Abstract
Lithium ion batteries (LIBs) are an essential energy-storage device for a majority of advanced electronics used in our everyday lives, from cell phones and laptops, to medical devices and electric vehicles. Despite their continued widespread adoption, methods to recycle and reuse end-of-life (EOL) LIB materials are still under active development. In the first part of this two-part review on LIB recycling, we review current commercial scale processes in practice for recycling or reusing EOL LIB components. Future waste projections estimate 4 million tons of cumulative EOL EV battery modules by 2030, which is above the current global recycling capacity. All of the processes in use today utilize a combination of pyrometallurgical and hydrometallurgical or mechanical and hydrometallurgical processing to recover mainly cobalt and nickel and copper, while other components are disposed as waste unless further processed. In this review, we highlight the need for recycling LIB material components based on resource availability and the current processes in practice to recover and recycle LIBs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
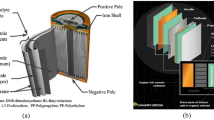
Lithium ion batteries (LIBs) are widely used for energy storage in many technologies from portable devices to electric vehicles. Compared to other types of batteries, lithium ion batteries present superior electrical performance. Several investigators proposed the concept of a lithium battery in the 1970s [1]. Seminal contributions to the development of rechargeable LIBs can be attributed to the works of Goodenough and coworkers [2]. One of the first reported patents of a rechargeable LIB is by Yoshino and coworkers [3, 4]. The chemistry of LIB involves a variety of materials including valuable metals, graphite, and organic compounds. Lithium is a crucial element to achieve high electrical performance for a battery. Lithium has the lowest reduction potential among all elements, which allows lithium-based batteries to have the highest possible cell potential. Lithium is light in weight and has one of the smallest ionic radii of a single-charged ion. This enables a larger quantity of lithium ions to be inserted into the electrode material compared to ions with larger radii [5]. A LIB cell functions by the reversible transportation of lithium ions and electrons between the anode and cathode. The anode and cathode are constructed by attaching a powder of active electrode materials on a current collector foil. For the anode and cathode powders, materials with a layered or tunnel structure, which allows fast and reversible lithium insertion, are used. Those materials must have a stable crystallographic structure during the lithium insertion [6]. Due to resource availability, powders of transition metal (e.g., cobalt, nickel, manganese, and iron) oxides and flake graphite are most commonly used for the cathode and anode, respectively. Copper and aluminum are used for current collector foils. The anode and cathode are separated by a porous separator which are generally made of plastics (e.g., polypropylene and polyethylene) and filled with organic electrolyte containing additive salts (e.g., dimethyl and ethylene carbonates with LiPF6) [7].
One lithium ion battery can have an operating potential of around 3.6 V or more [8], and has high energy and power densities for its weight. The energy and power densities of the present LIB cell are reported to be 70–250 Wh/kg and 200–3000 W/kg, respectively, depending on the cell chemistry [9]. These energy and power densities are more than double the values of other traditional batteries, e.g., lead–acid batteries have energy and power densities of up to 35 Wh/kg and 250 W/kg, and nickel–metal hydride batteries have up to 85 Wh/kg and 250 W/kg [8]. Other advantages of LIBs include minimal maintenance, no memory effect (loss of capacity after shallow–depth discharging), and low self-discharge in contrast to nickel-based batteries [7]. Due to their excellent energy and power densities, LIBs have made a significant contribution to the advancement of high-performance portable electronics such as cell phones, laptop computers, and medical devices. A list of major active cathode materials used for lithium ion batteries is shown in Table 1. Using LIBs as an energy-storage device for renewable energy sources has been promoted as a future solution for reducing anthropogenic global warming effects. The demands of LIBs for electric vehicles and stationary energy-storage systems are expected to increase rapidly in the future. Bloomberg New Energy Finance projected that electric vehicle sales would increase dramatically between 2025 and 2030, and 33% of the global car fleet would become electric vehicles by 2040 [10]. They forecasted that annual demands for LIBs for new electric vehicles would increase from 123 GWh (2020) to 408 GWh in 2025, and to 1293 GWh in 2030 [11]. They also expected that the stationary storage capacity would grow rapidly from 1 GWh (2017) to 81 GWh by 2024 [11]. In other market research, Avicenne Energy forecasted that the global LIB market would grow from 78 GWh in 2016 to 210 GWh in 2025 [12]. Herein, the LIB market share of electronic devices was estimated to grow from 31.2 GWh (40% share, 2016) to 54.6 GWh in 2025 (26% share), while that of automobiles would grow from 33.5 GWh (43% share in 2016) to 105 GWh (50% share) in 2025. The share for industrial use and energy-storage systems was estimated to grow from 4.7 GWh (6% share, 2016) to 16.8 GWh (8% share) in 2025. The growth of other markets including medical device, power tools, and e-bikes would be also significant, 8.6 GWh (11% share in 2016) to 33.6 GWh (16% share) in 2025 [12].
The production of LIBs has been increasing continuously especially due to presently growing demands for applications in electric vehicles and electronics. Major LIB manufacturers, such as LG Chem, Panasonic, and BYD, have announced to construct more manufacturing plants in the near future [11]. The global LIB manufacturing capacity is expected to increase from 103 GWh (2017) to 273 GWh by 2021 according to the research by Bloomberg New Energy Finance [11]. Along with increasing manufacturing capacity and technology improvements, the price of LIB packs for electric vehicles has been falling continuously; for example, it was $1000/kWh in 2010 and decreased to $273/kWh in 2016 [11]. Bloomberg New Energy Finance predicted that this price would drop to around $73/kWh by 2030 [11]. With the increasing global production rate, LIB technology also continues to improve in electrical performance, battery life, and safety in a wide range of performance characterizations for various energy-storage applications. Given the current LIB technology and projected growth, LIBs are expected to become only more commonplace.
Reasons to Recycle Spent Lithium Ion Batteries
Resource Scarcity
Considering the fast growth of global demands of LIBs, end-of-life (EOL) LIBs are most likely to become important secondary sources for various materials in the future. For example, a recent study estimates that various scrap metals will become the main sources for iron, aluminum, and copper within the next 30 years [14]. A summary of global availability of the main raw materials in advanced LIBs is given in Table 2. Among these elements, lithium, cobalt, and natural graphite are of the most concern based on geological availabilities, geopolitics, and/or market limitations.
Lithium is produced from either lithium-bearing ore (e.g., Spodumene) or brines [19, 27]. Due to the strong economic growth in rechargeable battery industries, the price and production of lithium have been increasing at a fast rate. The global mine production of lithium was 43,000 tons in 2017, while the global reserves were estimated to be 16 million tons. The majority of the world lithium production was accounted for by the productions from three Spodumene operations in Australia, and two brine operations each in Argentina and Chile [19]. To satisfy the increasing global lithium demands, new lithium mine operations and brine operations have been under development around the world, including Argentina, Australia, Bolivia, Canada, China, and the United States [27].
However, although high-quality lithium brines and almost half the total global lithium resources are located in South American countries, geopolitics in those countries may cause lithium supply scarcity (similar to rare earth elements in China). Environmental stakeholders highly criticize the brine lithium production activities, which involve intense evaporation processes. There are strong concerns of the adverse local environmental impacts such as groundwater pollution, dust and gas emissions, deteriorating ecosystems, and human sanity [27]. Technology advancement in mining activities is definitely required to mitigate these issues [28].
Among the main LIB raw materials, cobalt is subject to the highest risk of resource scarcity. The estimated global reserves of cobalt are only 7.1 million tons, while its demand has been increasing due to the strong growth in the rechargeable battery and aerospace industries. The global production was 110,000 tons in 2017. Cobalt is produced as a by-product of copper or nickel, except for productions in Morocco and Congo [16] where half the global reserves of cobalt are estimated to be. Congo is the world’s largest cobalt producer, which accounted for 58% of the global production in 2017. However, Congo is a politically vulnerable country, and many locations where cobalt reserves exist are not under strict government regulation, attributing to price volatility. Besides, legally controversial mining activities including child labor are also a serious concern [29]. China is the world’s leading consumer of cobalt and 80% of its consumption is used by the rechargeable battery industries [16]. The cobalt market is expected to be further limited in the near future due to limited resources and possible monopolization by a few countries.
Natural graphite differs in purity and morphology, and is categorized into three types: (1) low-quality amorphous, (2) natural flake graphite, and (3) high-quality but rarer lump graphite [8, 21, 30]. Among these types, natural flake graphite, which is a resource for battery-grade graphite, has become a recent concern for resource scarcity. China is the largest graphite producer, and without any new policy interventions, China's graphite resources will be depleted within 20 years [31]. Moreover, graphite mining activities have led to many environmental problems, such as groundwater and soil contaminations, air pollution, and mining solid wastes [31]. To be used as an anode in a LIB cell, natural graphite is purified by thermal or chemical treatment, and mechanically processed to generate battery-grade spherical graphite, which has a carbon purity of more than 99.9% and a desirable morphology allowing for high rate capacity [30, 32]. However, the yield of spherical graphite is only about one-third of the original natural flake graphite [30]. The other possible raw material, vein graphite, cannot be considered as a raw material for commercial LIBs, as it is expensive and has limited commercial availability. Annual commodity report of natural graphite by USGS reported that flake graphite was 30% of China’s total natural graphite production. Other main producers of flake graphite are Canada, Brazil, and Madagascar, but their productions are much smaller than China’s flake graphite production [21]. In countries that do not have natural flake graphite resources (e.g., United States), synthetic graphite is also used as an anode raw material. However, the price of synthetic graphite could be 1.5–3 times higher than natural graphite [30], which may hinder commercial production of large size LIB packs. Besides, the production of synthetic graphite from petroleum coke and coal tar pitch is energy intensive, which requires high-temperature treatment at 2500–3000 °C [32]. Due to its limited availability and the large amount of raw material required to produce battery-grade graphite, sustainable supply chain of natural flake graphite will be critical for the future LIB production.
Among the cathode types which contain cobalt, which is one of the most critical materials of LIBs, lithium cobalt oxide (LCO), lithium nickel–cobalt–aluminum oxide (NCA), and lithium nickel–manganese–cobalt oxide (NMC) will be popular cathode types for smart phone/tablets, power tools, and electric vehicles (EVs), respectively, in the future [12]. Especially, the market share of NMC is expected to grow dramatically in the next 10 years due to the increasing demands of EVs. With regard to the demand by mass, NMC cathode will be the most dominant cathode type of LIBs in the world [12]. Considering the large mass, batteries from EOL EVs will be a critical secondary metal source to secure LIB materials supply in the future. Recoverable masses of various metals from EOL EV batteries were estimated based on the prediction of global EV sales evaluated by Bloomberg New Energy Finance [10]. In this estimation, it was assumed that all recycled LIBs were NMC type (LiNi0.33Mn0.33Co0.33O2 cathode–graphite anode) and the battery weight was 540 kg (battery weight from Tesla Model S [33]). Fractions of battery components were referred from the study done by the ELIBAMA project [34]. Secondary metal sources were assumed to be the same amount as the metals required for the LIB production and available after 10 years of EV battery life [35]. Recovered metals from spent LIBs were accumulated every year as the total mass of available sources. Figure 1 shows the total masses of secondary metal and graphite sources. Global reserves estimated in 2017 by USGS are also shown for lithium and cobalt. The result of nickel was the same as cobalt as they have almost the same mass.
Secondary metal and graphite sources from end-of-life electric vehicle batteries estimated based on the prediction of global electric vehicle sales estimated in 2017 by Bloomberg New Energy Finance [4] (LIB pack weight: 540 kg, LIB type: nickel–manganese–cobalt (NMC) cathode and graphite anode [34]). The y-axis is in millions of tons. (Color figure online)
This estimation clearly indicates that recycling of cobalt from LIBs in EOL EVs is inevitable to satisfy future cobalt supply demands for LIB production. In the figure, the total secondary cobalt source from EV batteries exceeds the current reserves by 2045. In other words, cobalt reserves will be depleted within the next 30 years unless cobalt consumption in LIBs is reduced substantially or cobalt is recycled with a high recovery rate for LIB production. As lithium reserves are more likely to increase in the future, lithium resource scarcity is not as severe as cobalt. However, lithium is lacking recycling technologies [28], and it is still possible to be a critical raw material for future batteries. For EV battery production, graphite consumption is also tremendous. Considering a low output of battery-grade spherical graphite from limited natural flake graphite, and lacking graphite recycling technologies [28], anode graphite supply will become severely tight in the next 50 years.
These estimations indicate that lithium, cobalt, and graphite from EOL EV batteries must be recycled to avoid resource scarcity. Other metals such as nickel, manganese, and copper have a risk of depletion within several decades considering their reserves and production rates. However, these metals do not have severe supply risks (i.e., regulation risks, political risks, concentration in limited countries) compared to lithium, cobalt, and graphite. Nevertheless, recycling rates of these metals must be improved as metal consumption in production of EV LIBs is tremendous (current recycling rates of nickel, manganese, and copper are 58%, 53%, and 53%, respectively) [28]. Lastly, it must be noted that this is a rough estimation, and is based only on the sales prediction of EVs by Bloomberg New Energy Finance, which projects a high production rate of EVs. For a more precise estimation, EOL LIBs from other uses must be included. Also, cobalt consumption for NMC batteries in EVs is expected to decrease due to the high price of cobalt. In the next 10 years, the common composition of NMC cathode used in EVs will be Ni:Mn:Co of 5:3:2 or 6:2:2, which reduces cobalt content. The mixture of NMC and lithium manganese oxide (LiMn2O4) with the mass ratio of 75:25 will also be common for EV battery cathodes [12].
In addition to resource perspectives, recycling of EOL LIBs from EVs is reasonable due to the easier logistics to collect EOL EVs. Also, the large quantities of EV LIBs with the same manufacturing model enable operations of recycling processes, which are designed for specific models. Meanwhile, the value of LCO cathode materials, which come from EOL portable electronics, will also be significant. Although the logistics will be more complicated, it is important to innovate recycling methods for small-size EOL LIBs from portable electronics such as cell phones and tablets.
Environmental Hazards in Landfill
Spent LIBs, which are not sent to a recycling process, are often disposed of in landfills. Direct disposal of large quantities of spent LIBs induces various environmental problems such as toxic substance release and fire/explosion hazards. Although a LIB itself is safe under normal operating conditions, causing physical damage or placing cells under harsh conditions readily triggers unwanted chemical reactions. Generally, a LIB cell contains heavy metals, conductive salts with fluoride, and organic solvents. Many of these elements do not degrade, and are often recognized as toxic pollutants to soil, water, plants, and the food chain [36]. Especially, leaching of heavy metals, such as copper, cobalt, and nickel can have adverse impacts on both human health and local ecotoxicity [37]. Stacking LIBs in landfills can result in mechanical abuse, which may lead to short-circuiting of battery cells. If batteries still have remaining energy/charge, this may increase the temperature inside the battery and cause exothermal reactions with the organic electrolyte, resulting in fire and/or explosion [38]. A high temperature in the cell causes the decomposition of fluoride compounds, such as a polyvinylidene fluoride (PVDF) binder and lithium hexafluorophosphate (LiPF6) under the moist air. This can result in the release of various toxic gases, especially hydrogen fluoride [39].
Energy Consumption and Greenhouse Gas Emissions During Battery Production
Life-cycle-analysis (LCA) has been extensively carried out on the production of LIBs to investigate the energy consumption, carbon dioxide (CO2) emissions, and other environmental impacts. Despite the numerous LCA studies on primary production of LIBs, only a few LCA studies on detailed recycling processes are available in literature (e.g., LCA study by Dunn et al. [40]). With regard to LCA for LIB production, IVL research group investigated past LCA’s of LIB manufacturing processes [41]. The LCA study conducted by IVL research group concluded that the most adequate range of estimated energy consumption and CO2 emissions during LIB manufacturing were 350–650 MJ/kWh-battery and 150–200 kg of CO2/kWh-battery, respectively. Also, they estimated that the production of battery-grade materials accounted for 60–70 kg/kWh-battery of CO2 emissions where 30–50% of this was from battery-grade cathode and anode productions. In the same analysis, the CO2 emissions from battery manufacturing (shaping and assembling battery-grade materials, and testing of battery function) were estimated to be 70–110 kg CO2/kWh [41]. Amarakoon et al. estimated that 33% of LIB production energy usage was from cathode paste production, 8.6% from anode paste production, 13% from electrolyte production, and 32% from pack manufacturing [41]. Notter et al. performed a LCA of LIB production with LiMn2O4 cathode. They concluded that both anode and cathode productions have large impacts on the energy consumption, global warming potential, abiotic depletion potential, and environmental burden of battery production [42]. Dewulf et al. performed a LCA of LiNixMnyCozO2 cathode production and compared two scenarios; (1) cobalt and nickel in the cell would be recycled by pyrometallurgical process to be reused and (2) cobalt and nickel extracted from mines would be used in LIB production. They concluded that 51% of the cathode production energy would be saved if cobalt and nickel were recycled from EOL LIBs to reproduce the cathode material [43].
Among production stages in the battery manufacturing process (shaping of battery materials, materials assembling, and testing of battery function), the dry room process consumes the largest amount of energy [40]. However, this process is inevitable during assembling to minimize contaminations as much as possible. Therefore, it is more reasonable to reduce the energy and CO2 emissions in raw and battery-grade materials production rather than the manufacturing process.
Lastly, it must be noted that the estimations of energy consumptions and CO2 emissions are quite different depending on LCAs; battery materials, production methods, definition of each production stage, boundaries, and nomenclature are different from LCA study to LCA study. Thus, it is quite difficult to directly compare all LCA results. Nevertheless, the overall conclusion from these studies indicates that recycling EOL LIB components has the potential to reduce production energy consumption and CO2 emissions.
Current Lithium Ion Battery Industrial Recycling Processes
Currently, there are few policy regulations for LIB recycling established in North America (e.g., States of California, Minnesota, Puerto Rico, and New York have LIB recycling policies). Heelan et al. estimated the LIB recycling rate in North America based on recycling rates of e-wastes, which were equipped with LIBs. They concluded that the actual annual recycling rate of LIBs in North America was only 3% [44]. They also concluded that laptop batteries were estimated to be recycled with higher frequency, compared to smaller-size cell phone batteries. Gu et al. investigated the current status regarding collecting and recycling LIBs from consumer electronics in China. They concluded that the currently estimated LIB collecting and recycling rate could be even less than 5%, although some of major LIB recycling companies (i.e., GEM High-Tech Co., Brunp Co.) set up their own waste-collecting network [45]. European countries have developed more strict regulations for battery recycling. Having begun in 2016, each EU member is required to meet the waste electrical/electronic equipment collection rate of 45 wt% and at a recycling efficiency of 50 wt% for non-lead–acid and non-nickel–cadmium batteries [46].
Industrial scale battery recycling operations which are capable of recycling LIBs are located in European, North American, and Asian countries [44, 47, 48]. LIB recycling companies include Umicore (Belgium, 7000 tons/year and China, 5000 tons/year, Pyrometallurgy), Retrieve (Canada, United States, 4500 tons/year, Hydrometallurgy), Glencore (Xstrata Nickel) (Canada, Norway, 7000 tons/year, Pyrometallurgy), GEM High-Tech Co. (China, 25,000–30,000 tons/year, Hydrometallurgy), Brunp Co. (China, 10,000 tons/year, Hydrometallurgy), Batrec (Switzerland, 1000 tons/year, Pyrometallurgy), Accurec (Germany, 6000 tons/year, Pyrometallurgy), Recupyl (France, 110 tons/year, Hydrometallurgy), Valdi (France, 20,000 tons/year, Pyrometallurgy), Akkuser Ltd. (Finland, 4000 tons/year, Mechanical), Inmetco (United States, 6000 tons/year, Pyrometallurgy), and JX Nippon Mining and Metals (Japan, 5000 tons/year, Pyrometallurgy) [45, 48, 49]. It should be noted that for the data collected, it is not clear whether the plant capacities cited here are for EOL LIBs only or include other types of spent batteries, mineral ores, and metal-manufacturing scraps. As well as these companies, companies such as SungEel HiTech (Korea), Taisen Recycling (Korea), 4R Energy Corp (Japan), and Onto Technology (United States), have been investing to expand their recycling capacities [50]. According to Shmuel De-Leon Energy Ltd., the estimated present global LIB recycling rate is 5–7% [50]. This is far below the recycling rate which will be required to recycle future EOL LIBs, especially generated from EOL EV battery modules which could reach 4 million tons by 2030 alone (based on the estimation in Fig. 1).
In the following sections, we will review present industrial processes for recovery and eventual recycling LIB components. However, this article only reviews processes for which detailed information was available.
Umicore Process
Umicore’s pyrometallurgical–hydrometallurgical process can take both lithium ion and nickel metal hydride batteries. The pyrometallurgical part of this process produces nickel–cobalt–copper–iron alloy, and subsequent hydrometallurgical processes further refine the metals [51]. A schematic diagram of the Umicore process is shown in Fig. 2.
Spent batteries are directly fed with metallurgical coke, slag formers, and some metal oxides into a shaft furnace. The shaft furnace is divided into three sections from the top: pre-heating zone ( < 300 °C), plastic pyrolyzing zone ( < 700 °C), and metal smelting/reducing zone (1200–1450 °C) [52]. Oxygen-enriched air is injected via tuyeres from the bottom of the furnace. In the pre-heating zone, the electrolyte is evaporated by slowly increasing the temperature, which reduces the risk of explosion. In the plastic pyrolyzing zone, plastics in the battery packs melt, oxidize, and provide energy to the offgas. In the melting zone, carbon and aluminum from the battery case are oxidized, and reduces cobalt and nickel. The metal oxide and the air are controlled so that the proper redox potential is obtained. The slag composition has at least a ratio of SiO2/CaO = 1 for sufficiently low viscosity and adequate melting point [52]. The product alloy contains nickel, cobalt, copper, and iron, where 35% of its weight is from cobalt and nickel. The slag contains aluminum, lithium, silica, calcium, and some iron [52]. The offgas from the furnace is heated by a plasma torch to above 1150 °C and sent to a post-combustion chamber, where halogens are captured by injection of calcium or sodium-based products or zinc oxide. Then the offgas is quickly cooled down by water vapor to avoid recombination of organic compounds with halogen or formation of dioxins and furans [52].
The nickel–cobalt–copper–iron alloy is treated by acid-leaching processes to remove copper, iron, zinc, and manganese prior to the solvent extraction process. The solvent extraction process with sulfuric acid separates nickel from cobalt with a high purity. Both nickel and cobalt may be recycled to reproduce a cathode precursor [51].
This process is relatively simple and has a big advantage; it does not require sorting or mechanical treatment of batteries and also utilizes organic components as an energy source. However, the bottleneck is that the economic feasibility of this process is strongly driven by the prices of cobalt and nickel [53]. The process will not be economical if cobalt and nickel-bearing batteries are less than 30% of the feed [52]. Besides, this process is energy intensive; Sonoc et al. estimated that this process requires around 5 GJ for the smelting and gas clean-up systems to process 1 ton of batteries [54]. Also, this process does not recover other valuable metals such as lithium. Recovery of metals from the slag is energy intensive and not economical, and thus the slag is generally disposed or sold as construction material.
Inmetco Process and Glencore (Xstrata Nickel) Process
Inmetco (International Metals Reclamation Company) operates a pyrometallurgical facility, which treats metal wastes as well as EOL batteries. A schematic diagram of the Inmetco process is shown in Fig. 3. The main purpose of this process is to recover cobalt, nickel, and iron for the production of iron-based alloys. Similar to the Umicore process, lithium and aluminum are slagged, and organic materials and carbons are utilized as an energy source and reducing agent [55].
This process was initially designed to treat wastes from stainless steel manufactures such as flue dust, mill scale, and swarf. Currently, the process can accept spent batteries as a secondary feed [48]. The main feed, which is composed of stainless steel wastes, is milled and screened. The screened wastes are then blended and pelletized with reductant carbon and liquid waste containing nickel and cadmium. Spent batteries as a secondary feed are opened, and plastic materials and electrolyte are removed. The remaining parts are calcined to evaporate and distil cadmium. Then the batteries are shredded, and fed with the main feed and organic materials to the reduction stage in a rotatory hearth furnace. The reduction stage is operated at 1260 °C [48]. The offgas from the rotary hearth furnace is scrubbed, and the scrub solution is sent to a wastewater treatment facility to recovery cadmium, zinc, and lead. The reduced metals are further melted in an electric arc furnace where iron–cobalt–nickel alloy and slag containing lithium and aluminum are produced. The offgas from the electric arc furnace containing zinc is treated in baghouses to recover zinc and small amounts of other metals [48].
Glencore (Xstrata Nickel) process, which is one of the major pyrometallurgical LIB recycling processes, is also not fully dedicated to LIBs. In the Glencore process, cobalt and nickel-bearing battery scraps are fed as a secondary feed to their conventional pyrometallurgical process, which extracts nickel, cobalt, and copper from ores. Herein, only nickel, cobalt, and copper are of interest for metal recovery from LIBs, and other battery components are slagged or used as an energy source or reducing agents [55].
Accurec Process
Accurec developed a facility, which can recycle a majority of components from LIBs, as outlined by the schematic diagram in Fig. 4. The LIBs are first disassembled to separate steel components, electronic parts, copper cables, and plastic components. The remaining cells are sent to an autothermal pyrolysis process, which operates at 250 °C. The electrolyte is safely evaporated and collected in a downstream condenser [55]. The multi-step subsequent mechanical treatments separate ferromagnetic steel, aluminum cases, and aluminum and copper foils from active electrode materials. Then active electrode materials are sent to the vacuum pyrolysis process to recover lithium. Lithium is recovered in pure metallic form by direct evaporation and distillation or recovered as lithium oxide by selective entraining gas evaporation of lithium. The remaining metal oxides in active electrode materials are further refined via existing industrial processes [56].
Retriev Process
Retriev Inc. (previously, Toxco) operates a facility, which recycles primary lithium batteries and secondary LIBs by a hydrometallurgical processing route. This process focuses on recycling of lithium as well as other components. A schematic diagram of the Retriev process is shown in Fig. 5.
Lithium-based batteries are first cooled cryogenically with liquid nitrogen to around − 200 °C, with liquid nitrogen being refurnished constantly. The batteries are cooled for several hours to deactivate. By the cryogenic process, the reactivity of lithium is decreased by 5 or 6 orders of magnitude compared to the reactivity at room temperature [57]. After which, the batteries are crushed in a shredder or hammer mill and screened. Large size fractions such as mixed plastics, steel cases, and copper and aluminum foils are separated from small-size fractions containing active electrode materials [54]. The active electrode materials are immersed in a solution containing lithium hydroxide (LiOH), which is used to dissolve lithium salts. The pH of the solution is maintained at 10 to prevent the formation of hydrogen sulfide (H2S). Hydrogen generated by the reaction is burned off on the surface of the solution by the limited amount of oxygen [57]. Undissolved metal oxides and graphite are separated from the solution via a carbon filter press and recovered [54]. The filtrate is sent to the evaporator and storage tank array, where lithium salts in the filtrate, such as lithium chloride (LiCl), lithium carbonate (Li2CO3), and lithium sulfate (Li2SO4), are precipitated when their production exceeds the solubility. Lithium salts are pumped and filtered by a filter press. Then, the lithium-containing filter cake is put in the hybrid electrolytic cell, which contains diluted sulfuric acid to separate lithium ions from anions and anionic compounds. Lithium passes through a membrane and reacts with base to form LiOH. The formed LiOH is either directly dewatered or converted to Li2CO3 with carbon dioxide [57]. Also, soda ash may be added to form Li2CO3 prior to the filtration stage. Instead of the hybrid electrolytic cell, Li2CO3 which was formed by the reaction with soda ash may directly be filtered by a filter press [54].
Although this process does not involve a high-temperature processing, cryogenic process itself is energy intensive and hazardous. Sonoc et al. estimated that the energy requirement would be 219 MJ for cooling batteries to − 200 °C and 565 MJ for the shredding process to process one ton of batteries [54].
Batrec Process
The Batrec process treats lithium-based batteries and is characterized by a mechanical processing step under carbon dioxide (CO2) environment [58]. A schematic diagram of the Batrec process is shown in Fig. 6. Batteries are crushed under a CO2 atmosphere within a tightly closed chamber. The oxygen level is strictly controlled to avoid exothermic reactions. Also, under CO2 atmosphere, lithium metal forms a passivation layer on its surface, which prevents further reaction of lithium with oxygen. The shredder or hammer mills are placed in an atmospheric chamber of CO2. Shredded batteries are neutralized by moist air and collected at the bottom of the chamber, where a bed of dry ice is placed under air-conditioned environment [58]. These shredded batteries are discharged from the chamber and sent to the pyrolyzing process to detach active electrode materials from foils and binder. It is then screened to separate fine active electrode materials from cases and foils. The fine electrode materials are further refined by hydrometallurgical processes such as leaching and solvent extraction [58].
Recupyl Process
The Recupyl process is similar to the Batrec process regarding the mechanical processing under inert environment and subsequent hydrometallurgical processes [36]. A schematic diagram of the Recupyl process is shown in Fig. 7.
Batteries are sorted and crushed in an inert atmosphere containing a mixture of CO2 and argon inside a tightly enclosed chamber. Crushing is performed in two steps; the first crushing is accomplished with a rotary mill at less than 11 RPM, and the second crushing is done with an impact mill at less than 90 RPM [36]. As well as in the Batrec process, a CO2 atmosphere during crushing initiates passivation of metallic lithium on the electrode surface. Meanwhile, the offgas from the crushing process is neutralized with water and soda. The mill discharge is then screened to separate fine active electrode materials from large size fractions such as steel cases, paper, plastics, and foils. A high induction magnetic separator separates steel components in the large fractions. Other non-magnetic materials are separated based on difference of densities by densimetric tables [36].
Separated active electrode materials are leached in heavily stirred water under a turbulent atmosphere with low oxygen levels. This type of atmosphere prevents the explosion of hydrogen released by the reactions and the formation of hydrogen fluoride gas. After soluble lithium is dissolved in the water, the remaining solid is filtered to separate the solution. The lithium dissolved in this solution is precipitated as Li2CO3 or Li3PO4 with an addition of CO2 or phosphoric acid. The filtered solid containing the remaining electrode materials is sent to a leaching process with 2 N sulfuric acid at 80 °C to recover cobalt. The solution is cooled down to 60 °C and any undissolved carbon is filtered. The solution is further purified by copper cementation and iron precipitation at pH 2–2.85 and pH 3.85, respectively. After purification, the solution is neutralized to pH 5.8, and cobalt is recovered by electrolysis at 55 °C with a current density of 400–600 A/m2 using a stainless steel cathode and antimony–lead alloy anode. In this process, manganese is also precipitated as oxyhydroxide or dioxide form. In case that cobalt is rich in the purified solution, the purified solution is oxidized with sodium hypochlorite at pH 2.3–2.8 to precipitate cobalt oxide(III). The remaining solution containing some lithium is neutralized to pH 8.5 and sent to lithium salt precipitation process described previously in this section [36].
EcoBat Process
The EcoBat process invented by Sloop et al. at Onto Technology LLC, is categorized as a “direct LIB recycling” process [40]. This process is designed to recover battery components without decomposing or converting into different compositions so that the recovered materials are directly reinserted into the battery supply chain with little additional processing [59]. A schematic diagram of the EcoBat process is shown in Fig. 8.
First, collected batteries are discharged and sorted manually by chemistry and functional potential. Labels, any dirt, oil, or moisture are also removed and batteries are cleaned with alcohol. Electronic circuits for over-discharging protection are also removed for reuse in refurbished batteries. The batteries are then placed in an enclosed electrolyte extraction container with liquid CO2 at − 56 to − 20 °C, and additives such as solubility enhancers and Lewis bases are added to prevent the formation of hydrogen fluoride gas [59]. The pressure and temperature are increased above 2000 psi and 31.1 °C to achieve supercritical condition of CO2. The battery cell cases inside the extraction container are breached at above 800 psi. At this point, the reaction of lithium in the anode with CO2 to produce passivating Li2CO3 and carbon monoxide (CO) may occur if the battery is still charged. Small quantities of dry air or oxygen may be added to form CO2 and prevent CO gas release. Supercritical CO2 can contact and soak in sub-micron size pores due to low surface tension. This allows supercritical CO2 to extract the electrolyte and chemicals composing the solid-electrolyte interphase on the anode, such as oligoether and oligocarbonate [59]. Extraction may either be a dynamic or static process; CO2 may be constantly pumped through the system or a fixed amount of CO2 is used for a period of time. The CO2 with extracted electrolyte is pumped out of the container and sent to the electrolyte precipitation process. The CO2 is evaporated and recycled for extraction, and electrolyte and interphase wastes are recovered by precipitation. The extraction process continues until quantitative extraction of electrolyte is achieved.
After the electrolyte extraction process, the batteries, composed of cases and remaining cell components, are discharged from the extraction container. Those batteries are reviewed to determine whether they are suitable for refurbishing based on the presence of physical damages or short-circuits in the cell [59]. The batteries, which are determined to be refurbished, are filled with new electrolyte and cell cases are repaired. If refurbished batteries do not satisfy 80% of original capacity, they are sent to a full recycling route along with cells that are determined to be unsuitable for refurbishing. These batteries are shredded under nitrogen environment to prevent contamination, and go through mechanical processes such as density and electronic conductivity separations, cyclone, fluidized bed, and decantation, to sort the various battery components [59].
Review of Commercial Lithium Ion Battery Recycling Processes
Currently, the main commercial LIB recycling routes are pyrometallurgical or mechanical processes followed by hydrometallurgical metal separation and refining processes. In this section, the advantages and disadvantages of pyrometallurgical, hydrometallurgical, and direct recycling processes are discussed.
Pyrometallurgical Process
The main purpose through pyrometallurgical routes is to produce cobalt or nickel-bearing alloys. The advantage of pyrometallurgical processes is that they can effectively process LIBs with ores or other types of batteries and industrial wastes simultaneously. In some processes, no pretreatment of LIB packs is required (e.g., Umicore), and charged batteries can directly be fed in the reactor. However, it still requires further processes to refine cobalt, nickel, and copper separately, which is generally performed by leaching and solvent extraction. The furnace requires offgas treatment to prevent toxic gas emissions (e.g., dioxins and furans). Volatile metals such as zinc and cadmium should be recovered by distillation. Most of lithium and aluminum are slagged and generally not recovered. It is possible to recover lithium in the flue dust and slag as lithium carbonate by leaching and precipitation after grinding to small particles (e.g., 100 µm) [51]. However, an extra lithium recovery process is not reasonable unless the price of lithium is high enough to compensate the operating cost. All organic components, graphite and aluminum, are used as an energy source or reductant during a smelting process. The slag composition must be modified depending on the feed materials to minimize the loss of target metals and maintain adequate slag viscosity [51]. The economic feasibility of pyrometallurgical process highly relies on the recovery yield and the prices of cobalt, nickel, and copper. Recently, LIB chemistry has been developing towards the reduction of cobalt and nickel, which might jeopardize this process as a primary LIB recycling technology. Still, LIBs can be effectively recycled by pyrometallurgical process as a secondary feed along with other industrial metals.
Hydrometallurgical Process
Hydrometallurgical processes accompanied with mechanical pretreatment can process lithium-based batteries and recover lithium and valuable metals as well as other battery components (e.g., graphite, aluminum). China currently has the two biggest LIB recycling companies (i.e., GEM High-Tech Co. and Brunp Co.) and applies a closed-loop hydrometallurgical process, which reproduces cathode materials. In the mechanical processing part, the waste batteries are shredded, sieved, and treated by physical processes to separate different components. As charged LIBs are explosive and flammable, deactivation of LIBs (i.e., discharging, cryogenic treatment) or strict inert environment during mechanical treatment is required. Cryogenic treatment of LIBs and maintaining flow of inert gas (e.g., CO2, argon) might consume significant amount of electrical energy. The yield of valuable fine electrode materials after mechanical treatment may not be sufficient (i.e., 75% according to laboratory experiment by Diekmann et al. [60]). The recovered fine electrode materials (i.e., cathode materials) are treated via a leaching process. The fine electrode materials are immersed in lithium brine to dissolve lithium and separate metal oxides and graphite (e.g., Retriev or Recupyl), or leached in an acidic solution to dissolve both lithium and other metals (e.g., GEM High-Tech, Brunp, Batrec). The amount of fine electrode materials, which can be processed, is limited by the kinetics of leaching and the solubility of metal compounds. A low solid–liquid ratio in the leaching process leads to a large volume of waste solution. Eventually, the metals are recovered as metal compounds or pure metal through precipitation, solvent extraction, and electrolysis. Especially for closed-loop cathode production, separated metals require extremely high purity. The complexity of LIBs and a large range of material selection make it extremely difficult to separate battery components completely. Therefore, the economics of those processes largely depend on the yields of cobalt and nickel in the product to compensate the product quality. Otherwise, the recovered materials hardly pay for the operating cost of the recycling process as most of LIB materials have a lower market value. Still, the development of a hydrometallurgical process to effectively recover all LIB components will be the key challenge in sustainable LIB production. Research development opportunities in hydrometallurgical process are further described in the second part of this review.
Direct Recycling Process
Direct recycling process, which recovers battery materials without changing their chemistry, is an attractive recycling process to achieve a low operating cost and have less environmental impact. This method would also contribute to achieve the LIB recycling efficiency target of 50 wt% in EU countries by recovering the electrolyte [46]. The energy consumption for LIB production would be reduced significantly (e.g., 40% energy saving for LiCoO2 cathode) by recycling components if the direct recycling process is successfully applied [61]. So far, this process is still in a pilot-scale development stage. The key challenge in this process is the quality of refurbished battery materials. It was reported that anode graphite after supercritical CO2 treatment exhibited comparably poor performance, which indicated that the high pressure caused exfoliation of graphite [30]. Meanwhile, flow-through liquid CO2 was proved to be effective to electrolyte extraction as well as improving anode performance [30]. However, the cathode may not fully recover to its original electrical performance due to partial decomposition of the cathode [62]. Besides, the condition of binder in anode and cathode would be unclear in refurbished batteries. This requires mechanical and hydrometallurgical processes to recycle the batteries, which is unsuitable for refurbishment. In such a case, energy consumption for LIB production would not be reduced as much. Investigation of spent LIB conditions and sorting before supercritical CO2 treatment may help assuring the quality of refurbished batteries. However, it would be labor-intensive and increase operating cost.
Concluding Remarks
Lithium ion batteries (LIBs) have become an essential energy-storage device for today’s high-performance electronics such as cell phone, tablet, and medical devices due to their superior electrical performance and long cycle life. As a solution for reducing anthropogenic global warming effects, the application of LIBs in electric vehicles (EVs) and energy-storage systems have been dramatically increasing. Due to the large mass application of LIBs for EVs and energy-storage systems, production of LIBs is expected to soar in the next 10 years with the reduction of LIB price. With rapidly increasing demands of LIBs, resource scarcity of valuable materials, especially lithium, cobalt, and natural graphite, will be of great concern. Recycling of end-of-life (EOL) LIBs is inevitable to secure the stability of LIB material supply chains. Especially, EOL LIBs from EVs will be a crucial secondary source due to the large mass of valuable materials contained (i.e., lithium nickel cobalt manganese oxide cathode) and reasonable logistics to collect EOL LIBs. Recycling of LIBs is also beneficial for environmental perspectives. It will eliminate the fire/explosion hazard and soil and water pollution issues caused by leaching of hazardous materials from disposed LIBs in landfills. Recycling of anode and cathode materials, especially, would save a significant amount of energy and reduce greenhouse gas emissions as productions of anode and cathode can account for one-fourth of total energy consumption during LIB production. However, the present global LIB recycling rate is estimated to be only 5–7%, which is far below the capacity, which is required for sustainable LIB production.
Considering the possible resource scarcity with the rapid increase of global LIB demands, recycling of lithium and graphite as well as cobalt will be crucial for sustainable LIB production. Therefore, the mechanical–hydrometallurgical and direct recycling routes are more attractive solution as primary LIB recycling, although pyrometallurgical routes are still effective to be used along with other industrial products. However, due to the complexity of LIBs and safety concerns, achieving high yield and quality of recycled product through these routes is complicated and costly with current technologies. Reducing the operating cost with achieving high product yield and quality will be a key challenge for LIB recycling processes as the price of LIBs will further decrease in the future.
References
Blomgren GE (2017) The development and future of lithium ion batteries. J Electrochem Soc 164:5019–5025. https://doi.org/10.1149/2.0251701jes
Mizushima K, Jones PC, Wiseman PJ, Goodenough JB (1981) 2-LixCoO2 (0). Solid State Ionics 3–4:171–174. https://doi.org/10.1016/0167-2738(81)90077-1
Yoshino A, Sanechika K, Nakajima T (1987) Secondary battery. Asahi Kasei Corp. U.S. Patent 4,668,595
Yoshino A (2012) The birth of the lithium-ion battery. Angew Chemie Int Ed 51:5798–5800. https://doi.org/10.1002/anie.201105006
Nitta N, Wu F, Lee JT, Yushin G (2015) Li-ion battery materials: present and future. Mater Today 18:252–264. https://doi.org/10.1016/j.mattod.2014.10.040
Molenda J, Mole M (2011) Composite cathode material for Li-ion batteries based on LiFePO4 system. In: Metal, ceramic and polymeric composites for various uses. InTech, Rijeka
Deng D (2015) Li-ion batteries: basics, progress, and challenges. Energy Sci Eng 3:385–418. https://doi.org/10.1002/ese3.95
Bryner M, Clarke GM, Jansen AM, et al (2013) Lithium-ion batteries. Chem Eng Prog 35–64
Perdu F (2016) Overview of existing and innovative batteries. In: Science and energy seminar. e-EPS, Les Houches
Bloomberg New Energy Finance (2017) Electric vehicle outlook 2017
Curry C (2017) Lithium-ion battery costs and market. Bloomberg New Energy Finance. https://data.bloomberglp.com/bnef/sites/14/2017/07/BNEF-Lithium-ion-batterycosts-and-market.pdf. Accessed 12 July 2019
Pillot C (2017) The rechargeable battery market and main trends 2016–2025. In: International battery seminar & exhibit, March 20th. https://www.avicenne.com/pdf/Fort_Lauderdale_Tutorial_C_Pillot_March2015.pdf. Accessed 30 May 2019
Song C, Wang W, Peng H, et al (2018) Improving the electrochemical performance of LiNi0.80Co0.15Al0.05O2 in lithium ion batteries by LiAlO2 surface modification. Appl Sci 8:378. https://doi.org/10.3390/app8030378
Sverdrup HU, Ragnarsdottir KV, Koca D (2017) An assessment of metal supply sustainability as an input to policy: security of supply extraction rates, stocks-in-use, recycling, and risk of scarcity. J Clean Prod 140:359–372. https://doi.org/10.1016/j.jclepro.2015.06.085
Lee Bray E (2018) Bauxite and alumina. US Geol Surv Miner Commod Summ, pp 30–31
Shedd K (2018) Cobalt. US Geol Surv Miner Commod Summ, pp 50–51
Flanagan D (2018) Copper. US Geol Surv Miner Commod Summ, pp 52–53
Tuck C (2018) Iron ore. US Geol Surv Miner Commod Summ, pp 88–89
Jaskula B (2018) Lithium. US Geol Surv Miner Commod Summ, pp 98–99. https://doi.org/10.3133/70194932
Corathers L (2018) Manganese. US Geol Surv Miner Commod Summ, pp 104–105. https://doi.org/10.3133/70194932
Olson D (2018) Graphite (Natural). US Geol Surv Miner Commod Summ, pp 72–73
McRae M (2018) Nickel. US Geol Surv Miner Commod Summ, pp 112–113
Jasinski S (2018) Phosphate rock. US Geol Surv Miner Commod Summ, pp 122–123
Schnebele E (2018) Silicon. US Geol Surv Miner Commod Summ, pp 148–149
Schuyler Anderson C (2018) Tin. US Geol Surv Miner Commod Summ, pp 172–173
Bedinger G (2018) Titanium mineral concentrates. US Geol Surv Miner Commod Summ, pp 176–177. https://doi.org/10.3133/70194932
Martin G, Rentsch L, Höck M, Bertau M (2017) Lithium market research—global supply, future demand and price development. Energy Storage Mater 6:171–179. https://doi.org/10.1016/j.ensm.2016.11.004
Helbig C, Bradshaw AM, Wietschel L et al (2018) Supply risks associated with lithium-ion battery materials. J Clean Prod 172:274–286. https://doi.org/10.1016/j.jclepro.2017.10.122
Chohan UW (2018) Blockchain and the extractive industries: cobalt case study. Available at SSRN: https://ssrn.com/abstract=3138271. Accessed 12 July 2019
Moradi B, Botte GG (2016) Recycling of graphite anodes for the next generation of lithium ion batteries. J Appl Electrochem 46:123–148. https://doi.org/10.1007/s10800-015-0914-0
Yang Q, Geng Y, Dong H et al (2017) Effect of environmental regulations on China’s graphite export. J Clean Prod 161:327–334. https://doi.org/10.1016/j.jclepro.2017.05.131
Jara AD, Betemariam A, Woldetinsae G, Kim JY (2019) Purification, application and current market trend of natural graphite: a review. Int J Min Sci Technol. https://doi.org/10.1016/j.ijmst.2019.04.003
Klemola K (2016) Life-cycle impacts of tesla model S 85 and volkswagen passat. Online Report: http://kimmoklemola.fi/data/documents/SF-comparison-USA-20160110.pdf. Accessed 12 July 2019
ELIBAMA project (2014) Li-ion batteries recycling. In: Electrodes and cells manufacturing white paper. ELIBAMA project, pp 239–264
Foster M, Isely P, Standridge CR, Hasan MM (2014) Feasibility assessment of remanufacturing, repurposing, and recycling of end of vehicle application lithium-ion batteries. J Ind Eng Manag 7:698–715. https://doi.org/10.3926/jiem.939
Tedjar F, Foudraz J-C (2010) Method for the mixed recycling of lithium-based anode batteries and cells. U.S. Patent No. US 7,820,317
Kang DHP, Chen M, Ogunseitan OA (2013) Potential environmental and human health impacts of rechargeable lithium batteries in electronic waste. Environ Sci Technol 47:5495–5503. https://doi.org/10.1021/es400614y
Zeng X, Li J, Singh N (2014) Recycling of spent lithium-ion battery: a critical review. Crit Rev Environ Sci Technol 44:1129–1165. https://doi.org/10.1080/10643389.2013.763578
Larsson F, Andersson P, Blomqvist P, Mellander BE (2017) Toxic fluoride gas emissions from lithium-ion battery fires. Sci Rep 7:1–13. https://doi.org/10.1038/s41598-017-09784-z
Dunn JB, Gaines L, Barnes M, Sullivan J, Wang M (2014) Material and energy flows in the materials production, assembly, and end-of-life stages of the automotive lithium-ion battery life cycle (No. ANL/ESD/12-3 Rev.). Argonne National Laboratory (ANL), Argonne
Romare M, Dahllöf L (2017) The life cycle energy consumption and greenhouse gas emissions from lithium-ion batteries: a study with focus on current technology and batteries for light-duty vehicles. IVL Swedish Environmental Research Institute, Stockholm
Notter DA, Gauch M, Widmer R et al (2010) Contribution of Li-ion batteries to the environmental impact of electric vehicles. Environ Sci Technol 44:6550–6556. https://doi.org/10.1021/es903729a
Dewulf J, Van der Vorst G, Denturck K et al (2010) Recycling rechargeable lithium ion batteries: critical analysis of natural resource savings. Resour Conserv Recycl 54:229–234. https://doi.org/10.1016/j.resconrec.2009.08.004
Heelan J, Gratz E, Zheng Z et al (2016) Current and prospective Li-ion battery recycling and recovery processes. Jom 68:2632–2638. https://doi.org/10.1007/s11837-016-1994-y
Gu F, Guo J, Yao X et al (2017) An investigation of the current status of recycling spent lithium-ion batteries from consumer electronics in China. J Clean Prod 161:765–780. https://doi.org/10.1016/j.jclepro.2017.05.181
Nowak S, Winter M (2017) The role of sub- and supercritical CO2 as “processing solvent” for the recycling and sample preparation of lithium ion battery electrolytes. Molecules 22:403. https://doi.org/10.3390/molecules22030403
Ellis T, Mirza A (2015) Battery recycling: defining the market and identifying the technology required to keep high value materials in the economy and out of the waste dump. https://www.researchgate.net. Accessed 30 May 2019
Saloojee F, Lloyd J (2015) Lithium battery recycling process. Department of Environmental affairs Development Bank of South Africa (Project No. DB-074 (RW1/1016))
Zeng X, Li J, Liu L (2015) Solving spent lithium-ion battery problems in China: opportunities and challenges. Renew Sustain Energy Rev 52:1759–1767. https://doi.org/10.1016/j.rser.2015.08.014
De-Leon S (2018) Lithium ion battery recycling market 2018. Shumuel De-Leon Energy Ltd, Hod-Hasharon
Yazicioglu B, Tytgat J (2011) Life cycle assessments involving umicore’s battery recycling process. In: DG Environment–Stakeholder Meeting, Umicore
Cheret D, Santen S (2007) Battery recycling. U.S. Patent No. 7,169,206
Gaines L (2014) The future of automotive lithium-ion battery recycling: charting a sustainable course. Sustain Mater Technol 1:2–7. https://doi.org/10.1016/j.susmat.2014.10.001
Sonoc A, Jeswiet J, Soo VK (2015) Opportunities to improve recycling of automotive lithium ion batteries. Procedia CIRP 29:752–757. https://doi.org/10.1016/j.procir.2015.02.039
Georgi-Maschler T, Friedrich B, Weyhe R et al (2012) Development of a recycling process for Li-ion batteries. J Power Sources 207:173–182. https://doi.org/10.1016/j.jpowsour.2012.01.152
Weyhe R. International Energy Agency Photovoltaic Power System Programme, Accurec Recycling Gmbh. http://ieapvps.org/fileadmin/dam/public/workshop/10_Reiner_Thomas_WEYHE.pdf. Accessed 12 July 2019
McLaughlin W, Adams TS (1999) Li reclamation process. U.S. Patent No. 5,888,463
Zenger T, Krebs A, van Deutekom HJH (2010) Method of and apparatus for dismantling and storage of objects comprising alkali metals, such as alkali metal containing batteries. U.S. Patent No. 7,833,646
Sloop, Steven E, Parker R (2011) System and method for processing and end-of-life or reduced performance energy storage and/or conversion device using a supercritical fluid. U.S. Patent No. 8,067,107
Diekmann J, Hanisch C, Frob L et al (2017) Ecological recycling of lithium-ion batteries from electric vehicles with focus on mechanical processes. J Electrochem Soc 164:6184–6191. https://doi.org/10.1149/2.0271701jes
Gaines L (2018) Lithium-ion battery recycling processes: research towards a sustainable course. Sustain Mater Technol 17:e00068. https://doi.org/10.1016/j.susmat.2018.e00068
Julien CM, Mauger A, Zaghib K, Groult H (2014) Comparative issues of cathode materials for Li-ion batteries. Inorganics 2:132–154. https://doi.org/10.3390/inorganics2020132
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
The contributing editor for this article was Brajendra Mishra.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pinegar, H., Smith, Y.R. Recycling of End-of-Life Lithium Ion Batteries, Part I: Commercial Processes. J. Sustain. Metall. 5, 402–416 (2019). https://doi.org/10.1007/s40831-019-00235-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-019-00235-9