Abstract
The Paris Agreement declares to hold the increase in the global average temperature to well below 2 °C above pre-industrial levels and to pursue efforts to limit the temperature increase to 1.5 °C above pre-industrial levels in 2050. The agreement includes the commitment to deliver long-term low-carbon development strategies. Accordingly, global warming has been regarded as a crucial issue in every industry. Since the long-term target was set on the basis of the Paris Agreement, innovative technologies to realize CO2 mitigation in 2050 are desired, including in the steel industry. Until now, many various technology developments have been carried out in the ironmaking area; however, more advanced progress beyond the past progressive developments is required in order to attain the long-term target in 2050. In addition to modification of the current blast furnace process, the ironmaking process will be diversified, and new concepts such as CCU (CO2 Capture and Utilization) process in collaboration with chemical industry and hydrogen-based ironmaking utilizing CO2-free renewable energy aiming at CDA (Carbon Direct Avoidance) will be pursued in order to intensify CO2 mitigation. This review focuses on the evaluation of the current technology development to date and the design of an ambitious ironmaking process for the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Paris Agreement declares to hold the increase in the global average temperature to well below 2 °C above pre-industrial levels while pursuing efforts to limit the temperature increase to 1.5 °C above preindustrial levels in 2050, recognizing that this would significantly reduce the risks and impacts of climate change. The agreement includes a commitment to deliver long-term low-carbon development strategies. Every country has been obliged to submit Nationally Determined Contributions (NDCs) based on the Paris Agreement. In order to keep the global climate temperature below 2 ºC above pre-industrial levels, greenhouse gas emissions must be reduced by 80% by 2050 compared to 1990.
Although the significance of global warming is well recognized in the steel industry, designing the pathway to 2050 while maintaining the sound activity of the steel industry is a crucial issue. The steel industry has made various efforts to decrease the reducing agent leading to CO2 mitigation, but more promising pathways to the long-term global goal for CO2 mitigation must be constructed. The concrete pathway to 2050 consistent with the active competitiveness in the steel market still seems uncertain. Until now, several projects for improvement of the blast furnace process, such as blast furnace top gas recycling, have been actively developed. However, considering the current situations, the steel industry is now in the stage of reconsideration of the past progressive technology development and must pursue more ambitious processes to attain the long-term goal. In order to intensify CO2 mitigation, the ironmaking process will be diversified, and new concepts such as the CCU (CO2 Capture and Utilization) process and hydrogen-based ironmaking utilizing CO2-free renewable energy aiming at CDA (Carbon Direct Avoidance) will be pursued in addition to modification of the blast furnace processes. This review describes the assessment of the technology developments to date and the future perspective for achievement of the long-term goal for CO2 mitigation in the ironmaking.
Current State of Steel Industry
Since a large amount of carbon is consumed in the steel production process, the steel industry is occasionally regarded as a CO2 producer. However, carbon is used extremely effectively in the steel works as a reductant through highly developed technology such as energy saving. The steel industry also provides high-grade steel required in promoting energy saving in various processes and effective energy use in many types of industrial plants such as power plants. Actually, looking at the critical part of the fuel-fired power plants, high-quality heat-resident steels which make it possible to increase the energy efficiency contribute to CO2 mitigation. Moreover, high-strength steels are instrumental in reducing the weight of modern transportation vehicles, leading to less fuel consumption and fewer CO2 emissions. Thus, the total evaluation of the contribution of steel products in society is needed as shown in Fig. 1.
The current ironmaking processes are classified as shown in Fig. 2. As is well known, the major process route used in steel manufacturing is a combination of blast furnace and BOF (basic oxygen furnace) as shown in Fig. 2a, which currently accounts for 75% of world crude steel production [1]. Although blast furnace requires specified coking coal and iron ore, the BF-BOF process can supply various steel products, including high-quality steel. Moreover, the process covers a wide production capacity and the annual pig iron production rate of a large blast furnace with 5000 m3 inner volume reaches 4.0 million tons. As a highly economized system, the BF-BOF process is considered to be suitable for a large modern steel works. In East Asia, new integrated steel work based on the BF-BOF processes have been built recently. Although the BF-BOF is characterized by a highly effective energy utilization process, the CO2 intensity for crude steel reaches 1.8–2.0 t-CO2/t-steel, because carbon is consumed as a reductant and energy source.
As shown in Fig. 2b, steel scrap-EAF (electric arc furnace) process starts from the steel melting stage omitting reduction of iron ore, and as a result, its energy requirement is much smaller than BF-BOF route. Electricity requirement of EAF is estimated to be 300–350 kWh/t-steel. Although the CO2 intensity of the power sector influences the CO2 emissions in EAF, CO2 intensity of the scrap-EAF process is approximately 30% of the BF-BOF route. As drawbacks, because the supply of steel scrap consists of home scrap generated in the steel works and scrap from end-of-life steel products in society, the available scrap supply is limited to some extent, and the steel scrap-EAF process cannot produce high-quality steel due to the impurities (tramp elements) in steel scrap, which cannot be eliminated with the current technology. Therefore, the main products of steel scrap-EAF process tend to be materials which are less sensitive to the presence of impurities, although clean steel scrap is available for special steel production. For example, reinforced steel bars for construction are typical products of scrap-EAF process. The possibility of expanding the use of scrap-EAF process depends on a favorable electricity price and stable scarp supply.
Looking at DRI (direct reduced iron) processes, historically, various processes based on coal and natural gas have been developed as alternative processes to the blast furnace (Fig. 2c). Among them, the DRI process based on natural gas is a major process worldwide. This is an attractive process for mitigation of CO2 emissions, because natural gas carbon is a carbon-lean reductant. In general, the CO2 intensity of DRI process is estimated to be 70% of BF-BOF route [2]. MIDREX and TENOVA-HYL are representative DRI processes, but the appropriate sites for DRI production are restricted to the natural gas-producing areas. Recently, large DRI plants have been constructed in the southern United States, taking advantage of the availability of natural gas derived from shale gas. For example, 2.0 million t/y MIDREX plant by the Voestalpine group in Austria was commissioned in Corpus Christi, Texas in the United States to supply DRI to the North American market [3, 4].
Development of smelting reduction as an alternative process to the blast furnace has a long history. Although most projects were abandoned, the FINEX and COREX processes are now in the stage of commercial scale. HIsarna at Ijmuiden of Tata Steel Europe is under development [5]. The FINEX process, shown in Fig. 2d, is characterized by the combination of the melter and the fluidized beds. Iron ore and coal can be used directly without pretreatment processes such as the sintering machine and coke oven which are required in the BF-BOF route. FINEX has various advantages compared with the blast furnace and has reached 2 million t/y production scale [6], but plants are currently limited to Pohang of POSCO. Further evaluation on the future status of FINEX in ironmaking is expected. Basically, CO2 intensity of the FINEX is estimated to be slightly lower than that of the blast furnace.
Recently, the combination of DRI process and blast furnace which enables metallic iron introduction into the blast furnace was proposed as shown in Fig. 3 [7]. In the future, the Voestalpine group is planning to charge DRI produced in the US to their domestic blast furnaces as described above, and forecasts lowering CO2 by 5%. TENOVA HYL proposed to install DRI process in the steel works and produce DRI by COG (coke oven gas) and BOF gas [8]. The energy shortfall is covered by electricity purchased from outside. Although the CO2 mitigation ratio depends on the metallic charge rate and the total energy balance, its effect can be easily estimated by conventional model of the blast furnace. While improvement of the current ironmaking process is surely desired, additional extensions, such as the combination of DRI and the blast furnace, may be a realistic way to mitigate CO2 emissions.
Combination of blast furnace and DRI [8] (Color figure online)
According to the statistics of WSA [1], the crude steel production in the world is shown in Fig. 4, where the BF-BOF and EAF processes are noted. As is well known, crude steel production exceeded 1600 million tons since 2013. This tendency is impacted by the rapid growth in China, where a succession of the large integrated steel works based on BF-BOF were constructed. The BF-BOF route reached 74.3% in 2016, increasing from 65.4% in 2013. The annual production rate of DRI remains around 60 million tons. Considering the CO2 intensity of each process, CO2 emissions from the steel industry are mainly derived from BF-BOF route. Therefore, the first concern regarding the current state of CO2 mitigation is a review of the blast furnace process.
CO 2 Emissions from Steel Works
The simplified carbon flow in an integrated steel works with a blast furnace can be represented as shown in Fig. 5. The steel works are divided into the upstream process, which consists of the blast furnace, coke oven, and sintering machine, and the downstream process, such as rolling mill. 22–24 GJ/t energy is input to the ironmaking process, which provides 4–5 GJ/t surplus energy to the downstream. Generally speaking, self-consistency of the total energy balance is maintained in the integrated steel works, and particular additional energy is not required. Naturally, the possible ways to mitigate CO2 emissions are carbon input saving and sequestration of CO2 by CCS (CO2 Capture and Storage), while keeping the energy balance in the integrated steel works, where the evaluation of CO2 emissions is defined within the system boundary indicated in Fig. 5. A decrease in the carbon input implies improvement of the reduction reaction in the blast furnace and replacement of carbon to hydrogen-rich material or carbon–neutral material such as biomass. Utilization of biomass is undeniably attractive from the viewpoint of CO2 mitigation; however, securing a stable supply and ensuring effective use of biomass are critical issues. Various aspects of the use of biomass in ironmaking have been examined in great detail by Jahanshahi [9].
Figure 6 shows the CO2 emissions from the typical integrated steel works quantitatively, as calculated by the carbon balance model based on the conventional BF [10]. Although the emission sites of CO2 are widely distributed, 70% of CO2 emissions are derived from the ironmaking process. Looking at the carbon flow in Fig. 6, it can be noted that most carbon passes through blast furnace as coke and coal. The amount of carbon required in the steel works is determined by blast furnace performance. Therefore, improvement and redesign of the blast furnace process are key points for carbon input saving leading to CO2 mitigation in the steel works.
Approaches to Low Carbon in Ironmaking Process
The reduction mechanism of iron oxide in the blast furnace is briefly shown in Fig. 7, which simultaneously provides the basic concept of approaches to achieving low carbon. It is well known that the distribution of reduction in the blast furnace is divided into three steps, i.e., CO gas reduction, H2 gas reduction, and direct reduction, respectively. The reducing agent depends on the distribution of these steps. On the basis of these reduction steps, the approaches to achieving low carbon can be represented in the upper part of Fig. 7. First, one favorable approach is the improvement of gas utilization. For example, this can be attained by use of high reactivity coke to lower the thermal reserve zone temperature. In this case, the gas utilization ratio will be improved in the lower temperature region due to the use of high reactivity coke, as shown by (A) route in Fig. 7. Many studies have examined the use of high reactivity coke such as ferro-coke [11]. It goes without saying that the metallic charge from a DRI plant is very effective for reduction of reducing agent.
Reducing gas injection suppresses direct reduction, leading to a decrease in the coke rate, and this implies the mitigation of carbon input to the steel works. Originally, hot reducing gas injection started in the United States, and then it attracted attention by the reduction of coking coal for economic reason. In Japan, the FTG and NKG processes were actively developed as gas-reforming processes [12, 13]. In FTG, a reducing gas is produced by partial oxidation of heavy oil. A short trial at a commercial blast furnace with an inner volume of 1691 m3 equipped with four auxiliary tuyeres in the lower shaft was carried out. The configuration of FTG and NKG process is shown in Fig. 8. The NKG process consists of a pair of BFG heaters and COG reformers, and operation is repeated periodically through heating and reforming periods [13]. The reducing gas is produced by reforming of COG with the CO2 included in BFG, that is, so called dry reforming, and is then injected to the lower shaft of the blast furnace. Since dense CO2-rich BFG is favorable for the reforming process, selective removal of the high CO2 peripheral gas at the blast furnace throat has been proposed to promote the reforming reaction effectively. As this process is based on dry reforming by CO2, it could lead to a carbon recycling system without CCS in the ironmaking process. As an actual example, a recent Korean project proposed an application process consisting of capture of CO2 by NH3 absorption and dry reforming of COG without CCS [14].
The NKG process was verified with a 4.0 m3 experimental blast furnace [15]. Researches on the NKG process provided informative results on the effect of shaft gas injection on lowering in the coke rate through the penetration behavior and diffusion of injected gas. Figure 9 shows the effect of reducing gas injection on the change in the coke rate by a simulation model. As the reducing gas volume increases, the direct reduction ratio decreases, and as a result, the coke rate decreases remarkably up to 200 kg/t, which is almost equivalent to the minimum coke input for combustion in the raceway and carbonization. These processes developed in the early stage require the gas-reforming process and additional energy for reforming, and thus are sensitive to energy prices and failed to reach the commercial stage. Nevertheless, the analytical results concerning the effect of shaft gas injection were very informative for improvement of the blast furnace.
Effect of reducing gas injection in NKG process [13] (Color figure online)
After the global warming issue attracted attention, the concept of the top gas recycling through CO2 capture became well known as the concept of the low-carbon blast furnace, because top gas recycling drastically decreases the coke rate by suppressing the direct reduction without an additional gas-reforming system. In particular, top gas recycling with nitrogen-free blast furnace is the basic process for lowering coke rate, as it leads to a drastic decrease in CO2 emissions. This concept is related with the basic idea of ULCOS-BF (ULCOS: Ultra Low CO2 Steelmaking) [16,17,18,19].
These above concepts can be illustrated by Rist diagram shown Fig. 10. Control of the reduction equilibrium by high reactivity coke can be considered as first approach. In this case, the W point in the Rist diagram in Fig. 10 shifts to the right due to lowering of the thermal reserve zone temperature. This implies a reduction of the reducing agent by maintaining constant shaft efficiency. As described above, use of a hot reducing gas injection system by reforming oil, COG or natural gas were attempted previously and in this case, point B in Fig. 10, corresponding to the direct reduction ratio, shifts downward as a result of an increase in the indirect reduction ratio by gas reduction. Top gas recycling based on cold oxygen injection can more effectively intensify the indirect reduction through shaft gas injection. Although the reducing agent apparently increases on a molar basis, a drastic reduction in the coke rate is possible. These tendencies can be seen visually in Fig. 10. The selection of hot blast or cold oxygen injection has an influence on the specific oxygen gas volume. In the case of cold oxygen injection, point E in Fig. 10 moves downward due to the smaller sensible heat of cold oxygen. Conversely, point E shifts upward in the case of reducing gas injection utilizing hot blast. The points E′ and Eʺ in Fig. 10 correspond to the respective cases. In all cases, point B, corresponding to the direct reduction ratio, moves downward from B to B′ or Bʺ. If top gas recycling with CO2 sequestration is applied, top gas without CO2 provides the driving force for the downward movement of point B, which implies a decrease in the coke rate. Summarizing the above approaches to achieving low-carbon, full oxygen injection is more advantageous because various injectants such as pulverized coal or natural gas can be effectively used up to the limit of combustibility in the raceway. Moreover, the nitrogen-free condition kinetically accelerates the reduction rate of iron oxide and thereby improves productivity. Based on these considerations, the desirable process for low carbon is obvious.
The total concepts for a low-carbon blast furnace can be summarized as shown in Fig. 11. Generally speaking, various processes can be picked up, but the possibility of each process will depend on the local condition of the steel works such as energy and resources supplies. On the left side of Fig. 11, although the current blast furnace is available, an additional production process for DRI or high reactivity coke is required. On the contrary, on the right side of Fig. 11, more effective decrease in CO2 emissions is possible, but the large-scale modification of blast furnace is inevitable. In the steel works where an economically optimized steel production system has already been implemented, conversion to low-carbon process is a serious issue, considering the competiveness of steel works.
Low-Carbon Blast Furnace
Research Achievement Related to the Oxygen Blast Furnace and Top Gas Recycling
The oxygen blast furnace and top gas recycling are basic processes for realizing low-carbon ironmaking. Historically, various types of such blast furnaces have been proposed, as shown in Fig. 12, including theoretical studies carried out around the 1980s [20,21,22,23,24,25]. Figure 12 shows the differences of each process regarding the application of shaft gas injection or preheated gas injection. Shaft gas injection systems by top gas recycling are accompanied CO2 sequestration equipment in order to reproduce the reducing ability of top gas. Although these processes undoubtfully achieve drastic CO2 mitigation, the energy balance is a matter of concern from the viewpoint of energy consistency in the integrated steel works. In this connection, it should be noted that blast furnace plays an important role as an energy supplier for downstream processes.
As a simple process, preheated gas injection has attracted attention [23,24,25]. Figure 13 shows a comparison of the oxygen blast furnace with preheated gas injection and the conventional blast furnace. This process was actually verified with an experimental blast furnace by JFE Steel (Previously, NKK) [23]. In any case, the nitrogen-free condition resulting from oxygen injection promotes indirect reduction, which is similar to the concept of reducing gas injection shown in Fig. 8. This kind of oxygen blast furnace has several features. First, the oxygen blast furnace can be operated with higher productivity because the concentrations of reducing gases such as CO and H2 increase, and the specific bosh gas volume decreases under a nitrogen-free condition. The productivity of the oxygen blast furnace is approximately two times higher than that of the conventional blast furnace, as the limitations of the ore reduction rate and slag flooding are loosened. It has been reported that the maximum productivity of 5.1 t/day m3 was actually achieved in a campaign with an experimental oxygen blast furnace [23]. The direct reduction ratio in the oxygen blast furnace decreases due to the intensified gas reduction under the nitrogen-free condition, leading to a reduction of the solution loss reaction. Owing to the high combustion capability in the raceway of the oxygen blast furnace, a large quantity of injectants can be blown into the tuyeres, and diversified injectants can be used. At present, in addition to pulverized coal, intensified natural gas injection is considered to be suitable for the oxygen blast furnace since the decomposition heat of natural gas can be utilized to control the flame temperature. Use of hydrogen-rich injectants such as natural gas also helps to decrease CO2 emissions from the steel works. Moreover, the oxygen blast furnace can produce top gas with a higher calorific value than that of the conventional blast furnace. In the examples shown in Fig. 13, the calorific values of the BFG produced by the conventional blast furnace and the oxygen blast furnace are 3.0 MJ/Nm3 and 6.4 MJ/Nm3, respectively [26]. The high-calorific top gas can be utilized effectively in other processes such as the power plant or as chemical resources.
The typical top gas recycling was developed as ULCOS-BF in ULCOS project [16,17,18,19], and its validity was confirmed at the LKAB experimental blast furnace (working volume: 8.2 m3) in Lureå. In this process, reducing gas derived from the top gas is recycled after CO2 removal by VPSA and reheating. Three different versions can be considered, as shown in Fig. 14 [17,18,19]. In versions 1 and 4, reducing gas from the top gas is injected into the lower shaft. Three campaigns were successfully carried out at the LKAB experimental blast furnace. The coke and coal saving effects by reducing gas injection obtained in these campaigns are illustrated in Fig. 15, showing that the carbon saving depends on the recycled gas volume in each version. According to the results with the experimental blast furnace, carbon saving reached about 25% at the recycled gas injection of 700 Nm3/thm, which means 120 kg/thm reduction in carbon input compared with the reference operation [17, 19].
Process flows in various versions in ULCOS-BF [17] (Color figure online)
Influence of recycled gas volume from top gas on carbon savings in LKAB experimental blast furnace [17] (Color figure online)
In the next stage, the deployment of ULCOS-BF was planned at the two sites in Florange in France and Germany. However, the next step of ULCOS-BF project was canceled for economic reason. Financial support is required in order to out this project on hold, and the future effect on the competitiveness of steel works with new processes should be deeply considered.
Improvement to the Advanced Oxygen Blast Furnace
Although top gas recycling has several advantages, it is necessary to use a large amount of shaft gas and co-injection of tuyere gas with pulverized coal and oxygen. Since a CO2 sequestration system will presumably require more sophisticated engineering techniques, it might be relatively complicated to construct the total process. Therefore, as a slightly simplified process, an advanced oxygen blast furnace design is proposed [26,27,28]. The advanced oxygen blast furnace is equipped with only preheating gas injection and co-injection of pulverized coal and natural gas. In particular, an advanced oxygen blast furnace is characterized by a downsized inner volume thanks to high productivity of oxygen blast furnace, as shown in Fig. 16. As mentioned above, the productivity of oxygen blast furnace is double that of the conventional blast furnace, and this high productivity makes it possible to reduce the furnace inner volume in comparison with a conventional blast furnace at the same pig iron output. Because the smaller inner volume of the advanced oxygen blast furnace reduces the load of burden materials from the upper furnace, it is also possible to relax burden property requirements related to physical strength, i.e., the strength of the sintered ore and coke, and the use of low-quality burden materials also enhances the economic advantage of the oxygen blast furnace. According to Takahashi [26], the maximum compressive stress on the burden is approximately 20–30% smaller than in the conventional blast furnace. This stress reduction means that the restriction of the burden physical properties can be relaxed, and low-grade burden can be used. In addition to the economic benefits associated with the use of low-quality burden materials, the relaxation of the burden strength requirements in the oxygen blast furnace will also lead to substantial energy savings in the sintering and coking processes.
Moreover, owing to a large amount of natural gas injection, the direct reduction ratio is minimized to the limit, and the solution loss reaction also becomes extremely small. Figure 16 shows the total characteristics of the advanced oxygen blast furnace. The distribution of the reduction steps in the blast furnace is shown in Fig. 17 [27, 28]. The hatched area including the “Base” case corresponds to the conventional blast furnace condition. Although direct reduction with a low coke rate can be controlled by injectants, the region for the conventional blast furnace is somewhat limited, as shown in Fig. 17, and the change of the reduction step is stagnant because the hot blast contains nitrogen. With intensified top gas recycling, the operating point corresponding to the reduction distribution moves upward, namely, in parallel with the increase in CO gas reduction, and direct reduction decreases to around 15% at a top gas recycling ratio of about 90%. In other words, CO gas reduction just replaces direct reduction. On the other hand, in the advanced oxygen blast furnace with natural gas injection, this point moves to the right. Unlike the top gas recycling process, hydrogen reduction replaces direct reduction in the process with natural gas injection. At 150 kg/thm injection rate of natural gas, the direct reduction ratio closely approaches 0%, while the CO gas reduction ratio remains almost constant [27, 28]. In this critical point, it is expected to suppress degradation of the coke particles in the lower part of the blast furnace due to the decrease in the solution loss reaction. Intensified hydrogen reduction accelerates the reduction rate and improves the permeability of the cohesive zone.
Evaluation on CO2 Mitigation by Top Gas Recycling and Advanced Oxygen Blast Furnace
Figure 18 shows the relationship between the energy supply to downstream processes and the CO2 emission ratio [27, 28]. These results were calculated by the material and heat balance model of the integrated steel works. The model elements consist of the sintering machine, coke oven, and blast furnace, and also include the power plant and oxygen plant. In addition to these, various other equipment facilities such as blowers for the blast furnace are also included in this model. The model of the blast furnace is based on Rist diagram.
Relationship between energy supply to downstream and CO2 emissions [19] (Color figure online)
As is obvious from Fig. 18, in the case of top gas recycling, the carbon input has a close relationship with surplus energy. In the top gas recycling process, a reduction of carbon input is achieved at the cost of the energy supply to downstream processes. In contrast to this, in the advanced oxygen blast furnace as represented by the hatched area in Fig. 18, the carbon input tends to decrease even though the energy supply to downstream processes increases. This implies that carbon-based reduction is gradually replaced by hydrogen reduction. Thus, the compatibility between the decreased carbon input and surplus energy supply is satisfied in the case of the advanced oxygen blast furnace.
In the top gas recycling process, it is estimated that the limit of top gas recycling is substantially equal to a 20% reduction in the CO2 emission ratio. However, under this condition, the energy supply to downstream processes approaches to 0 GJ/thm. The critical point in top gas recycling is actually determined by the relationship between carbon input reduction and energy makeup for the downstream processes by securing additional external energy sources. This implies that application of top gas recycling will be limited to certain steel works which have small-scale downstream processes. In the integrated steel works with large downstream processes, top gas recycling is not a suitable approach. Figure 18 also shows the CO2 emissions ratio corresponding to the case with CCS. Although a remarkable decrease in CO2 emissions can be expected, CCS requires additional energy and costs. Thus, the technological applicability and economic efficiency of CCS must be solved from the global viewpoint. Both CO2 mitigation and a sufficient energy supply can be achieved with the advanced oxygen blast furnace in the right part of Fig. 18. As is obvious from Fig. 18, the conventional approach to intensification of the energy supply means an increase in carbon input. However, in the advanced oxygen blast furnace, CO2 mitigation is satisfied by energy conversion to a hydrogen-rich injectant, and the CO2 mitigation ratio reaches a reduction of about 10% in comparison with the conventional blast furnace. The optimal point for CO2 mitigation and energy use will depend selectively on the situation of the actual steel works. Excess gas including hydrogen can be utilized as available energy for producing electricity at an outside power plant or as a chemical resource.
In summary, various processes such as top gas recycling and the oxygen blast furnace can be proposed for mitigating CO2 emissions, but the achievable range by those blast furnace arrangements is estimated to be 10–15% CO2 reduction in the integrated steel works so long as ironmaking process depends on carbon. Although CCS is frequently mentioned as end of pipe, CCS technologies require a large amount of investment and include many uncertainties in technology and environmental issues, which still remain to be confirmed. Moreover, these efforts will be accompanied by increased production cost resulting from capital expenditure and operating expense of CCS.
Perspective on Long-Term Goal
Road Map to 2050 by EUROFER
As described in the Introduction, the long-term goal in 2050 is equivalent to an 80% reduction in CO2. It is not certain whether the target will be attained by the deployment of the present and growing technology in the future. In 2013, EUROFER (The European Steel Association) proposed a report titled “A Steel Roadmap for Low Carbon Europe 2050” [29, 30]. This review provides a realistic technical view of the CO2 mitigation potential, examining which reduction technologies will be available by 2050 and how much impact they can have between now and 2050. It also examines the economic dimension and how far such considerations will affect decisions on investment in emission-reducing technologies. Although this review focuses on the situation of European steel industry, various aspects are applicable and helpful for examining other steel industries in mature countries.
The roadmap was prepared by the EUROFER Low Carbon Steel Roadmap Working Group in collaboration with Steel Institute VDEh and BCG (Boston Consulting Group). In the roadmap, several cases were proposed, including incremental technology development, conversion to DRI-EAF and introduction of top gas recycling with CCS. Then, the effect of CO2 mitigation and the cost sensitivity in each scenario were quantified for long-term goal in 2050. These forecasts on the CO2 emissions trajectory up to 2050 are shown in Fig. 19, and the technologies included in these scenarios are shown Fig. 20.
CO2 intensity pathway to 2050 in EUROFER roadmap [29] (Color figure online)
Scenarios to 2050 in EUROFER roadmap [29] (Color figure online)
According to the roadmap as shown in Figs. 19 and 20, at best, the implementation of cost-effective CO2 mitigation technologies could decrease the CO2 intensity of the steel sector by 15% in 2050 compared to 2010. The conversion of BF-BOF to DRI-EAF makes it possible to decrease CO2 emissions to 40%, and application of BF-TGR in combination with CCS enlarges CO2 mitigation to 56% based on 2010. However, in order to realize the technologies achieving mitigation beyond the 15% level, it would be necessary to resort to yet unproven technologies in combination with CCS, hence involving huge investment in infrastructure and higher operating costs. In the roadmap, the production costs resulting from introduction of DRI-EAF and CCS were calculated on the basis of CAPEX (Capital Expenditure) and OPEX (Operating Expense), and they showed an unfavorable economic situation, even if such a scenario would lead to a reduction of absolute CO2 emissions of ca. 60% in 2050 compared to 1990. The abatement cost for CO2 for the same investment cycle would have to range between €260 and €700 per ton of CO2, depending on the input-factor price increase (and also excluding decommissioning costs).
According to the road map suggested by EUROFER, public funds will be indispensable, as potential breakthrough technologies will involve huge investments and high financial risks. Moreover, their keen concerns are unfair social system in steel production; that is, if competing regions do not be submit to such constraints, the uptake of “breakthrough” technologies by the EU steel industry will not be affordable.
Discrepancy Between Goal and Reality
The roadmap of EUROFER shows the possible range with the current technology and the aspects related to breakthrough technology on the basis of forecast. On the other hand, Paris Agreement shows the ideal goal for prevention of global warming. Comparing the two approaches, we can actually see the discrepancy between goal in 2050 and reality from the technology viewpoints. These situations can be illustrated as Fig. 21. Summarizing the analysis of the top gas recycling and oxygen blast furnace, the actual range on CO2 intensity reduction is located within 10–15%. Even in the Roadmap of EUROFER, the cost-effective improvement of CO2 intensity is estimated to be at most 15%.
Going beyond this level of reduction would require resorting to yet unproven technologies in combination with CCS, and would involve a huge investment in infrastructure and higher operating costs. Although such a scenario would lead to a reduction of ca. 60% in absolute CO2 emissions in 2050 compared to 1990, it would be accompanied by increased production cost. Climate change is a global issue which requires a global response. In an ever increasing globalized economy, this should be achieved through the enforcement of a comprehensive international agreement. In addition to global social system, from the technological viewpoint, more innovative approaches are desired in the steel industry in order to bridge the gap between the goal and reality.
Alternative Approach to Long-Term Goal by CO 2 Capture and Utilization
In order to restrict global warming to not more than 2 °C, more efforts will be needed. It is uncertain that the modified ironmaking based on blast furnace can reach the long-term goal. To go beyond the present level, alternative approaches to solve such constrains have emerged recently, mainly in Europe.
Thyssenkrupp initiated “Carbon2Chem” project, the aim of which is to convert the off-gases from steel works including CO2 into chemical products [31,32,33,34,35]. Chemical products mean methanol, NH3, synthetic fuel, and others, as shown in Fig. 22. The energy required for the conversion process is to come from renewable sources. At present, gases from steel production are burnt to produce electricity and heat for the production process. In contrast, Carbon2Chem puts the gases at the start of a chemical production chain. This is possible because steel mill gases include hydrogen, nitrogen, and carbon, which are the basic elements for numerous chemical products. CO2 can be used as a raw material by splitting its molecules. This requires hydrogen, which in part is already present in the steel mill gases. Additional hydrogen is to be produced by using renewable energies. The processes in the steel mill will be modified in such a way that parts of the process gases are diverted to chemical production when CO2-free and low cost electricity is available from renewable sources.
Concept of Carbon2Chem project [35] (Color figure online)
This project has a relationship with Power to Gas [36], which implies the production of green hydrogen from renewable energy. Since Carbon2Chem requires additional hydrogen to chemical reaction, CO2-free hydrogen obtained through electrolysis of water is favorably supplied through the hydrogen supply chain designed by Power to Gas as shown in Fig. 23. Thyssenkrupp emphasizes that Carbon2Chem is characterized by a broad new concept; “Cross-industrial network” or “Integrated CO2 capture”. They say that it creates a new concept by creating a network of steel production, electricity generation, and chemical production.
Concept of Power to Gas [36] (Color figure online)
Carbon2Chem’s technological prospects are evaluated to be hopeful because the basic chemical processes and the required technologies are largely known. It is already technically possible to convert process gases from steel production into ammonia as a starting product for fertilizers. The process would also utilize some part of the CO2 contained in the steel mill gases. Another possibility would be to produce methanol from mill gases, a process which would utilize almost all the CO2 they contain.
Germany’s minister of education and research announced funding of more than €60 million for the first stage of the Carbon2Chem project at the kick-off meeting in 2016. A consortium of 16 partners from the areas of basic and applied research and various sectors of industry are involved in the project. ThyssenKrupp, the Max Planck Institute, Fraunhofer, chemical companies, and universities which are noted for chemical energy conversion, will carry out preparatory planning and the scientific work. It is reported that at least ten years of development work will be needed before the process is ready for industrial-scale use. A technical center will be built on the premises of Thyssenkrupp Steel Europe in Duisburg to test the Carbon2Chem processes on a pilot scale once the first phase of the project is complete.
As another CCU approach, conversion of the off-gas from steel mill to alcohol by gas fermentation process is proposed [37,38,39,40,41,42]. At its core, the Chicago-based company, LanzaTech, provided the original gas fermentation technology which uses carbon-containing gases as both a nutrient and energy source for microorganisms that, in turn, produce fuels and chemicals as shown in Fig. 24. In the LanzaTech process, off-gas from a steel mill is introduced into the bottom of a bioreactor vessel, and fermentation proceeds in a liquid medium where the microbes grow and produce specific products. These naturally occurring microbes are entirely contained in the bioreactor and have no direct interaction with the outside environment. These particular microorganisms are very tolerant of gas contaminants, removing the need for expensive scrubbing technology. Microbial fermentation of carbon- and hydrogen-rich off-gases, such as COG, BFG, and BOFG to produce ethanol or other basic chemicals, substantially mitigates CO2 emissions. In the steel industry, carbon is used primarily as a chemical reactant to reduce iron oxide to metallic iron. This is an important distinction from the typical industrial use of carbon as a fuel.
LanzaTech process by fermentation of various off-gases from industry [41] (Color figure online)
The first test plant was built in BlueScope Steel in New Zealand in 2008. Subsequently, Baosteel built a demonstration plant in Shanghai, and Shougang Steel group constructed a similar-scale demonstration plant. The demonstration facility in Baosteel has been operational since 2012 and successfully running at an annualized rate of 100,000 gallons of ethanol. The low-temperature, low-pressure gas fermentation route benefits from tolerance to a wide variety of impurities and pollutants, eliminating the need for extensive gas clean-up or conditioning. The microbes used in the gas fermentation process convert carbon to ethanol at very high selectivity compared to the conventional chemical synthesis routes. In 2018, the Shougang Steel group have announced the start-up of the commercial facility with a capacity of 54 million l/y at the Jingtang Steel Mill, in which BOFGs are converted to ethanol. In Europe, ArcelorMittal in collaboration with Primetals has begun construction of a new plant at its site in Ghent, Belgium, to house a pioneering new installation which will convert carbon-containing gas from steel mills in Ghent steel works into bioethanol. This project is called “Steelanol” [37, 42]. Annual production of ethanol at Ghent is expected to reach around 21 million gal/y (80 million l/y). Funding was obtained from various sources, including the European Union's Horizon 2020 program, to carry out further research and development and scale up the project.
“Carbon2Chem” and gas fermentations such as “Steelanol” open up a long-term vision of integrated steel and chemical complexes, where all gas streams can be efficiently utilized to create products and maximize value. This will create a relationship between the steel and chemical industries similar to that between oil refining and chemicals today, where steelmakers are an upstream feedstock and utilities are suppliers to chemical manufacturers.
Hydrogen-Based Ironmaking Toward Carbon Direct Avoidance
HYBRIT Process
Hydrogen-based ironmaking implies to replace the blast furnaces with an alternative process, using CO2-free hydrogen produced from “clean” electricity derived from renewable energy. SSAB, LKAB, and Vattenfall announced the launch of a project that can solve the steel industry’s carbon dioxide challenge—HYBRIT (Hydrogen Breakthrough Ironmaking Technology) [43,44,45]. HYBRIT is a joint venture between SSAB, LKAB, and Vattenfall, aiming to replace coal with hydrogen in the steelmaking process and to reduce CO2 emissions from ironmaking to zero by eliminating the need to use fossil fuel for iron ore reduction. In HYBRIT, iron metal is produced by using hydrogen gas as the main reductant. The concept of HYBRIT is shown in Fig. 25. The production route is similar to DRI shaft furnace processes, except for the CO2 emissions: In the HYBRIT process, hydrogen reacts with iron oxides to form water instead of CO2. Hydrogen gas is produced by electrolysis of water using fossil-free electricity. The fossil fuel in ore processing will be eliminated with the increased level of energy efficiency and by switching to carbon neutral energy. The EAF process is used for heating and melting to produce molten steel from charged materials by means of electric current derived from renewable energy. The use of EAF allows steel to be made from up to 100% scrap metal, or as in the HYBRIT concept, from a mix of direct reduced iron and scrap. Similar to the reference process, the liquid steel is tapped into a ladle where its final chemical composition and the temperature of the steel are adjusted, and is then cast into crude steel slabs in the continuous caster.
Concept of HYBRIT process [45] (Color figure online)
A pre-feasibility study, conducted 2016–2017, gave the positive aspects for the next phase of HYBRIT. The pre-feasibility study considers that fossil-free steel will in future be able to compete in the market with traditional steel thorough the European Union Emissions Trading System (EUETS). It is reported that 2018 is used for planning and designing the construction of a globally unique pilot plant for fossil-free steel production in Luleå and in the Norrbotten iron ore fields, 250 km north west of Luleå. The pilot phase is planned to last until 2024, after which that project will advance to the demonstration phase in 2025–2035. The Swedish Energy Agency contributed to the feasibility study and research project. If conditions allow, construction of the commercial plant will be carried out by 2040. Sweden has set a national target to reach zero net emissions of CO2 by the year 2045, defining the future pathway for the country’s steel industry. From these backgrounds, it is hoped that HYBRIT will be a significant part of the road toward SSAB’s goal of being fossil-free by 2045.
H2FUTURE Project and SALCOS
H2FUTURE is also a hydrogen-based ironmaking project which utilizes CO2-free hydrogen by electricity from renewable energy sources [46,47,48]. This project is being promoted by Voestalpine, Siemens, and VERBUND. Siemens is a key technology supplier for the proton exchange membrane (PEM) electrolyzer, and VERBUND, the project coordinator, will provide electricity from renewable energy sources and is responsible for the development of grid-relevant services. Other partners in the project are the research institute, Energy Research Centre of the Netherlands (ECN) in the Netherlands, and K1MET in Austria. The concept of H2FUTURE is shown in Fig. 26. A large-scale 6 MW PEM electrolysis system provided by Siemens will be installed and operated at the Voestalpine Linz steel plant in Austria. The reduction process is based on existing shaft furnace such as MIDREX. The H2FUTURE project has started on January 1, 2017 and has a duration of 4.5 years. The Fuel Cells and Hydrogen Joint Undertaking (FCH JU) of the European Commission provides funding of this project.
H2FUTURE project as a hydrogen-based ironmaking [48] (Color figure online)
The large PEM electrolysis device supplied from Siemens, with a power consumption of 6 MW, will use renewable energy sources to produce hydrogen, and this project is expected to provide valuable insights into the use of renewable energy resources to produce hydrogen- and carbon-free steel production process. The project will be one of the ambitious researches on “breakthrough technologies” in order to fulfill long-term global climate protection targets.
SALCOS (Salzgitter Low CO2 Steelmaking) project initiated by Salzgitter group and the VTT Technical Research Center of Finland [49, 50]. This project is associated with GrInHy (Green Industrial Hydrogen via reversible high-temperature electrolysis) project, which focuses on producing hydrogen by CO2-free renewable energy [50]. The concept of reduction process of iron oxide as shown in Fig. 27 is similar to other hydrogen-based ironmaking process. However, it is characterized by high-temperature electrolysis through a solid oxide cell provided from Sunfire GmbH in GrInHY project. Therefore, the system has reversibility by producing hydrogen from steam and electricity in the solid oxide electrolysis cell (SOEC) mode and generating electricity and heat using either hydrogen or natural gas as fuels in solid oxide fuel cell (SOFC) mode. During electrolysis operation with the highest electrical efficiency levels, the technology converts industrially generated steam from waste heat into hydrogen; in the reverse case of fuel cell operation, it produces electricity and heat from hydrogen or natural gas. The adjunct project GrInHy has received funding from the Fuel Cells and Hydrogen 2 Joint Undertaking and support from the European Union’s Horizon 2020 research and innovation program.
Concept of SALCOS [49] (Color figure online)
Concluding Remarks
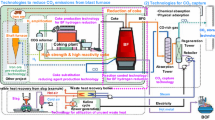
The steel industry is a key sector in every country, and steel is regarded as a growing market in the world. The steel industry has already contributed to the creation of environmental-friendly society by providing high-quality steel and making efforts to develop carbon-lean processes. Until now, a number of studies and research projects have already examined approaches for further improving energy efficiency and reducing CO2 emissions in the sector. However, based on current climate change forecast, it is predicted that the steel industry will face greater challenges which cannot be solved with the past incremental technologies in the future. In response to the Paris Agreement, the pathway to the long-term goal for CO2 mitigation in 2050 is a crucial issue in the steel industry. As described in previous chapters, the steel industry has pursued various technology developments in the ironmaking process, but only progressive technologies will not reach the target for 2050. Although modifications of blast furnace such as top gas recycling can realize a certain improvement in CO2 mitigation, more innovative technology will be required in the future to achieve the long-term goal. The steel industry must step up toward the long-term goal. Figure 28 shows the past transition and the future perspective in blast furnace process. As shown in Fig. 28, the directions toward long-term goal will be diversified. In addition to modification of blast furnace from conventional blast furnace to top gas recycling, new paradigms such as CCU- and hydrogen-based ironmaking based on CO2-free renewable energy are being pursued. On the other hand, this direction will be more costly than the existing ironmaking plants. Thus, a more comprehensive social system to support clean steel production will be required. Climate change is a global issue which requires a global response and responsibility. There still exist many subjects to be considered. The optimal solutions cannot be seen, and the discussions to find a better pathway to attain the long-term goal has just started. In order to search for the promising directions, this review has summarized the past development to date and the ambitious researches based on the suggestive projects in the new framework.
References
World Steel Association (2018) Word Steel in Figures 2018
Schenk J, Lüngen HB (2016) Evaluation of the capabilities of direct and smelting reduction processes to enhance the energy efficiency and to reduce the CO2 emission of the steel production in Europe. In: 7th European coke and ironmaking congress—ECIC 2016, Sept 12–14, 2016, Linz, Austria
Millner R, Ofner H, Boehm C, Ripke SJ, Metius M (2017) Future of direct reduction in Europe-Medium and long-term perspective. In: European steel technology and application days 2017—3rd ESTAD 2017, June 26–29, 2017, Vienna, Austria
Buergler T, Kofler I (2016) Direct reduction technology as a flexible tool to reduce the CO2 intensity of iron and steelmaking. In: 7th European Coke and Ironmaking Congress—ECIC 2016, Sept 12–14, 2016, Linz, Austria
van der Stel J, Meijer K, Teehuis C, Zeijlstra C, Keilman G, Ouwehand M (2013) Update to the development of HIsarna—an ULCOS alternative ironmaking process. In: IEAGHG/IETS iron and steel industry CCUS and process integration workshop. Nov 4–7, 2013, Tokyo, Japan
Yi SH (2016) Updates on the FINEX, A commercially proven alternative ironmaking process. In: 7th European coke and ironmaking congress—ECIC 2016, Sept12–14, 2016, Linz, Austria
Voestalpine AG, Primetals Technologies (2017) Opening of the HBI plant in Texan marks start of a new era at Voestalpine, MPT International, Feb, pp 30–32
Pauluzzi D, Martinis A, Martinez J (2016) High-carbon DRI the feeding material to improve performances and decrease CO2 emission in both BF and EAF. In: 7th European Coke and Ironmaking Congress—ECIC 2016, Sept.12–14, 2016, Linz, Austria
Jahanshahi S, Mathieson JG, Michael Somerville MA, Haque N, Norgate TE, Deev A, Pan Y, Xie D, Ridgeway P, Zulli P (2015) Development of low-emission integrated steelworks. J Sustain Metall 1:94–114
Ariyama T (2009) Future perspective on low carbon and decarbonizing in ironmaking process. Bull Iron Steel Int Jpn 14:781–789
Yamamoto T, Sato T, Fujimoto H, Anyashiki T, Fukada K, Sato M, Takeda K, Ariyama T (2011) Reaction behavior of ferro coke and its evaluation in blast furnace. Tetsu-to-Hagané 97:501–509
Yatsuzuka T, Nakayama K, Ohmori K, Hara Y, Iguchi M (1972) Injection of reducing gas into blast furnace (FTG Process). Tetsu-to-Hagané 58:624–636
Nishio H, Miyashita T (1973) On the top gas recycled reforming processes and the injected gas distributions. Tetsu-to-Hagané 59:1506–1522
Lee MS (2013) CCU technology development in RIST. In: IEAGHG/IETS iron and steel industry CCUS and process integration workshop, Nov 4–7, 2013, Tokyo, Japan
Miyashita T, Nishio H, Shimotsuma T, Yamada T, Ohotsuki M (1972) Reducing gas injection into furnace stack in an experimental furnace. Tetsu-to-Hagané 58:608–623
Birat JP, Lorrain JO, de Lassat Y (2009) The CO2 tool–CO2 emissions & energy consumption of existing & breakthrough steelmaking route. La Revue de Métallurgie CIT Sept, pp 325–335
van der Stel J, Louwerse G, Sert D, Hirsch A, Eklund N, Pettersson M (2013) Top gas recycling blast furnace developments for green and sustainable ironmaking. Ironmak Steelmak 40:483–489
Hirsch A, Korthas B, Hülstrung J, Grant M, Berthelemot A, Sert D, Hanrot F, Harp G, Adam J, van der Stel J, Hattink M, Jak H, Veerman A, Ansseau O, Danloy G, Küttner W, Eklund N, Pettersson M, Zuo G, Sköld BE, Sundqvist L, Simoes JP, Dimastromatteo V, Zagaria M, Babich A, Lin R, Feiterna A, Smith A, Bürgler T, Habemann A, Feilmayr C, Hitchinson C, Ritz V (2013) European Commission, research fund for coal and steel-new blast furnace process (ULCOS), 2013
van der Stel J, Hattink M, Zeilstra C, Louwersem G, Hirsch A, Janhsen U, Sert D, Grant M, Delebecque A, Diez-Brea P ,Adam J, Ansseau O, Feiterna A, Lin R, Zagaria AM, Küttner W, Schott R,Eklund N, Pettersson M, Boden A, Sköld BE, Sundqvist L, Simoes JP, Edberg N, Lövgren J, Bürgler T, Feilmayr C, Sihvonen M, Kerkkonen O, Babich A, Born S (2014) European Commission, research fund for coal and steel-ULCOS top gas recycling blast furnace process-final report, 2014
Fink F (1986) The floss furnace-A new concept to smelt iron in blast furnace. In: Proc European ironmaking conf VDEh, Düsseldorf, vol 3, pp 55–56
Qin M, Yang N (1986) A blast furnace process with pulverized coal oxygen and gas circulation for reduction. Scan J Metall 15:138–142
Lu WK, Kumar RV (1984) The feasibility of nitrogen-free blast furnace operation. Trans Iron Steel Soc AIME 5:25–31
Ohno Y, Hotta K, Matsuura M, Mitsufuji H, Saito H (1989) Development of oxygen blast furnace process with pre-heated gas injection into upper shaft. Tetsu-to-Hagané 75:1278–1285
Ohno Y, Matsuura M (1990) Heating-up and reduction characteristics of burdens in oxygen blast furnace process. Tetsu-to-Hagané 76:1262–1269
Ohno Y, Matsuura M, Mitsufuji H, Furukawa T (1992) Process characteristics of a commercial-scale oxygen blast furnace process with shaft gas injection. ISIJ Int 32(1992):838–847
Takahashi K, Nouchi T, Sato M, Ariyama T (2015) Perspective on progressive development of oxygen blast furnace for energy saving. ISIJ Int 55:1866–1875
Sato M, Takahashi K, Nouchi T, Ariyama T (2015) Prediction of next-generation ironmaking process based on oxygen blast furnace suitable for CO2 mitigation and energy saving. ISIJ Int 55:2101–2114
Ariyama T, Sato M, Nouchi T, Takahashi K (2016) Evolution of blast furnace process toward reductant flexibility and carbon dioxide mitigation in steel works. ISIJ Int 56:1681–1696
The European Steel Association EUROFER (2013) A steel roadmap for a low carbon Europe 2050
The Boston Consulting Group, The Steel Institute VDEh (2013) Steel’s contribution to a low carbon Europe 2050—technical and economic analysis of the sector’s abatement potential.
Achatz R, Oles M (2015) Chemische Verwertung von Hütten gasen. In: Aachen Stahlkolloqiom 2015, Sept 3–4, 2015, Aachen, Germany
Carbon2Chem https://www.thyssenkrupp-steel.com/de/newsroom/highlights/carbon2chem.html/ Accessed Sept 16, 2018
Fraunhofer-Gesellschaft: Press Information, Projektstart Carbon2Chem, June 27, 2016
Max-Planck Institute für Chemische Energie Konversion: Pressemitteilung, June 27, 2016
Schmöle P (2016) The blast furnace-fit for the future? In: 7th European coke and ironmaking congress—ECIC 2016, Sept 12–14, 2016, Linz, Austria
DNV KEMA Energy & Sustainability (2013) Final report—systems analyses power to gas, Groningen 20, June 2013
van der Werf W (2014) Advanced fuels & chemicals from waste—waste in a circular economy joint workshop: AMF-IEA bioenergy infrastructure compatible fuels, May 20, 2014. Copenhagen, Denmark
Fleischanderl A, Plattenr T, Puschitz (2015) The circular economy: carbon recycling and the steel industry. European steel technology and application days 2015, METEC & 2nd ESTAD 2015, June 15–19, 2015, Düsseldorf, Germany
Plattner T, Fleischnderl A, Haselgruebler M, de Mare C, van der Stricht W, Nair P, Wolf C (2017) Carbon recycling at its best utilization of by-products from process-gas fermentation. In: European steel technology and application days 2017, 3rd ESTAD 2017, June 26–29, 2017, Vienna, Austria
Fleischanderl A, Plattner T, Nair P, Schultz M (2016) Carbon recycling from metallurgical waste gases into bio-fuel and chemical. SCANMET V, June 5–6, 2016, Luleå, Sweden
LanzaTech http ://www.lanzatech.com/ Accessed Sept. 16, 2018
Steelanol https://www.steelanol.eu/en/ Accessed Sept. 16 2018
Karsberg V, Lindblad E, Kryssare M SSAB, LKAB and Vattenfall to build a globally-unique pilot plant for fossil-free steel. HYBRIT press release, Feb 1, 2018
Tottie M (2016) HYBRIT-Iron ore to steel based on hydrogen. US Sweden Sustainable Agenda, Oct 14, 2016, Luleå, Sweden
SSAB, LKAB, Vattenfall (2017) HYBRIT Fossil-free steel, Summary of findings from HYBRIT pre-feasibility study 2016-2017
Verbund, Voestalpine, Siemmens (2017) European commission fund H2FUTURE project-Voestalpine, Siemens and Verbund are building a pilot facility for a green hydrogen at the Linz location. Press release, Feb 7, 2017, Vienna
Zauner R (2017) Innovative use of clean hydrogen in an industrial application. In: The H2FUTURE project and the role of utilities Verbund AG presentation Apr 27, 2017
H2FUTURE https://www.h2future-project.eu/ Accessed Sept 16, 2018
Hille V (2017) Dekarbonisierung der Stahlproduktion durch siginifikanten Einsatz von Wassestoff-das Project SALCOS. VJK-Jahrestagung, Nov 21, 2017, Berlin, Germany
GrInHy https://www.green-industrial-hydrogen.com/home/ Accessed Sept 16, 2018
Acknowledgements
We wish to express our appreciation to the persons from the following projects for the permission to reprint figures of the project materials: Carbon2Chem, EUROFER, ULCOS, HYBRIT, H2FUTURE, LanzaTech, and SALCOS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflicts of interest.
Additional information
The contributing editor for this article was I. Sohn.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ariyama, T., Takahashi, K., Kawashiri, Y. et al. Diversification of the Ironmaking Process Toward the Long-Term Global Goal for Carbon Dioxide Mitigation. J. Sustain. Metall. 5, 276–294 (2019). https://doi.org/10.1007/s40831-019-00219-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-019-00219-9