Abstract
A flue dust that contains valuable metals is generated during smelting operation for e-waste treatment. However, metal recovery from this flue dust has not been extensively studied. The flue dust can contain valuable metals such as gold, silver, copper, and iron. In this study, the flue dust from e-waste was investigated as the potential metal resource for obtaining valuable metals, including copper and iron, using physical and chemical methods. In the physical processes, size separation was performed, and the mass and composition of each fraction were analyzed. Major valuable elements of the flue dust are copper and iron with small levels of gold and silver in all size fractions. The iron is present mostly as magnetite, as determined by XRD. The magnetite was removed by magnetic separation, and the removal efficiency was about 90%. The chemical processes included leaching in various acid solutions (HNO3, H2SO4, and HCl) for copper recovery. The highest recovery efficiency of over 99% was obtained after 8 h in 1 M nitric acid and was optimized at a pulp density of 20 g/L and a stirring speed of 200 rpm at 20 °C temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The demand of electronic equipment has increased with the advances in technology. As a result, electronic wastes (e-waste) are rapidly increasing in the world [1]. E-waste or electronic scrap (e-scrap) contains valuable metals such as copper (Cu), iron (Fe), zinc (Zn), aluminum (Al), gold (Au), and silver (Ag). Therefore, the recycling of e-waste is an important subject not only from the point of view of waste treatment but also from that of the recovery of valuable materials.
For recovery of metals from e-waste, various treatment options based on physical and chemical processes are proposed [2]. E-waste contains a variety of materials with different physical properties, and metals from nonmetals are easily separated by physical methods [3, 4]. Many physical separation methods have been conducted for the separation of metals from e-waste, such as gravity, magnetic, electrostatic, and eddy-current methods [5,6,7,8]. After this physical process, generally, chemical processes, including pyrometallurgy and hydrometallurgy, are carried out for the extraction of the base and precious metals. Pyrometallurgy is a traditional technology for recovery of nonferrous metals as well as precious metals from e-waste. Smelting in furnaces, incineration, combustion, and pyrolysis are the typical pyrometallurgical processes [3, 4]. The hydrometallurgical process is based on the traditional hydrometallurgical technology of metals extractions from their primary ores [9]. It can be separated into three general processes; leaching, solution concentration, and purification and metal recovery.
Most of the flue dust is generated during the pyrometallurgical process, and the elements and compositions of flue dust depend on the characteristics of materials that are processed. Researchers have investigated the recovery of Cu from Cu smelter flue dust [10, 11]. Vítková et al. reported the leaching behavior of electrostatic precipitator dust from the Cu smelter using a pH-static leaching experiment [12]. However, processing of flue dust from the e-waste smelting process is not well known because there is insufficient information about the constituents of the dust. The analysis shows that it contains valuable metals such as Au, Ag, Cu, Fe, Zn, etc. The development of Cu recovery process from e-waste flue dust can also be applied to mixed waste from other smelting processes.
In this study, a size separation process was carried out as a first concentration step and then, chemical composition and phase identification were measured by inductively coupled plasma-optical emission spectrometry (ICP-OES) and X-ray diffraction (XRD). In the second step, magnetic separation process, with a variation of applied current was conducted to remove the ferrous materials. In the chemical process, Cu leaching experiments were applied to the nonmagnetic fraction for Cu recovery.
Experimental
The flue dust studied in this work was provided by an e-waste recycling company, and the material was generated in an e-waste smelting operation. The compositional analysis of samples, as a function of the particle size, is an important aspect to know which element(s) concentrate in which specific size fraction. Through these analyses, the proper physical separation method can be selected depending on the sample composition. Sieving was carried out as the first step. Ro-tap sieve shaker (RX-29, W.S. Tyler) was used as a size separator, and six different sizes of metal sieves (45, 75, 125, 250, 335, and 600 µm) were used for each sample.
Magnetic separation was carried out next to remove the ferrous materials. The LB-1 magnetic barrier separator (S.G. Frantz Co.) was used, and it consists of an electromagnet having two long pole pieces. For the separation of ferromagnetic materials from other magnetic and nonmagnetic materials, an additional Low-field control (LFC-3) equipment was used. The LFC-3 can generate and control low magnetic fields of the currents from 0 to 100 mA. The forward slope (20°) and the side slope (15°) of equipment were fixed, and the separation effect was analyzed according to the applied current from 5 to 25 mA.
After size classification and magnetic separation, the compositional analysis of the flue dust was conducted. Samples were fused with a lithium borate mixture (40 wt% lithium tetraborate, 60 wt% lithium metaborate) at 1100 °C for 1 h. After the borate fusion process, the melt was dissolved in HNO3 for ICP-OES (Optima 8000, Perkin Elmer) analysis.
Acid solutions, such as HNO3 and H2SO4, have been investigated to recover metals from e-waste [13]. In this study, first, leaching experiments were carried out in the acid solution to confirm the possibility of recovery of Cu from the flue dust. The leaching temperature was also set at 20 °C (room temperature) for a cost-effective recovery process. HNO3 (68–70%, GR ACS), H2SO4 (95–98%, J. T. Baker), and HCl (36.5–38.0%, GR ACS) were used as leaching reagents. During leaching, solids/liquid content (pulp density) was fixed at 20 g/L. A magnetic stir bar was also used to mix the solution, and the rotational speed was fixed at 200 rpm. After leaching, the solid residue was separated from the leachate by vacuum filtering, and the leachate was analyzed by the ICP-OES.
Results and Discussion
The size separation process was carried out as a first recovery step on the flue dust. The cumulative particle size distribution of the flue dust is shown in Fig. 1. Six different sizes of metal sieves (45, 75, 125, 250, 335, and 600 µm) were used, and after size separation, each particle fraction weight was measured. Material over 600-µm particle size was composed of plastic and other nonmetallic light components, and the composition of this coarse fraction was not measured. In the flue dust, 80% of the particles were smaller than 500 µm.
The results of ICP-OES analysis of the flue dust with particle size are presented in Table 1. Major elements of the flue dust were Fe and Cu in all particle size fractions, and the amounts of Fe and Cu were about 26 and 6 wt%, respectively. Therefore, the fractions were recombined because the content ratios of Fe and Cu were similar in all sizes. Therefore, the used particle size for the experiments was less than 600 µm, with the Fe content varying from 22.1 to 25.7 wt% and Cu content from 3.6 to 5.8 wt%.
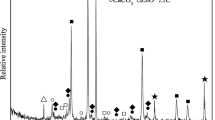
The XRD pattern of flue dust is shown in Fig. 2. In this case, fine material of less than 45 μm size was analyzed to know the exact metal peak, especially Fe phase, because peaks overlapped due to impurities, such as organic materials and other oxides in the larger size fractions. As shown in Fig. 2, the iron was confirmed to be in magnetite (Fe3O4) phase. Fe3O4 is ferromagnetic and can be magnetized to become a permanent magnet [14]. Therefore, it can be easily separated by magnetic separation. Yavuz et al. also reported that Fe3O4 nanocrystals were separated by low-field magnetic separation [15]. Through XRD results, the magnetic separation was selected as the second process.
In magnetic separation, the forward slope (20°) and the side slope (15°) of equipment were fixed, and the separation effect was analyzed according to the applied currents (5, 10, 15, 20, and 25 mA). Mass analysis of samples after magnetic separation is shown in Fig. 3. The weight of magnetic fraction increased with the increasing applied current. In 5 mA, a small amount of magnetic material was transferred to the magnetic fraction because a low magnetic field was created. On the other hand, at 30 mA, all materials were stuck to the pole pieces due to the high magnetic field providing no separation. Therefore, the maximum applied current was fixed at 25 mA for separation. Compositional changes in Cu and Fe, after magnetic separation, are shown in Fig. 4. In the nonmagnetic fraction, the composition of Cu was increased after magnetic separation, while the composition of Fe was lowered. At 25 mA current, it had the highest content of copper and the lowest iron. Fe recovery after magnetic separation at 25 mA was calculated as follows:
where C i and C n are the concentrations of Fe of initial sample and nonmagnetic sample, respectively. Mi and M n are the masses of the initial sample and nonmagnetic sample, respectively. The recovery of Fe calculated by Eq. (1) was about 90%.
Acid leaching experiments, after the magnetic separation process, were performed for Cu recovery. Acid leaching is often used as the first-stage leaching for the extraction of base metals, Cu in particular [16,17,18]. Acid leaching of metals from e-waste has been extensively investigated using various acids [19]. In order to investigate the effects of various acid solutions, leaching experiments were performed with H2SO4, HNO3, and HCl, separately.
The results of metal leaching with different acid solutions are shown in Fig. 5. All experiments were conducted under same conditions for 1 h at 20 °C. The Cu dissolution in HNO3 was higher than in other acids, and the value was about 24 wt%. On the other hand, the Cu dissolution in HCl was the lowest. Based on the results, additional studies on Cu dissolution in HNO3 were conducted. The effect of time on Cu dissolution in HNO3 is shown in Fig. 6. The Cu dissolution gradually increased with the increasing time, and was completely dissolved after 8 h. After Cu leaching experiment, a filtration process was also carried out to analyze the residue (solid) materials. The residue was about 23 wt% compared to the initial feed amount. Residue was subsequently dried at 110 °C for 2 h and analyzed by ICP. From the results of ICP and XRD, about 6.7 wt% Fe was found in Fe2O3 form and Fe compound in the residue, and other compositions were mostly that of nonmetallic materials.
On the basis of the above investigations, a process for Cu recovery can be proposed. A schematic design of the process is shown in Fig. 7. The flue dust is divided into two particle sizes as greater than and less than 600 µm, followed by magnetic separation of under 600-µm fraction to remove ferrous materials. In the chemical process, Cu leaching experiments, using the nonmagnetic fraction, are performed for Cu recovery. The leaching efficiency (Cu dissolution) can reach over 99% in 1 M HNO3 solution in 8 h. At this stage, copper can be recovered by standard process of electrowinning practiced industrially.
Conclusion
The physical and chemical processes were investigated for Cu recovery of flue dust generated during smelting of e-waste. Flue dust below 600 µm showed most of the Fe and Cu concentrations. A magnetic separation at an applied current of 25 mA showed the highest content of copper with a low iron content. The magnetic fraction separated 90% of the contained iron in the dust. For the recovery of Cu, dissolution in HNO3 showed almost 100% copper recovery after 8 h of leaching.
References
Robinson BH (2009) E-waste: an assessment of global production and environmental impacts. Sci Total Environ 408:183–191. https://doi.org/10.1016/j.scitotenv.2009.09.044
Cui J, Zhang L (2008) Metallurgical recovery of metals from electronic waste: a review. J Hazard Mater 158:228–256. https://doi.org/10.1016/j.jhazmat.2008.02.001
Cui J, Forssberg E (2003) Mechanical recycling of waste electric and electronic equipment: a review. J Hazard Mater B99:243–263. https://doi.org/10.1016/S0304-3894(03)00061-X
Tuncuk A, Stazi V, Akcil A, Yazici EY, Deveci H (2012) Aqueous metal recovery techniques from e-scrap: hydrometallurgy in recycling. Miner Eng 25:28–37. https://doi.org/10.1016/j.mineng.2011.09.019
Veit HM, Juchneski NCDF, Scherer J (2014) Use of gravity separation in metals concentration from printed circuit board scraps. REM R Esc Minas 67:73–79. https://doi.org/10.1590/S0370-44672014000100011
Veit HM, Diehl TR, Salami AP, Rodrigues JDS, Bernardes AM, Tenório JAS (2005) Utilization of magnetic and electrostatic separation in the recycling of printed circuit boards scrap. Waste Manag 25:67–74. https://doi.org/10.1016/j.wasman.2004.09.009
Wu J, Li J, Xu Z (2008) Electrostatic separation for recovering metals and nonmetals from waste printed circuit board: problems and improvements. Environ Sci Technol 42:5272–5276. https://doi.org/10.1021/es800868m
Li J, Jiang Y, Xu Z (2017) Eddy current separation technology for recycling printed circuit boards from crushed cell phones. J Clean Prod 141:1316–1323. https://doi.org/10.1016/j.jclepro.2016.09.144
Khaliq A, Rhamdhani MA, Brooks G, Masood S (2014) Metal extraction processes for electronic waste and existing industrial routes: a review and Australian perspective. Resources 3:152–179. https://doi.org/10.3390/resources3010152
Morales A, Cruells M, Roca A, Bergó R (2010) Treatment of copper flash smelter flue dusts for copper and zinc extraction and arsenic stabilization. Hydrometallurgy 105:148–154. https://doi.org/10.1016/j.hydromet.2010.09.001
Okanigbe DO, Popoola API, Adeleke AA (2017) Characterization of copper smelter dust for copper recovery. Procedia Manuf 7:121–126. https://doi.org/10.1016/j.promfg.2016.12.032
Vítková M, Ettler V, Hyks J, Astrup T, Kříbek B (2011) Leaching of metals from copper smelter flue dust (Mufulira, Zambian Copperbelt). Appl Geochem 26:S263–S266. https://doi.org/10.1016/j.apgeochem.2011.03.120
Kumar M, Lee JC, Kim MS, Jeong J, Yoo K (2014) Leaching of metals from waste printed circuit boards (WPCBs) using sulfuric and nitric acids. Environ Eng Manag J 13:2601–2607
Wasilewski P, Kletetschka G (1999) Lodestone: natures only permanent magnet—what it is and how it gets charged. Geophys Res Lett 26:2275–2278. https://doi.org/10.1029/1999GL900496
Yavuz CT, Mayo JT, William WY, Prakash A, Falkner JC, Yean S, Li Cong, Shipley HJ, Kan A, Tomson M, Natelson D, Colvin VL (2006) Low-field magnetic separation of monodisperse Fe3O4 nanocrystals. Science 314:964–967. https://doi.org/10.1126/science.1131475
Kumari A, Jha MK, Lee JC, Singh RP (2016) Clean process for recovery of metals and recycling of acid from the leach liquor of PCBs. J Clean Prod 112:4826–4834. https://doi.org/10.1016/j.jclepro.2015.08.018
Vračar RŽ, Vučković N, Kamberović Ž (2003) Leaching of copper (I) sulphide by sulphuric acid solution with addition of sodium nitrate. Hydrometallurgy 70:143–151. https://doi.org/10.1016/S0304-386X(03)00075-6
Choubey PK, Panda R, Jha MK, Lee JC, Pathak DD (2015) Recovery of copper and recycling of acid from the leach liquor of discarded printed circuit boards (PCBs). Sep Purif Technol 156:269–275. https://doi.org/10.1016/j.seppur.2015.10.012
Habbache N, Alane N, Djerad S, Tifouti L (2009) Leaching of copper oxide with different acid solutions. Chem Eng J 152:503–508. https://doi.org/10.1016/j.cej.2009.05.020
Acknowledgements
The authors are thankful to the members of the NSF I/UCRC (WPI: 233110) on Resource Recovery and Recycling (CR3) and to the National Science Foundation for their support to this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
The contributing editor for this article was B. Friedrich.
Rights and permissions
About this article
Cite this article
Lee, H., Lee, E., Jung, M. et al. Recovery of Copper from Flue Dust Generated in e-Waste Processing Using Physicochemical Methods. J. Sustain. Metall. 4, 260–264 (2018). https://doi.org/10.1007/s40831-017-0150-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-017-0150-4