Abstract
Rare earth production from ion-adsorption deposits constitutes an important rare earth production route, and the most important production route for heavy rare earths such as dysprosium and terbium. The demand for dysprosium has experienced substantial growth in recent years, mainly due to its use in neodymium–iron–boron (Nd–Fe–B) magnets, the demand for which is increasing largely due to their use in efficient motor applications. Hence, the analysis of environmental impacts associated with rare earth mining and processing is gaining importance. In this study, a life cycle inventory for rare earth production from ion-adsorption deposits was compiled through a detailed analysis of the literature and with help from industry experts. A detailed review of the literature on environmental impacts associated with the mining process was also conducted, and impacts not covered by the current impact assessment methods are discussed. Despite the detailed study, data uncertainties remain. Therefore, recommendations for further research are given, including further investigations into the fate of emissions from in situ leaching of rare earths in the proximity of the mining site, and development of the methods used to assess resource extraction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rare earth elements (REEs) play a key role for green technologies. Nd–Fe-–B magnets commonly used in energy-efficient motors constitute one of the most important REE applications, which has shown substantial growth in recent years. The installation of efficient Nd–Fe–B motors can contribute to large efficiency gains [1, 2]. The growth in demand for Nd–Fe–B magnet material used in motors has led to an increase in demand for heavy rare earths (HRE), especially dysprosium.
Rare earth (RE) primary production is associated with notable environmental and social impacts [3]. The recent increase in demand for rare earth elements and the expected continuation of this market trend have sparked a growing interest in the assessment of environmental impacts associated with their production. A life cycle inventory of light rare earth production, based on the process route from bastnaesite conducted at the main production site in Bayan Obo, has been implemented in the ecoinvent database for some time [4]. Further life cycle assessment studies have been published in recent years [5,6,7,8,9]. However, the process routes addressed by the above-mentioned studies are not representative of the main production route for HREs: Most HREs are mined from ion-adsorption deposits in Southern China [10]; in situ leaching is now the legally required production method for REE from these deposits [11].
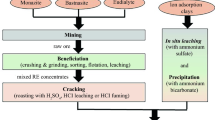
First, a detailed process description of REE production from ion-adsorption deposits (i.e., deposits in which RE ions are adsorbed to aluminosilicate clay particles) through in situ leaching and solvent extraction is presented ("Production of Individual Rare Earth Oxides from Ion-Adsorption Deposits via In Situ Leaching and Solvent Extraction" section). The deposits are also sometimes referred to as ionic deposits or ionic clays. Environmental impacts linked with this process are discussed (section "Environmental Impacts Associated with REO Production from Ion-Adsorption Clays Discussed in the Literature"). Then, a life cycle inventory to represent this process is compiled ("Life cycle Assessment of Rare Earth Oxide Production from Ion-Adsorption Clay Deposits" section). Vahidi et al. [12] have compiled a dataset for in situ leaching from ion-adsorption deposits. Their study, based on information from Chinese literature, covers the production of a rare earth concentrate from the in situ leaching process. In this study, the system boundaries are extended to include another key downstream processing step into the dataset: the separation of REE concentrates into individual REEs through solvent extraction. In section, "Discussion, Conclusions, and Recommendations for Further Work", the technical difficulties of REE production from decreasing ore grades which constitute a topic for research in China are discussed, and recommendations for continued research to further improve the dataset are provided. Finally, the suitability of common life cycle impact assessment (LCIA) methods to assess the environmental impacts associated with this rare earth production route is discussed.
Methods
A life cycle inventory dataset was compiled for rare earth production from ion-adsorption clays with the aim to provide these data for use in further life cycle assessment studies. This was done according to the current ISOFootnote 1 14040/14044 standards [13, 14]. All stages of the life cycle assessment were conducted in order to be able to analyze the dataset and compare it against impact assessment results for other rare earth production routes. The modeling was done using the OpenLCA software (V.1.5) and with background datasets from ecoinvent 3.2.
Prior to the data compilation, a review of both peer-reviewed and gray literature was conducted to obtain both qualitative and quantitative information on the current practice of rare earth production (mining and processing) from ion-adsorption deposits. The literature was also reviewed regarding environmental impacts associated with rare earth production to assist the interpretation of our dataset results, and to ascertain whether the life cycle inventory adequately reflected the issues. The information found in the literature was complemented by way of interviews with industry experts.
Production of Individual Rare Earth Oxides from Ion-Adsorption Deposits via In Situ Leaching and Solvent Extraction
Characterization of Deposits
Most HREs are mined from ion-adsorption deposits in Southern China [10]. RE3+ ions contained in weathered rocks (sands) of magmatic origin are mobilized under tropical conditions and absorbed to aluminosilicates [15,16,17]. A small percentage, 5–25% of total rare earth content, is contained in the mineral phase and colloid sediment, but this fraction is not accessible with the current mining practice [18, 19]. The ores occur close to the surface and are characterized by low thorium/uranium contents. The ore is low‐grade, generally only 0.05–0.5% REO (rare earth oxides), with higher content in HREs [15]. Mining is viable only above a certain concentration, with deposits between 0.2 and 0.4% currently mined (Ding, pers. comm.). The deposits are sediments with higher variability in composition than other ore types, due to the weathering [10, 15, 16]. The ion-adsorption deposits can be broadly classified into two ore types, i.e., light and heavy ion-adsorption deposits [16], but variations in between (with both significant light and heavy fractions) can also be found. Low cerium contents are characteristic for these deposits, and can be explained by the formation process [20].
Ion-adsorption deposit mining started in Longnan province, where pond leaching was the technique applied. The deposits in Longnan with high yttrium contents (65% of rare earth content) are no longer operational (Ding, pers. comm.). Areas with current or recent operational mines include Ganzhou and Xinfang where deposits with 20 and 30% of yttrium are mined (Ding, pers. comm.). A composition representative of ores currently mined is shown in Table 1 [21]. The composition represents an official Chinese estimate [22]. Due to the variability of the deposits, the average composition of deposits mined could shift in future.
REO Concentrate Production Process from Ion-Adsorption Clays—In Situ Leaching
While heap leaching used to be common, in situ leaching is now the mandatory production technique for rare earth mining from ion-adsorption deposits in China [11]. Heap leaching has been banned due to environmental concerns. Flow sheets on in situ leaching of ion-adsorption clays and the following solvent extraction process are available from the literature [12, 19, 23]. The leachate is directly inserted into the ore to extract the rare earths in an ion-exchange reaction, and then processed on site to re-extract the rare earths. The mines operate for 1–5 years (Ding, pers. comm.).
Site Preparation
The site preparation involves the drilling of holes into the ore body required to insert the leachate and the excavation of precipitation ponds. Equipment needs to be installed for the circulation of the leachate, extraction of the rare earths from the leachate, and the removal of impurities, and the heating of the extracted rare earth material to obtain oxides.
Leaching
The leaching solution (with 1–5% ammonium sulfate) is pumped into holes drilled into the ore body, remains in the ground for 150–400 days and is then re-extracted at the bottom, followed by washing of the ore with water [12, 23]. However, since the ion-exchange kinetics are fast [17], the time span can be much shorter, and washing may not always be practiced (Ding, pers. comm.). Ion exchange with ammonium sulfate:
Approximately 8–20% of the leachate is lost in the ore ([12] and Ding, pers. comm.), possibly due to the heterogeneity of the mined layer in the ore body. In addition to this loss, the NH4 + ions which are exchanged against the rare earth ions stay in the ore, i.e., they are emitted to soil. Cations other than REEs (Al3+, Ca2+, Mg2+ and Fe3+) are also unintentionally extracted in the process, i.e., NH4 + ions additional to those required to exchange the accessible rare earth content are exchanged [24,25,26]. Sulfate anions from the leachate are also emitted to soil.
Typical compositions of the leaching solution contain 1–8 g/l of RE2O3 and impurities—see Table 2 [26]. The percentage of impurities contained in the leachate generally increases with decreasing ore grades.
Removal of Impurities in Leachate (Through Selective Precipitation)
After extraction from the ore, the pregnant leachate is moved to one of the ponds to undergo precipitation to remove impurities. Aluminum ions in particular cause a technical challenge since they disturb the REE precipitation process by causing flocculation (formation of agglomerated rather than crystalline particles), and are therefore removed at pH 5, before rare earth precipitation [27]. Fe3+ and Mg2+ ions precipitate under very similar conditions and can therefore be removed in the same process [26]. Ammonium bicarbonate is added to the leachate for two reasons: to achieve the required conditions for precipitation by increasing the pH of the leachate from ~3.5 to ~5, and as the precipitating agent (providing the OH− ions from the reaction of bicarbonate ions with water) [26].
The removal rate for aluminum ions depends on the pH of the solution, but also on the concentrations of Al3+, other impurities and REE in the leachate [26, 27]. Around 95% of Al ions and 5% of RE ions are typically extracted in this process [27]. Problems occur particularly in leachates with low RE contents (<2 µg/l) where the Al ion content is high relative to the RE content: only ~70% of Al are precipitated, and ~25% of RE content is lost [27]. As a consequence, the process is less efficient and consumes more ammonium bicarbonate for lower ore grades, and also gets less efficient throughout the operation of the mine, since the leachate is recirculated and rare earth content in the leachate decreases during the operation. Equations 2 and 3 from Chi et al. [26] show the intended precipitation reaction (2) and the unintended side reaction (3).
The aluminum hydroxide impurities are filtered off after the precipitation to remove them from the leachate. The impurities which cannot be extracted in this process (mainly Ca2+ ions) end up in the REE concentrate after heating, which contains around 7% of impurities by mass, and are then removed during SX (solvent extraction) [25].
Extraction of REE from Leachate Through Precipitation and Heating
After filtering off the metal hydroxides, the REE and remaining Ca2+ impurities are extracted through another precipitation process. Both ammonium bicarbonate and oxalic acid can be used as precipitants [12]. However, ammonium bicarbonate is cheaper and much more common for the precipitation of rare earths before separation [12, 26, 28]. (Oxalic acid is commonly used for rare earth precipitation after separation [28]).
Precipitation with ammonium bicarbonate [26]
Carbonic acid decomposition [26]
Heating of rare earth carbonates to obtain oxides [29]
Rare earths are precipitated from the leachate as carbonates. The efficiency of the reaction is pH dependent: The pH determines the fraction of ammonium bicarbonate present as carbonate ions, and therefore the overall amount of reagent needed to precipitate all RE ions as carbonates [26]. The required pH (>8) is achieved through further addition of ammonium bicarbonate (which here again acts as precipitant and pH regulator). Furthermore, ammonium bicarbonate is consumed for carbonic acid decomposition (Eq. 5) (without which the solution would gradually get more acidic [26] [see (Eq. 4)]. The extraction rate for rare earths in this step is around 95% (around 5% of REE content is lost in each precipitation step), according to lab experiments [26].
Usually, two or three precipitation ponds are placed next to each other to manage overflow (Ding, pers. comm.). Agitation is achieved by the continuous pumping of the REE containing leachate from the ore, and the leachate is recirculated back into the ore until the rare earth content in the leachate is so low that further processing is no longer viable (Ding, pers.comm). The purity of the REE after precipitation is around 91–93% [3, 12]. RE carbonates are then heated to 800–900 °C to convert them to mixed rare earth oxides [26], (Eq. 6). After a leaching step, they are then separated in a multistage SX process in a centralized location.
Management of Leachate/Waste Water Treatment
During the mining operation, the leachate is pumped into the ore body. Part of the leachate is lost in this process, i.e., cannot be re-extracted.
In the leaching process, ammonium (NH4 +) ions from the leachate are transferred to the ore body, and RE ions and impurities are taken up into the leachate. First impurities and then rare earths are precipitated in nearby ponds; the leachate composition is adjusted (pH, ammonium bicarbonate content) and pumped through the ore body again. This process is stopped when the RE content gets so low that further processing of the leachate is no longer viable. Once the operation ceases, the leachate is collected and can be reused at another mining site (Ding, pers. comm.). Ponds are refilled with the excavated material and planted with vegetation.
During the operation, almost all of the leachate is recirculated, and waste water leaving the system is not usually generated (Ding, pers. comm.). This is possible because of the constant removal of the metal impurities throughout the operation. The reuse of the leachate at other sites may not always be practiced, e.g., in the case of illegal mining. The practice of reusing the leachate at other mining sites and the possible avoidance of waste water generation are mentioned in the literature, but so are high concentrations of ammonium in the discharge water, far exceeding standards [19]. It can be seen in Fig. 1 that the potential reuse of leachate after the mining operation finishes has a big influence on the net (NH4)2SO4 consumption.
Recovery Rates and Decreasing Ore Grades
Both ore grades (content of the desired metal in the ore) and recovery rates (the percentage of the desired metal contained in the ore which can be extracted) are difficult to determine for in situ leaching processes. Some literature sources report high rare earth recovery rates for REE mining from ion-adsorption clays, (e.g., 85–90% [17], however, these recovery rates can only be achieved with the heap-leaching technique, which is no longer being applied (Ding, pers. comm.). For in situ leaching, only around 40–70% of the mined are currently recovered (Ding, pers. comm; Lartigue-Peyrou, pers. comm). Tian et al. [18] state that REE recovery rates from in situ mining of ionic deposits are low [18]. Illegal mining of ion-adsorption deposits is a cause for decreasing ore grades in the ore bodies left to the legal miners. Illegal miners selectively focus on higher-grade fraction of the inhomogeneous deposits, thereby lowering their own production costs and reducing the economic viability of the remaining degraded deposit [21]. Although an effort is made to curb illegal production activities, the illegal fraction of the market is still notable at around 30–40% [21], which illustrates the importance of the issue.
It has been reported that the processing costs per unit of output increase with decreasing ore grades, since larger inputs of chemicals and energy are required [18, 19, 30]. The mining of leaner ores is technologically challenging and associated with lower leachate recovery rates and higher contents of ammonium in discharge water, exceeding environmental standards [19]. The leach selectivity is lower, i.e., larger quantities of elements other than rare earths are involuntarily extracted [31]. Due to the higher content in impurities, the processing of pregnant leachates from leaner ores with lower rare earth contents reduces the efficiency of the precipitation process, with increased consumption of precipitants and decreased rare earth recovery rates [18, 31]. The decreased recovery rates are associated with the unintended side reaction during the aluminum removal from the leachate (see section "Removal of Impurities in Leachate (Through Selective Precipitation)").
The recovery of the rare earth fraction which is not present as exchangeable ions in the clays would require a leachate with pH <3 [32]. This would, however, mean that colloidal sediment phase aluminum would also be liberated, and the amount of aluminum present in this phase far exceeds the amount of rare earths [32]. This would cause issues in the currently practiced precipitation process, since more Al would need to be precipitated, which would further increase the total ammonium bicarbonate requirements during precipitation per unit of REE.
Production of Individual Rare Earth Oxides
Separation of individual rare earths is technically difficult, especially for neighboring elements. Solvent extraction (SX) is commonly used for industrial-scale separation of rare earths [33, 34]. Figure 2 shows the typical processing route for rare earth concentrates obtained from ion-adsorption deposits [35]. The SX process for rare earths from ion-adsorption deposits starts by leaching the rare earth concentrates, typically with HCl, sometimes with HNO3 or H2SO4 solutions [19, 33], to bring the rare earths into solution. Mixer-settlers are used as the standard equipment for the separation process [34]. The separation is typically conducted with P507 and naphthenic acid as extractants and kerosene as diluents [3, 19, 35]. P507 only features small separation factors for some elements, which is why different systems are currently used to separate the 15 REE from ion-adsorption clays to 3N–5N purity [35]. For the separation of yttrium, naphthenic acid in kerosene is added to iso-octanol [19, 35]. The organic phase is regenerated, and the aqueous phase is neutralized and discarded [35]. H+ ions which are exchanged for REE need to be neutralized (saponified) with NaOH to maintain the pH required for the exchange reactions [15, 36]. After separation, the individual rare earth fractions are precipitated with oxalic acid, washed, and calcinated; and the waste water from precipitation is neutralized before discharge [35].
Separation of REEs from concentrates from ion-adsorption deposits—simplified process diagram based on [35] (color figure online)
SX—general exchange reaction for organophosphorus acids [36], [15]
(HA denotes extraction agent in diluent).
Precipitation with oxalic acid [12]:
Calcination of rare earth oxalates (heating in furnace)
Environmental Impacts Associated with REO Production from Ion-Adsorption Clays Discussed in the Literature
Environmental impacts arise at various processing stages at the mining site and during the rare earth separation process. It should be noted that ion-adsorption deposits are advantageous over other rare earth deposits in terms of their lower radioactivity levels [16, 21]. Environmental impacts associated with the separation process are attributable to the energy consumption associated with the operation of the mixer-settler units and calcination furnaces, water consumption, and the salt contained in the neutralized waste waters.
Environmental Impacts Associated with In Situ Leaching of Ion-Adsorption Type Deposits
Effects on Ore Body and Lower-Lying Agricultural Areas
The in situ leaching practice requires less surface vegetation clearing than the previously practiced tank/heap leaching techniques, but increases the risks of mine collapses and landslides [11, 24]. In the leaching process, Ca and Mg cations situated “alongside” REE on the surface of the ore minerals are unintentionally replaced with ammonium ions, which causes soil nutrient depletion [24]. Sulfate ions contained in the leachate are also emitted into the ore body. In humid regions, sulfate ions are taken up by soils through three main mechanisms: immobilization through microbial activity in the top soil layer; where the ions are built into organic compounds, adsorption to the surface of soil particles, usually associated with the release of OH− or H2O ions to the solution, or precipitation of insoluble aluminum sulfates [37]. Adsorption is likely to be the most relevant mechanism in the section of the ore body from where the rare earths are extracted. Ion-adsorption deposits are often found in hilly landscapes, with surface areas of the deposits themselves generally unsuitable for agriculture. Due to high precipitation volumes in the area, ions are quickly washed out from the ore body itself. Ammonium emissions contribute to the eutrophication of nearby fields.
Effects on Ground- and Surface-Water Near the Mining Site
Both ammonium and sulfate ions affect freshwater ecosystems. High concentrations of ammonium have been reported for the ground- and surface-water near ion-adsorption deposits [11]. Vahidi et al. [12] and Yanfei et al. [32] highlight eutrophication associated with direct emissions of ammonium [12, 32]. According to ILCD, nitrogen emissions contribute to marine eutrophication, whereas freshwater eutrophication is limited by phosphor [38]. This is reflected in the recommended method pack, which only incorporates phosphor emissions for the freshwater eutrophication category. Terrestrial (soil) eutrophication takes into account N emissions to air only. N emissions to freshwater, ground, and marine waters (but not soil) are captured by the characterization factors for marine eutrophication. A quick review of the literature was undertaken to find out if these assumptions are valid in the Chinese context. Eutrophication is a problem in Chinese lakes, estuarine, and coastal regions [39,40,41]. According to the literature, the growth of algae is not always strictly limited by P in Chinese lakes [42]. Eutrophication assessments in Chinese lakes consider both N and P content; and significant regional differences of algae communities to different nutrients are reported [43]. According to Conley et al. [41] who assessed eutrophication alleviation in an international context, site-specific factors need to be considered, and a balanced approach to control both P and N is required [41]. Hence, the generic eutrophication category from CML,Footnote 2 which takes into account emissions of P and N to different soil, water, and air compartments, was used.
Effects of emissions from mining activities on surrounding water bodies are reported in the literature. Secondary (anthropogenic) salinization of rivers in mining areas is known to reduce aquatic biodiversity and disturb freshwater ecosystem functions, e.g., reducing the breakdown of organic matter [44, 45]. Sulfate ions in particular stimulate the microbial production of HS− ions, which are phytotoxic, and contribute to eutrophication (by inhibiting nitrification and by contributing to the release of phosphor) [45].
Heavy metals are mobilized from soils at acidic ph levels [46] —the pH of the leachate is around 3 [26]. According to one study, high levels of heavy metals have been reported in waste water from ion-adsorption deposits [11, 12]. Fe3+ and small amounts of Fe2+, Mn2+, and Pb2+ can be found in the leachates [25, 26]. Fe2+, Mn2+, and Pb2+ are toxic to humans and aquatic organisms. Fe3+ has a very low solubility at neutral ph and is therefore generally not considered a species of concern (although potential damage to fish eggs and gills has been reported [47]).
With ore grades getting decreased, the leaching recovery rates are getting lower, which means that higher amounts of ammonium salts are used and potentially emitted, exceeding emission standards by far [18, 19]. Research is being conducted in China to improve the recovery rates, and decrease chemical consumptions and emissions in the in situ mining process. One study has looked into additives which help make the lixivant (leachate) more hydrophilic and to help diffusion through the ores, which are characterized by small porosity and low permeability [18]. The permeability also poses a barrier to using more concentrated ammonium sulfate solutions (which might otherwise be a means to increase the recovery rates). Another study suggested the replacement of the precipitation step with a technique involving SX, ion exchange, and a liquid membrane [25]. Both options are said to improve the efficiency and reduce the environmental impacts of the process. Alternatives to ammonium sulfate reagents for the ion-exchange process are also a research topic in China [32].
Environmental Impacts Associated with the Solvent Extraction Process
After the in situ mining and processing stage, four extraction systems are currently combined in SX, resulting in high levels of chemical consumption and emissions [35]. It has been suggested to work with only one extraction system, namely P507, to enable the recirculation of the phases (“hyperlink process”), which would decrease chemical consumption and emissions during rare earth separation [35]. Water usage/emissions are above the required standards in current mining practice [35]. There is also an economic motivation to decrease water usage in the industry, due to the high expenditure [34]. Salt concentrations in the waste water are also an issue because the separation process is undertaken inland, and effluents are hence emitted to freshwater [34, 35]. Salinization negatively affects aquatic biodiversity [45].
Life cycle Assessment of Rare Earth Oxide Production from Ion-Adsorption Clay Deposits
Goal, Scope, Functional Unit, and System Boundaries
The goal of this study is to compile a dataset representative of the typical rare earth oxide production process from ionic deposits in Southern China which can be used in future studies. Due to uncertainties associated with the dataset, recommendations for further research are provided.
The functional unit of our dataset is the production of one metric ton of separated rare earth oxides after solvent extraction. The datasets reflects an estimate for a production situation in which all individual rare earth elements are separated. The functional unit corresponds to one metric ton of separated rare earth oxide with the rare earth composition of the ore, given in Table 1. (It is assumed that the full separation of Ho2O3, Tm2O3, Yb2O3, and Lu2O3 into individual elements is not conducted, due to the currently low demand for these elements). Separate inventories for individual rare earths are not presented, since this was not the aim of the study—see section "Beyond the Scope of this Study: Assigning Environmental Impacts to Individual Rare Earth Elements" on assigning impacts to individual REEs.
The system boundaries comprise in situ leaching from ion-adsorption deposit ores in Southern China, and separation into individual rare earth oxides, starting with the obtained concentrate. Upstream processes such as the production of chemicals or electricity used in the mining and separation process are approximated with generic datasets (see Tables 3 and 5). A description of the process is given in section "Production of Individual Rare Earth Oxides from Ion-Adsorption Deposits via In Situ Leaching and Solvent Extraction".
Life Cycle Inventory Analysis
The life cycle inventory was compiled from the literature and complemented with information obtained in expert interviews conducted in April and July 2016 (see Tables 3, 4, 5, and 6). The dataset comprises the mining and separation of the rare earth concentrate into individual rare earth oxides, and is presented both per metric ton of REE concentrate and per metric ton of separated REO (Fig. 3).
Vahidi et al. [12] present life cycle inventory data for in situ leaching of rare earths from ionic deposits, but do not include the production of individual rare earth oxides through solvent extraction (Fig. 3) [12]. This part to the life cycle inventory was added. It was largely based on material requirements and emissions reported in Chun-Sheng et al. [35]. Amendments to the data for the in situ leaching part are presented in section “Additions and Modifications to the In Situ Leaching Dataset Presented by Vahidi et al. [12]”.
Additions and Modifications to the In Situ Leaching Dataset Presented by Vahidi et al. [12]
Relating Low and High LCI Values to Ore Grades
Material and energy requirements are a function of ore grades. It was assumed that the high consumption figures presented in the dataset by Vahidi et al. [12] refer to the lower end of the economically viable ore grade, and the lower consumption figures to the higher end of the ore grade [12]. This is a rough assumption and not based on a quantitative model to describe the relationship between grades and material efficiencies.
Furthermore, the rare earth recovery rate is also a function of the ore grade (which is again related to the material and energy requirements). For the low impact estimate, the higher recovery rate is assumed, and vice versa. Mining is economically viable for concentrations of around 0.2–0.4% (Ding, pers. comm.). The reference unit of the output in Vahidi et al. [12] corresponds to 91% REO and 9% impurities (functional unit: 1 kg of 90–92% purity mixed REO) [12]. Of the 910 g REO per kg output, the metal content is 764 g. The (maximum) accessible fraction of rare earths in the deposits, adsorbed to the clays in ionic form, amounts to approximately 80% of total rare earth content [19]. We assume a 40–70% recovery of the accessible REE content for this dataset.
Elementary/Resource Flows
Estimates on resource flows, amount of tailings produced, and land occupation, were calculated based on the recovery rate and ore grade range assumptions, and ore density and geometry of the mined deposits described in the literature. An average composition of REE in ion-adsorption deposits is assumed, representative of the output from the major producing mines, and based on the most recent source (Table 1).
To account for resource depletion, the elementary flows are modeled for 100% of the ore content, since it is assumed that after mining, the remaining content is no longer viable to mine in the near future. The aluminosilicate fraction of the clay is approximated with the resource consumption of kaolinite—in practice, there are a variety of minerals present in the ores [48]. The quantity of drilling slurry, from which no rare earths are extracted, is estimated based on Yang et al. [11], who provide typical sizes of drilling holes and distances between them [11]. The area mined per metric ton of REO is estimated based on ore density and depth of the regolith layer [11, 24]. Estimates for the area required for the ponds and the storage for the excavated materials from the ponds and ore drillings were added.
Process Energy Consumption During In Situ Leaching
The estimates for electricity presented in the dataset by Vahidi et al. [12] are maintained. The authors state that the large range presented (0.5–5.3 MWh/t REO concentrate), which they have calculated from electricity costs for the mines reported in Chinese literature, is likely to be associated with the specific site conditions and the associated differences in the electricity requirements for the pumps. The energy consumption for heating of carbonates is likely to consume energy quantities of the same magnitude as the calcination step in the separation process. This figure is adjusted as follows: The energy carrier used for heating is assumed to be heavy fuel oil, which is commonly used in China for metallurgical processes [5]. Alternatively, natural gas or coal could be used. For the heating of the carbonates, filtering, and mechanical pressing, 6–7.5 MWh/t rare earth concentrates are estimated, based on discussions with industry experts. The energy consumption for site preparation is covered by the excavation dataset.
Precipitating Agents and CO2-Release from Precipitation
Ammonium bicarbonate is commonly used for the precipitation of rare earths before separation [12, 28]. It was therefore assumed that only ammonium bicarbonate is used in this step, and the dataset was adjusted accordingly to account for the associated material usage and emissions.
Chi et al. [26] provide a detailed analysis of the ammonium bicarbonate consumption during precipitation—(see also section “Removal of Impurities in Leachate (Through Selective Precipitation)”) [26]. Using the formulae given in Chi et al. [26], an ammonium bicarbonate consumption per t REO concentrate of around 3–4 metric tons was calculated for the impurity percentage reported in Table 2, as well as for an assumed double impurity content [26]. The estimate includes ammonium bicarbonate consumption as reagent, pH regulator, and reagent for carbonic acid decomposition for both impurity removal precipitation and rare earth precipitation. The consumption is influenced by the REE recovery rates during precipitation.
Direct CO2 emissions from precipitation of impurities and removal of carbonic acid, and heating of carbonates were added to the dataset. The quantities of CO2 emitted in the process depend on the ratio of impurities to REE, REE recovery rates, and the reagent used. Direct CO2 emissions are in the range of 1 t per metric ton of rare earth concentrate.
The actual composition varies. According to Jun [25], the leach liquor typically contains (NH4)2SO4 (~2000 mg/L), Al3+ (~1000 mg/L), and Ca2+ (~1000 mg/L), Fe3+ (~100 mg/L) [25].
Ammonium Sulfate Consumption and Emissions
The ammonium sulfate estimate given by Vahidi et al. [12] was interpreted as the ammonium sulfate weight, not the weight of the N content as presented in the dataset. This interpretation is based on the emission quantities given in the dataset. The net usage of ammonium sulfate is dependent on the recovery rate and ore grade, but most importantly on whether the leachate is captured and reused at another site when the mining operation ceases—see 3.2.5 and Fig. 1. The dataset given by Vahidi et al. [12] assumes that the waste water is emitted, with or without prior removal of NH4 + ions.
An extra column was added to Tables 3 and 4 which shows data for the reuse case. In the reuse situation, the NH4 + input during precipitation corresponds approximately to the magnitude of NH4 + losses to the ore body, suggesting a net usage of (NH4)2SO4 is could be low, or close to zero if infinite reuse was possible in practice, (which seems unlikely). As a rough estimate, one-third of the lower (NH4)2SO4 figure was assumed for the reuse situation. In all three modeled cases, approximately 20% of the leachate is lost in the ore, and some leachate is spent on ion exchange for the unintended extraction of impurities.
Emissions to soil have been calculated by Vahidi et al. [12] from the ion exchange Eq. (1), and relate to the corresponding quantity of rare earths extracted. Since NH4 + ions also exchange other metal ions, 30% are added for the emissions to soil for a rough estimate, based on the composition in Table 2. It is assumed that the differences between chemical input, NH4 + emissions, and leachate losses, and possible leachate reuse are emitted to water (it should be noted that it does not make a difference whether ammonium ions are emitted to soil or water in the CML baseline category ‘eutrophication, generic’).
Ammonium Bicarbonate—Consumption and Emissions
The ammonium bicarbonate consumption was calculated, based on the equations given in Chi et al. [26]—see also 3.2.3. The figures (3–4 t NH4HCO3 per metric ton REO) are in line with the figures given by Vahidi et al. [12] (2.2–4.5 t/t).
Estimate for Al3+ Emissions to Freshwater
As discussed in Removal of Impurities in Leachate (Through Selective Precipitation), one of the main impurities in the leachate is aluminum [27], a constituent of the clay minerals [16] which is unintentionally extracted in the ion exchange process. Since the process of impurity removal is not 100% effective, some of the ions remain in the leachate. It is, however difficult to derive estimates for Al emissions since the process is continuous, with changing conditions during the course of the mining operation. The leachate is continuously recirculated in the in situ leaching process, until the additional rare earth extraction becomes too low to make the mining operation worthwhile (Ding, pers. comm.)—i.e., the concentrations of rare earths and impurities decrease during the operation time of the mine. The ratio of individual elements extracted to the leachate is different for different ore grades, as higher quantities of impurities are extracted from lower ore grades, and also due to the geological variability of the deposits.
An estimate for Al3+ emissions was derived as follows: 95% of Al3+ ions are typically removed during precipitation before the concentrations get too low, i.e., 5% are potentially emitted [27]. The mass ratio between Al3+ and RE3+ ions is around 1:10 [26] to 1: 16 Al3+:RE3+ [27]. If the leachate is fully emitted to surface waters with the remaining 5% Al3+, this corresponds to 3–5 kg Al3+ ions per metric ton of REO concentrate.
Solvent Extraction—Transforming Rare Earth Concentrate to Individual REE Oxides
Energy and Material Efficiency in Solvent Extraction
The concentration of the input solution has an important influence on the process efficiency which can be achieved and affects the required size of the equipment [34], (Ding, pers. comm.). Rare earth loadings of ~180 g REO/l in the solvent or extractant are possible. The loading capacity depends on the system used.
The process can be targeted to the required output and purity [34]. The number of processing stages required in SX depends on the extractant used, which determines the separation factor between individual rare earth elements, and therefore the number of processing steps required [34]. As mentioned in section, "Environmental impacts associated with REO production", the use of different extraction systems for different parts of the system decreases the overall number of separation steps required (and process energy consumed by the mixer settlers per metric ton of output), but results in a higher consumption of reagents and consequently higher emissions per unit of output [35]. Another important factor which influences the energy consumption is whether and how much heating is required to achieve the required processing temperatures. To reach a purity for individual rare earths between 99 and 99.999%, ~30 to 100 separation stages are required to separate two groups of rare earths, or individual rare earths [34]. The separation of heavy rare earths is generally more complex than the separation of light rare earths, and associated with a larger number of processing steps [34].
Life Cycle Inventory Compilation
For the solvent extraction process which follows after in situ leaching, the inventory is based on the process described in Chun-Sheng et al. [35], which is commonly applied for the separation of rare earths from ionic deposits in China (see also [19]). The available information does not allow for a detailed analysis of the different aspects discussed in section “Energy and Material Efficiency in Solvent Extraction”, but represents an estimate.
The consumption of chemicals used in SX of REE separation from ion-adsorption deposits is based on Chun-Sheng et al. [35]. The source does not provide a detailed analysis of chemical usage; so estimates regarding the quantities of different reagents had to be made. Overall quantities of acid, neutralizing agent, water, and emissions are given in the document. For water usage and emissions, 30 m3/t REO was used as a lower estimate of consumption and emission (based on the Chinese emission standard), and 120 m3/t REO for the higher consumption and emission estimate (based on typical process values) [35]. The oxalic acid consumption and CO2 emissions arising from the calcination were estimated based on chemical equationsFootnote 3 (Eqs. 6 and 7). Oxalic acid is modeled as citric acid—(Table 5) (see also 12]). Besides oxalic acid, HCl is used in the SX process. The difference to the total acid consumption (~10.5 t) stated by Chun-Sheng et al. [35] is assumed to be HCl, and the neutralizing agent used in the process to be NaOH [35]. When comparing the overall input and output quantities for salts and acids stated in [35], it is possible that the input quantity for acid used during extraction refers to the diluted acid. The undiluted quantity is included as a high estimate; the diluted quantity is included as a low estimate for the acid consumption (HCl (36%) is assumed). The salt output is adjusted accordingly in the inventory. (However, it should be noted that the emissions of salts are not currently captured by the common impact assessment methods).
The organic phase (P507, kerosene, other extractants) is recirculated. The losses (net consumption) are estimated at 1 kg–20 kg/ton of REO [49] and (Ding, pers. comm.). According to the data presented by Vahidi et al. [49] and Schmidt [50]; the net consumption for the extractant is 30–35 kg/t REO for a different route. The impact assessment results from Vahidi and Zhao [49] for P204, a different extractant which is commonly used for the separation of light REE, indicates that the impact of the solvent losses is not negligible. The authors of this conference paper only present impact assessment results, not the LCI dataset itself. The environmental impacts are around 50 times of “solvent, organic at plant” for the global warming potential (GWP-100a), and in this range for most other impact categories. We created our own proxy dataset for P204, which is manufactured by reacting phosphorous pentoxide with 2-ethylhexanol (Eqs. 10 and 11). The production processes for P204 and P507 are similar, and the dataset for P204 is used here as a proxy for P507.
This is done by using the closest proxy datasets for the reactants available in ecoinvent. Phosphorus (process: market for phosphorus, white, liquid | phosphorus, white, liquid | APOS,Footnote 4 U) is used as precursor for P4O10. The oxidation of phosphorus is exothermic and therefore does not need any further energy input. 1-Butanol (process: market for 1-butanol | 1-butanol | APOS, U) is used as a proxy for butanal (the aldehyde form of butanol). The process yield was based on Li (2009) and own estimates: 45% for the last synthesis step to Di-(2-ethylhexyl)phosphoric acid, 80% yield for the hydrogenation and 80% yield for the aldol condensation. Process energy consumption and infrastructure was adopted from organophosphorus-compound production, unspecified | organophosphorus-compound, unspecified| APOS, U as an estimate. According to our calculation, impacts of the net solvent use are very small (<1.5% in each category and for each scenario).
During SX, electricity is used for stirring and pumping. The rare earths are first separated into groups of REE with similar properties which are then further separated into individual rare earth fractions. Individual RE fractions are then calcinated to obtain REO, which is commonly done with electric tunnel furnaces. Detailed information on off-gas composition was not available. The energy consumption for the full SX process is in the same order of magnitude as the consumption for the calcination process (Ding, pers. comm.). According to Talens Peiro [10], the energy consumed for SX is between 4 and 6 MWh/t REE [10]. 5 MWh/t REO are assumed for this study (Table 5).
Impact Assessment Results
The life cycle impact assessment results were calculated with the CML baseline methodFootnote 5 [51]. The modeling was done with OpenLCA 1.4.2 and ecoinvent 3.2 background datasets. Results are presented here for attributional background datasets with “APOS” allocation (Table 7). The resource depletion category was adjusted to include characterization factors for REE (see Table 8).
Impact assessment results are presented per metric ton of separated rare earth oxide for a low and a high estimate scenario, and a “leachate reuse” scenario (Table 7). (Results have not been allocated to individual rare earth elements.) The leachate reuse scenario provides similar results to the “low estimate” scenario. A lower input of ammonium sulfate per t REO concentrate, and adjusted sulfate and ammonium emissions, constitute the only differences made to the “low estimate scenario” life cycle inventory (see Tables 3 and 4). The largest difference at LCIA level appears in the generic eutrophication category, which indicates that the management of leachate emissions is crucial for the impacts in this category. Besides eutrophication, the other category impacts are slightly lower under the “leachate reuse” scenario, due to the partially avoided ammonium sulfate production.
CML Baseline and ADP Elements, Economic Reserves, with Characterization Factors for REE Added for ADP Elements, and Ultimate and Economic Reserves (see Table 8)
Contribution Analysis for Selected Impact Categories
A contribution analysis is shown for global warming potential (GWP 100a), eutrophication and abiotic resource depletion (ADP).
GWP 100a
The GWP-100a values for the production of 1t of individually separated REO produced through the analyzed route range from 34 to 58 t CO2 equ./t REO. A contribution analysis for the global warming potential (GWP100a) shows that both mining (concentrate production) and SX are important production stages, with around 31–39% of GWP-100a attributable to the production of the concentrate. Details can be found in the electronic supplementary material (Tables 1, 2, 3 of electronic supplementary material). Around 36–42% of the GWP-100a is down to process energy consumption, around 4–5% is from direct emissions from CO2 releases during heating (after precipitation) and calcination, and the remainder is from the upstream impacts of chemical inputs.
When compared to the production of REE from mineral deposits rich in light REE, the GWP100a values per metric ton of separated REO are higher for the production of REO from ion-adsorption deposits.Footnote 6 Values per metric ton of separated REO for the production of predominantly light rare earths from Bayan Obo are around 12–16 t CO2 equ./t separated REO after SX [5], or 23–35 t CO2 equ./t REO, according to a different study [9]. The ranges presented in [9] account for process- specific differences between light, medium and heavy rare earth production [9].
Eutrophication
The production of 1t REO, separated by individual REO, after SX, is associated with 0.5–1.7 t \({\text{PO}}_{4}^{-}\,{\text{equ.}}/{\text{t}}\) REO. 92% (leachate reuse scenario) to 96% (high estimate scenario) of this is attributable to the production of the RE concentrate. 90–95% of the total can be attributed direct process emissions of NH4 + to soil and freshwater. The CML baseline category ‘eutrophication, generic’ has the same characterization factors for ammonium ion emissions, regardless of the receiving compartment. The results in this category are significantly lower when the leachate is reused and/or wastewater emission standards are maintained.
Abiotic Resource Depletion
The impact category ADP elements, ultimate reserves (as implemented in OpenLCA method pack 1.5.5Footnote 7 in CML baseline, V.4.4.) was adjusted to include characterization factors for rare earth elements. The lack of some characterization factors for rare earths in ADP elements, ultimate reserves has previously been pointed out by Sutter and Merz [52] and Walachowicz et al. [53] who derived characterization factors for dysprosium and neodymium [52, 53]. The depletion potential is a function of the resources available, and the annual extraction rates [56]. For each element, the annual production figure is divided by the reserves squared; and the value is then divided by the corresponding ratio for antimony, which serves as reference substance for this impact category [38, 54].
To calculate the factors for ADP ultimate reserves, REE concentrations were adopted from USGS [55], in accordance with the figures for other elements [38]. The mass of the earth’s crust was taken from Guinée [56] (2.31 × 1022 kg). The rare earth production figures were adopted from JRCFootnote 8 [57] and correspond to the global production in the year 2012 (converted to metallic weights).
According to the impact assessment results (ultimate reserves, see electronic supplementary material, Tables 3 and 4), the rare earth elementary flows contribute with around 16–18% to the category; indicating that they are relatively abundant in the earth’s crust.
In addition, characterization factors were calculated for REE for “ADP, economic reserves” based on figures provided by Krishnamurthy et al. [19] and EC [57] for REE, and USGS [58] and van Oers [54] for reserves and annual extraction rates of antimony. Economic reserves constitute the part of the reserve base which can be economically extracted.Footnote 9 Known reserves are largely from primary deposits in which the REE are contained in minerals (~80% bastnaesite, 20% monazite) [19]. The composition of the reserve estimate reflects this.
Results for ADP, economic reserves, range from 57 to 115 kg Sb- equivalent per metric ton of separated REO. The rare earths contribute to “ADP, economic reserves” with 67% (high estimate scenario) to 77% (low estimate scenario) (see electronic supplementary material, Tables 5 and 6). The contribution from individual rare earths to this category does, however, not correspond to REE criticality: Thulium and lutetium dominate the contribution to this category at 47–54% for the two elements. These elements are among the geologically least abundant REE, but do not currently play an important economic role [57]. The issue that needs to be highlighted here is that the ADP factor by definition is based on annual extraction rates, rather than annual demand for the metals. In the case of thulium, holmium, lutetium, and ytterbium, the supply–demand imbalance is striking, with around 75 metric tons used and 1740 metric tons extracted annually [57]. These observations support previous observations by Adibi [59] who compared ADP values for REE production against those of copper production and highlighted that REE criticality was not reflected by the results. Criticality does, however, not strictly fit into environmental LCA, as discussed in [60].
Kaolinite contacted with the leach solution was included in the life cycle inventory, since it is not sure if the clays could still be used for other purposes after the RE mining operation. The result showed a contribution from kaolinite of 14% for the low estimate and 25% for high estimate scenario). Clay reserves are generally large [61].
Discussion, Conclusions, and Recommendations for Further Work
Main Findings
A life cycle inventory for the production of individual rare earth oxides from ionic deposits through in situ leaching and solvent extraction was compiled. The dataset can be used when assessing environmental impacts of various rare earth applications. It was based on data from the literature and help from rare earth industry experts. The in situ leaching data was largely adapted from Vahidi [12]. The system boundaries of our dataset cover the separation of the concentrate into individual oxides, and both steps are shown to contribute significantly to the LCIA results. Life cycle inventory data for the separation process was based on data representative of typical process consumption for solvent extraction in China, starting with concentrates from ion-adsorption deposits [35].
LCIA results, calculated with CML baseline, are presented per metric ton of separated REO. They refer to the process as a whole and are not yet allocated to individual elements. Marine, freshwater, and human toxicity are all associated with upstream chemical and energy production. Eutrophication is largely caused by direct ammonium emissions at the mining site. The GWP-100a for the production of 1t of individually separated REO produced through the analyzed route ranges from 34 to 58 t CO2 equ./t REO. Of this, ca. 30–40% is attributable to the production of the concentrate with the remainder going to the separation processes. When compared to the production of rare earths from deposits rich in light rare earths, the GWP-100a values per metric ton of separated REO from ion-adsorption deposits tend to be higher, with 12–35 t CO2 equ. per metric ton of separated REO after SX from deposits such as Bayan Obo [5, 9]. (Due to the differences in the output mix, the results are, however, not directly comparable).
The influence of ore grades and management practices in the mining process was discussed, and the associated impact on the LCI dataset were estimated. Decreasing ore grades and associated higher impurity-to-REE ratios in the leachate affect the consumption of ammonium bicarbonate used as a reagent during precipitation. This also affects the rare earth extraction efficiency and energy consumption per t of concentrate. The high eutrophication potential associated with ammonium emissions from in situ leaching (compared with REE production from deposits rich in light REE) has been highlighted by Vahidi [12]. The difference between the scenarios for production from ion-adsorption clays shows that this can be greatly reduced with appropriate management practices such as leachate reuse or waste water treatment. Leachate reuse also brings down the net consumption of ammonium sulfate.
Further Research Needs in Light of the Scope of this Study
Research Needs Regarding the Life Cycle Inventory
In order to better understand the additional requirement for precipitants and energy, it would be helpful to conduct further lab experiments to gain insights on the quantitative relationship between ore grades and precipitate consumption. Furthermore, it would be interesting to collect energy consumption data from mines of different ore grades and relate them to production output quantities. Further research is required to understand the fate of emissions from in situ leaching of REE in the proximity of the mining site, and how these link with different management practices such as wastewater treatment and management of overflow or leakage from pools. To better understand the environmental impacts associated with in situ leaching, site-specific environmental assessments are recommended.
For the solvent extraction, detailed information on the production routes for individual rare earths was not available. The analysis of the available data has shown that this processing stage is an important contributor to the overall impact and warrants more detailed investigations.
Research Needs Regarding the Quantification of Environmental Impacts Associated with Rare Earth Extraction
Some impacts to the environment could not be sufficiently quantified, either due to missing information required to derive a reliable life cycle inventory, or since they are not addressed by current impact assessment methods. Impacts reported in the literature, but not assessed by current LCIA methods include freshwater salinization, and associated impacts on ecosystems, risks of landslides, and the depletion of minerals (Ca, Mg) in the soil [11, 24]. Stream biodiversity is affected by ammonium sulfate emissions, and the elevated ph [11]. A detailed fate model for ammonium sulfate emissions was not available, which introduces some uncertainty to the LCI, but the emissions are said to pollute streams in the area long after mine operation is discontinued. Sulfate ions are converted to hydrogen sulfide by microorganisms, which is toxic to aquatic organisms [11], citing [62, 63].
The question of how to assess resource use in LCIA is the subject of an ongoing debate. The abiotic resource depletion category in CML (ADP) has not been developed to assess criticality, and it is debated whether this should be included in environmental LCA studies [60]. Methods which incorporate criticality assessment into LCIA are in development [64, 65]. The ADP category in CML relates annually extracted quantities to the overall availability of a specific resource in nature. It thereby only considers one out of three categories of resource criticality as defined by Graedel et al. [66], namely the supply risks. For REEs, the vulnerability to supply risks associated with their unique chemical properties; their importance in green applications, as well as the environmental impacts associated with their production constitute important criticality aspects. ADP characterization factors for REEs were calculated, which showed that annual extraction rates may not be a suitable measure for resource depletion in case of large supply–demand imbalances, such as in the case of thulium, holmium, lutetium, and ytterbium. Those elements are extracted as byproducts, but no large-scale commercial applications exist.
Beyond the Scope of this Study: Assigning Environmental Impacts to Individual Rare Earth Elements
In some cases, the environmental impacts of individual REE might be of interest. During SX, the rare earths are first separated into groups of rare earths with similar properties which are then further separated into individual rare earth fractions [35]. Hence, the processing routes for the production of individual rare earths are not equally long for each element, and the material and energy requirements could be separated out by element. Ideally, a model would be constructed for each pair of elements which is separated at the last step. The separation of co-occurring elements would only be included in the route to a point where they are partitioned off as groups, in accordance with the production system (Fig. 2). For example, to achieve a separation between Pr and Nd, it is necessary to separate the rare earth mix into La/Ce, Pr/Nd, and “others,” then La/Ce and Nd/Pr, and then Pr and Nd. However, the separation between Gd, Tb and Dy is not necessary to produce separated Pr and Nd, since they are separated off as a group in an earlier processing step. This procedure would be the preferred option according to ISO 14044, which states that the unit processes should be divided into subprocesses, and the input and output data relevant to these subprocesses should be collected if the environmental impact attributable to the individual process outputs are of interest [13]. It should be noted that this process structure, as shown in Fig. 2 is also simplified representation –the subprocessing routes can be crossed in practice [67]. Also, this process subdivision is different for different separation systems, which are different for different starting materials—see e.g., [19, 33].
Furthermore, the market values of individual rare earths differ notably due to the imbalance between demand and supply for individual rare earths caused by the coproduction situation. In order to denote the energy and material requirements associated with the separation process for each individual rare earth element, detailed process information would be required. However, to our knowledge, the information on the exact number of processing steps required for each element, and the values of semi-finished products (different rare earth concentrates) are not publicly disclosed.
If the solution described here is not feasible, the second best solution (for an attributional LCI dataset) is to apply economic allocation factors to individual elements (Table 1).
Conclusion
A dataset for the production of rare earth oxides from ion-adsorption deposits via in situ leaching and solvent extraction was compiled. The dataset was based on a previous study. To our knowledge, a dataset for this route including the separation step has not previously been published. However, it is of interest due to the increasing demand for heavy rare earths such as dysprosium, for which this is currently the most important production route. Our dataset includes the separation of rare earth concentrates into individual rare earth oxides, which is essential for many rare earth applications and, as our results show, not negligible in terms of its impact. This study helps to further the knowledge about rare earth production from ion-adsorption deposits. Despite the detailed study, data uncertainties associated with the dataset remain. Therefore, further research needs to be carried out, both regarding the life cycle inventory dataset and the impact assessment methods.
Notes
ISO: International Organization for Standardization.
CML: Centrum voor Milieuwetenschappen, (Institute of Environmental Sciences, Leiden University).
Assuming 10–30% above the theoretical consumption.
APOS: allocation at point of substitution (see website of the database provider for details - http://www.ecoinvent.org).
(version 4.4. of January 2015, as implemented in OpenLCA method pack 1.5.5, updated by Greendelta as described in [51]).
Please note that the figures are not directly comparable, since they refer to different processing routes for different types of ores, and produce different mixes of rare earth oxides.
See [51] for details on updates by Greendelta.
JRC: Joint Research Centre (of the European Commission).
The ILCD midpoint method, category ADP reserve base, contains characterization factors for rare earths, but they are generic for all REE (except for yttrium, which has been assigned a different factor) [54 ].
References
Waide P, Brunner CU (2011) Energy-efficiency policy opportunities for electric motor-driven systems: OECD/IEA. http://www.iea.org/Textbase/npsum/ee_for_electricsystemssum.pdf. Accessed 29 July 2015
Buchert M, Manhart A, Sutter J (2013) Untersuchung zu Seltenen Erden: Permanent magnete im industriellen Einsatz in Baden-Württemberg: Öko-Institut e. V., Freiburg
Schüler D, Buchert M, Liu R et al (2011) Study on rare earths & their recycling: Final Report for the Greens/EFA Group in the European Parliament, Darmstadt
Althaus H-J, Chudacoff M, Hischier R et al (2007) Life cycle inventories of chemicals: Ecoinvent Report No 8. Data V2.0, Dübendorf
Sprecher B, Xiao Y, Walton A et al (2014) Life cycle inventory of the production of rare earths and the subsequent production of NdFeB rare earth permanent magnets. Environ Sci Technol 48:3951–3958. doi:10.1021/es404596q
Koltun P, Tharumarajah A (2014) Life cycle impact of rare earth elements. ISRN Metall 1–2:1–10. doi:10.1155/2014/907536
Browning C, Northey S, Haque N et al (2016) Life cycle assessment of rare earth production from monazite. REWAS 2016: towards materials resource sustainability. The Minerals, Metals & Materials Society, Warrendale; Wiley, Hoboken. doi:10.1002/9781119275039.ch12
Graf R (2012) Ökobilanzielle Betrachtung von Seltenen Erden: Diplomarbeit. Universität Stuttgart, Stuttgart
Zaimes GG, Hubler BJ, Wang S et al (2015) Environmental life cycle perspective on rare earth oxide production. Sustain Chem Eng 3:237–244. doi:10.1021/sc500573b
Peiró LT, Méndez GV (2013) Material and energy requirement for rare earth production. JOM 65(10):1327–1340. doi:10.1007/s11837-013-0719-8
Yang XJ, Lin A, Li X-L et al (2013) China’s ion-adsorption rare earth resources, mining consequences and preservation. Environ Dev 8:131–136. doi:10.1016/j.envdev.2013.03.006
Vahidi E, Navarro J, Zhao F (2016) An initial life cycle assessment of rare earth oxides production from ion-adsorption clays. Res Conserv Recycl 113:1–11. doi:10.1016/j.resconrec.2016.05.006
DIN (2006) Environmental management—Life Cycle assessment—Requirements and guidelines (ISO 14044:2006) [Umweltmanagement—Ökobilanz—Anforderungen und Anleitungen (ISO 14044:2006)]; German and English version EN ISO 14044:2006
DIN EN ISO (2006) Environmental management—Life cycle assessment—Principles and Framework (ISO 14040:2006) [Umweltmanagement—Ökobilanz—Grundsätze und Rahmenbedingungen (ISO 14040:2006)]; German and English version EN ISO 14040:2006
Gupta CK, Krishnamurthy N (2005) Extractive metallurgy of rare earths. CRC Press, Boca Raton
Voßenkaul D, Stoltz NB, Meyer FM et al (2015) Extraction of rare earth elements from non-Chinese ion adsorption clays: Proceedings of EMC 2015
Papangelakis V (2014) Recovery of rare earth elements from clay minerals: ERES2014: 1st European Rare Earth Resources Conference. Milos, 04–07 Sept 2014
Tian J, Tang X, Yin J (2013) Enhanced leachability of a lean weathered crust elution-deposited rare-earth ore: effects on Sesbania gum filter aid reagent. Metall Mater Trans 44(5):1070. doi:10.1007/s11663-013-9871-3
Krishnamurthy N, Gupta CK (2016) Extractive metallurgy of rare earths, 2nd edn. CRC Press, Boca Raton
Haschke M (2016) In-situ recovery of critical technology elements: “SYMPHOS 2015”, 3rd International Symposium on Innovation and Technology in the Phosphate Industry. Procedia Engineering (138): 248–257
Packey DJ (2016) The impact of unregulated ionic clay rare earth mining in China. Resour Policy 48:112–116. doi:10.1016/j.resourpol.2016.03.003
Lartigue-Peyrou F (2016) Rare earth industry expert statement. Personal communication via e-mail, 6.07.2016
Navarro J, Zhao F (2014) Life-cycle assessment of the production of rare-earth elements for energy applications: a review. Front Energy Res. doi:10.3389/fenrg.2014.00045
Yanfei X, Zongyu F, Xiaowei H et al (2016) Recovery of rare earth from the ion-adsorption type rare earths ore: II. Compd Leach Hydrometall 163:83–90. doi:10.1016/j.hydromet.2016.03.016
Jun T (2011) Extraction of rare earths from the leach liquor of the weathered crust elution-deposited rare earth ore with non-precipitation. Int J Min Process 98:125–131. doi:10.1016/j.minpro.2010.11.007
Chi R, Zhou Z, Xu Z et al (2003) Solution-chemistry analysis of ammonium bicarbonate consumption in rare-earth-element precipitation. Metall Mater Trans B 34(5):611–617. doi:10.1007/s11663-003-0031-z
Luo X-P, Zou L-P, Ma P-L et al (2015) Removing aluminum from a low-concentration lixivium of weathered crust elution-deposited rare earth ore with neutralizing hydrolysis. Rare Met. doi:10.1007/s12598-015-0621-3
Packey DJ (2016) Question re. composition of ionic deposits given in paper. Personal communication via e-mail, 12.07.2016
Lin G, Zhang L, Yin S et al (2015) Study on the calcination experiments of rare earth carbonates using microwave heating. Green Process Synth. doi:10.1515/gps-2015-0040
Calvo G, Mudd G, Valero A et al (2016) Decreasing ore grades in global metallic mining, a theoretical issue or a global reality? ECI Conference (Abstract)—life cycle assessment and other assessment tools for waste management and resource optimization, Cetraro, Calabria
Jun T (2013) Process optimization on leaching of a lean weathered crust elution-deposited rare earth ores. Int J Min Process 119:83–88. doi:10.1016/j.minpro.2013.01.004
Yanfei X, Zongyu F, Xiaowei H et al (2015) Recovery of rare earths from weathered crust elution-deposited rare earth ore without ammonia-nitrogen pollution: I. Leaching with magnesium sulfate. Hydrometallurgy 153:58–65. doi:10.1016/j.hydromet.2015.02.011
Xie F, Zhang TA, Dreisinger D et al (2014) A critical review on solvent extraction of rare earths from aqueous solutions. Min Eng 56:10–28. doi:10.1016/j.mineng.2013.10.021
Leveque A (2014) Extraction and separation of rare earths. EREAN Summer School, Leuven
Chun-Sheng L, Fu-Xiang C (2016) Green separation of rare earth resources in China: State Key Lab of Rare Earth Materials Chemistry and Applications of Peking University; Minmetals (Beijing) Research Institute of RE Co., Ltd
Elwert T, Goldmann D, Schmidt F et al (2013) Hydrometallurgical recycling of sintered NdFeB magnets. World Metall 66(4):209–219
Sokolova TA, Alekseeva SA (2008) Adsorption of sulfate ions by soils (a review). Eurasian Soil Sci 41(2):140–148. doi:10.1134/S106422930802004X
EC-JRC (2010) International reference life cycle data system (ILCD) handbook—general guide for life cycle assessment—detailed guidance, 1st edn. Publications Office of the European Union, Luxembourg
Strokal M, Yang H, Zhang Y et al (2014) Increasing eutrophication in the coastal seas of China from 1970 to 2050. Mar Pollut Bull 85(1):123–140. doi:10.1016/j.marpolbul.2014.06.011
Le C, Zha Y, Li Y et al (2010) Eutrophication of lake waters in China: cost, causes, and control. Environ Manag 45(4):662–668. doi:10.1007/s00267-010-9440-3
Conley DJ, Paerl HW, Howarth RW et al (2009) Ecology. Controlling eutrophication: nitrogen and phosphorus. Science 323(5917):1014–1015. doi:10.1126/science.1167755
Yi Q, Wang X, Wang T et al (2014) Eutrophication and nutrient limitation in the aquatic zones around Huainan coal mine subsidence areas, Anhui, China. Water Sci Technol 70(5):878–887. doi:10.2166/wst.2014.293
Huo S, Ma C, Xi B et al (2013) Establishing eutrophication assessment standards for four lake regions, China. J Environ Sci 25:2014–2022
Linarić M, Markić M, Sipos L (2013) High salinity wastewater treatment. Water Sci Technol 68(2013):1400–1405. doi:10.2166/wst.2013.376
Cañedo-Argüelles M, Kefford BJ, Piscart C et al (2013) Salinisation of rivers: an urgent ecological issue. Environ Pollut 173:157–167. doi:10.1016/j.envpol.2012.10.011
Humsa TZ (2015) Impact of rare earth mining and processing on soil and water environment at Chavara, Kollam, Kerala: a case study. Proc Earth Planet Sci 11:566–581. doi:10.1016/j.proeps.2015.06.059
Vuori K-M (1995) Direct and indirect effects of iron on river ecosystems. Ann Zool Fenn 32:317–329
Tian J, Chi R-A, Yin J-Q (2010) Leaching process of rare earths from weathered crust elution-deposited rare earth ore. Trans Nonferrous Met Soc China 20:892–896. doi:10.1016/S1003-6326(09)60232-6
Vahidi E, Zhao F (2016) Life cycle analysis for solvent extraction of rare earth elements from aqueous solutions. REWAS 2016: Towards materials resource sustainability. The Minerals, Metals & Materials Society, Warrendale; Wiley, Hoboken, pp 113–120
Schmidt G (2013) Description and critical environmental evaluation of the REE refining plant LAMP near Kuantan/Malaysia—Radiological and non-radiological environmental consequences of the plant’s operation and its wastes. Report, prepared on behalf of NGO “Save Malaysia, Stop Lynas” (SMSL), Kuantan/Malaysia. https://www.oeko.de/oekodoc/1628/2013-001-en.pdf
Acero AP, Rodríguez C, Ciroth A (2017) LCIA methods Impact assessment methods in Life Cycle Assessment and their impact categories: 1.5.6. http://www.openlca.org/download/
Sutter J, Merz C (2015) Characterization factors for REE in LCIA method ADP elementary. Personal communication via e-mail, 7.1.2015
Walachowicz F, March A, Fiedler S et al (2014) Verbundprojekt: Recycling von Elektromotoren—MORE: Teilprojekt: Ökobilanz der Recyclingverfahren. Projekt gefördert im Rahmen des Programms “Schlüsseltechnologien für die Elektromobilität (STROM) des BMBF
van Oers L, de Koning A, Guinée J et al (2002) Abiotic resource depletion in LCA: improving characterization factors for abiotic resource depletion as recommended in the new Dutch LCA Handbook. Institute of Environmental Sciences, Leiden
Long KR, Van Gosen BS, Foley NK, Cordier D (2010) The principal rare earth elements deposits of the United States—a summary of domestic deposits and a global perspective. Department of the Interior, US Geological Survey, Reston
Guinée J (1995) Development of a methodology for the environmental life-cycle assessment of products: with a case study on margarines. Dissertation, Promotor: Udo, de Haes H.A.
EC (2014) Report on critical raw materials for The EU—critical raw materials profiles: For DG Enterprise and Industry
USGS (2016) Antimony. Statistics and Information. http://minerals.usgs.gov/minerals/pubs/commodity/antimony. Accessed 29 June 2016
Adibi N (2014) Introducing a multi-criteria indicator to better evaluate impacts of rare earth materials production and consumption in life cycle assessment. J Rare Earths 32(3):288–292. doi:10.1016/S1002-0721(14)60069-7
van Oers L, Guinée J (2016) The abiotic depletion potential: background, updates, and future. Resources. doi:10.3390/resources5010016
USGS (2016) Clays. Statistics and Information. http://minerals.usgs.gov/minerals/pubs/commodity/clays. Accessed 29 June 2016
Palmer MA, Bernhardt ES, Schlesinger WH et al (2010) Mountaintop mining consequences: American Association for the Advancement of Science. Sciencemag 327:148–149. doi:10.1126/science.1180543
van der Welle ME, Roelofs JG, Lamers LP (2008) Multi-level effects of sulphur–iron interactions in freshwater wetlands in The Netherlands. Ecol Effects Diffus Pollut 406(3):426–429. doi:10.1016/j.scitotenv.2008.05.056
Mancini L, Benini L, Sala S (2016) Characterization of raw materials based on supply risk indicators for Europe. Int J Life Cycle Assess. doi:10.1007/s11367-016-1137-2
Sonnemann G (2015) From a critical review to a conceptual framework for integrating the criticality of resources into Life Cycle Sustainability Assessment. J Clean Prod 94:20–34. doi:10.1016/j.jclepro.2015.01.082
Graedel TE, Harper EM, Nassar NT et al (2015) Criticality of metals and metalloids. Proc Natl Acad Sci USA 112(14):4257–4262. doi:10.1073/pnas.1500415112
Yan C, Jia J, Liao C et al (2006) Rare earth separation in China. Tsinghua Sci Technol 11(2):241–247. doi:10.1016/S1007-0214(06)70183-3
Acknowledgements
The research leading to results of this study has received funding from the European Community’s Seventh Framework Programme (FP7/2007–2013) under Grant Agreement No. 607411 (MC-ITN EREAN: European Rare Earth Magnet Recycling Network). This publication reflects only the authors’ views, exempting the Community from any liability. Project website: http://www.erean.eu. The authors would like to thank Solvay for enabling the expert interviews and for their help with the compilation of this dataset; Winfried Bulach, Bo Weidema, Lauran van Oers, Mikhail Tyumentsev, and members of the EREAN Steering Group, and two anonymous reviewers for valuable comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
The contributing editor for this article was Markus Reuter.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schulze, R., Lartigue-Peyrou, F., Ding, J. et al. Developing a Life Cycle Inventory for Rare Earth Oxides from Ion-Adsorption Deposits: Key Impacts and Further Research Needs. J. Sustain. Metall. 3, 753–771 (2017). https://doi.org/10.1007/s40831-017-0139-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-017-0139-z