing Taiwan. Soc Sci J 43(3):453–459
Moffat K, Zhang A (2014) The paths to social licence to operate: an integrative model explaining community acceptance of mining. Resour Policy 39(1):61–70
European Union (2018) DIRECTIVE (EU) 2018/851 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 30 May 2018 amending Directive 2008/98/EC on waste. Official Journal of the European Union. (Online). https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018L0851&from=EN. Accessed 16 June 2019
Reuter MA, Eksteen JJ, Van Schaik A (2003) Pyrometallurgical reactors—closers of the recycling material cycle. In TMS annual meeting Yazawa international symposium on metallurgical and materials processing: principles and technologies, no 1, pp 1005–1018
Acknowledgements
This research has received funding from the European Commission’s H2020—Marie Sklodowska Curie Actions (MSCA)—Innovative Training Networks within SOCRATES (Website http://etn-socrates.eu) project under the Grant Agreement No. 721385. This work reflects only the author’s view, exempting the Community from any liability. The authors also acknowledge the reviewers for their comments that greatly improved the manuscript, especially also prompting the drawing of the revised Fig. 1, which now nicely reflects the inconvenient truth around the circular economy and captures succinctly the message of this paper.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
The contributing editor for this article was S. Kitamura.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Rights and permissions
About this article
Cite this article
Abadías Llamas, A., Bartie, N.J., Heibeck, M. et al. Simulation-Based Exergy Analysis of Large Circular Economy Systems: Zinc Production Coupled to CdTe Photovoltaic Module Life Cycle. J. Sustain. Metall. 6, 34–67 (2020). https://doi.org/10.1007/s40831-019-00255-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-019-00255-5