Abstract
This study tried to investigate the effect of Co/SiO2 NPs on CO2 absorption in a single raising bubble column (20 °C and 1 atm). Co-doped SiO2 nanoparticles were first synthesized through the chemical vapor deposition (CVD) method, then several nanofluids, including different weight percentages of the synthesized NPs (0.001, 0.01, 0.02, 0.05, and 0.1 wt%) were prepared. Comprehensive experimental studies examined the effect of NPs concentration and nanofluid volume on CO2 absorption rate. The stability of nanofluids, as an affecting factor on nanofluid efficiency, was investigated over 10 days. It was tried to obtain mass transfer parameters, including Sherwood (Sh), and Schmidt (Sc) numbers, incorporating the CO2 diffusivity into the Co/SiO2 nanofluid. Results showed that increasing NPs concentration from 0.001 to 0.02 caused the CO2 absorption rate to reach a maximum point followed by a downward trend. Increasing nanofluid volume was not beneficial for increasing gas absorption, which is attributed to the fact that the predominant mechanism of CO2 absorption was the Brownian motion of NPs. Results confirmed that the prepared nanofluids had acceptable stability over 10 days, and the nanofluid (80 mL), including 0.02 wt% of NPs, had the maximum CO2 absorption, which was 28% more than the base fluid. Findings indicated that the magnitude of the CO2 mass transfer coefficient in the nanofluid was 1.953 * 10− 4 (m.s− 1), which was 1.89 times more than that for the base fluid. Finally, a comprehensive correlation (R2 = 0.99) was introduced to predict the CO2 mass transfer coefficient in the Co/SiO2 nanofluid.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carbon dioxide (CO2) absorption refers to the process of capturing and storing carbon dioxide from the atmosphere or industrial processes. It is a crucial component of mitigating climate change, as CO2 is a potent greenhouse gas that contributes to global warming (Åhlén et al. 2023; Giorgetta et al. 2013; Small et al. 2014). During the last decades, many ways introduced to remove and absorb CO2 from gas streams like using membranes (Jung, Lee, and Lee 2023; Hamalová et al. 2023; Fu et al. 2023), cryogenics (Kim et al. 2023; He et al. 2023; Meng et al. 2023), absorption (Gautam and Mondal 2023; Huhe, King, and Chuang 2023; Sharif et al. 2023), and adsorption (Skjervold et al. 2023; Al-Absi et al. 2023). Currently, absorption and adsorption methods have been developed to a practical stage but absorption methods are more common (Zhang, Borhani, and Olabi 2020; Ochedi et al. 2021; Fang et al. 2020). The absorption methods are divided into physical and chemical types of absorbents. Chemical absorption using amine series solutions is a mature and well-developed technology (Dutcher, Fan, and Russell 2015; Koytsoumpa, Bergins, and Kakaras 2018) but enormous costs for energy supply to regenerate solvent is a major drawback of this method (Wang et al. 2015; Raynal et al. 2011). Degradation of amine absorbents, side reactions, and corrosion are some other problems (Ünveren et al. 2017; Wu et al. 2020). Although the physical absorption is a relatively low CO2 recovery capacity, it is more suitable than the chemical methods for high-pressure systems such as the integrated gasification combined cycle (IGCC) (Ban, Keong, and Mohd Shariff 2014; Zhang, Borhani, and Olabi 2020).
There are two important vital factors for an ideal solvent to be used in CO2 absorption; high rate of reaction and high CO2 absorption capacity (Mota-Martinez, Hallett, and Mac Dowell 2017). The presence of nanoparticles (NPs) dispersed homogeneously in a base fluid, typically water or some other liquid, can form a stable colloidal solution namely “Nanofluid”. These nanoparticles can be a metallic, metal oxide, or carbon-based and typically have dimensions in the range of 1 to 100 nanometers. When these nanoparticles are added to the base fluid, they can significantly alter their properties, including their thermal conductivity, viscosity, and absorption characteristics (Tavakoli et al. 2022; Zhang et al. 2018; J.S. Lee, Lee, and Kang 2015). Several attempts have been made to use nanofluids as a suitable candidate for the physical absorption of CO2 due to the mass transfer enhancement (Mohd Rozaiddin and Lau 2022; Zhang et al. 2018; Hussin et al. 2023). The addition of nanoparticles to the base fluid can enhance the absorption of CO2 due to several properties of the nanoparticles, including their high surface area, high reactivity, and ability to form stable dispersions in the base fluid. Additionally, nanofluids can improve the overall efficiency of the CO2 absorption process by reducing the energy required to separate CO2 from other gases.
Over the past decades, many studies have been carried out to improve the mass transfer characteristics of nanofluids. For example, Kim et al. (W.-g. Kim et al. 2008) performed CO2 absorption experiments in a bubble-type absorber with water-based nanofluids using SiO2 nanoparticles. It was concluded that the addition of nanoparticles led to an increase in the total CO2 absorption by 24%. Jiang et al. (Jiang et al. 2013) found that the CO2 absorption rate could be enhanced by up to 8% with the presence of nanoparticles in comparison to pure monoethanolamine (MEA) solution. Pang et al. (Pang et al. 2012) studied the NH3/H2O bubble absorption performance with Ag nanoparticles and found that the absorption rate of a nanofluid including 0.02 wt% Ag NPs were enhanced as high as 55% compared to the base fluid. Pineda et al. (Pineda et al. 2012) measured CO2 absorption in a tray column absorber. They reported that the maximum absorption rate enhancements were 9.4% for Al2O3 and 9.7% for SiO2 nanoparticles. Nanoparticles also show a drastic enhancement in low-temperature CO2 absorption (Zarei and Keshavarz 2023; Lashgarinejad et al. 2023). Amaris et al. (Amaris, Bourouis, and Vallès 2014) studied the effects of carbon nanotubes on the performance of the NH3/LiNO3 absorber. They reported that the maximum enhancement in the absorption performance with carbon nanotubes (CNTs) was 1.64 and 1.48 times for cooling water at 40 and 35 °C, respectively. Lee et al. (J.W. Lee et al. 2016) examined a bubble absorber’s CO2 absorption performance enhancement. They reported that the CO2 absorption rate was enhanced up to 4.5% at 0.01 vol% of Al2O3 nanofluid at 20 °C, and 5.6% at 0.01 vol% of SiO2 nanofluid at 20 °C. Lee and Kang (J.W. Lee and Kang 2013) investigated enhancement in CO2 absorption of NaCl aqueous solution-based Al2O3 nanofluid. They measured the CO2 solubility in Al2O3/NaCl nanofluid for different Al2O3 concentrations and solvent temperatures. The results showed that the CO2 solubility enhancement ratios at 0.01 vol% of Al2O3 nanoparticle concentration, were 11.0% at 30 °C, 12.5% at 20 °C, and 8.7% at 10 °C.
Although several studies have shown that nanofluids can improve the performance of CO2 absorption compared to conventional fluids, the use of nanofluids for CO2 absorption is still in the experimental stage, and more research is needed to fully understand their potential. One advantage of the nanofluids is attributed to the high surface area of the nanoparticles, which provides more contact area between the CO2 and the fluid. This leads to faster reaction rates and higher CO2 absorption capacity. Moreover, the presence of nanoparticles can increase the mass transfer coefficient, which is the rate at which CO2 is transferred from the gas phase to the liquid phase. But nanoparticle concentration, nanoparticle size and shape, nanoparticle surface chemistry, fluid properties, temperature, and pressure are deciding factors in using nanofluids for CO2 absorption. The effectiveness of nanoparticles can be limited by issues such as aggregation and sedimentation.
Computational models can be used to simulate the absorption of CO2 using nanofluids. The computational models for simulating CO2 absorption using nanofluids typically involve solving the mass and energy balances for the system. The equations are solved numerically using a variety of techniques such as finite element analysis, finite difference methods, or computational fluid dynamics. The models can be used to predict the performance of different nanofluids for CO2 absorption under different conditions such as temperature, pressure, and concentration. The models can also be used to optimize the design and operation of absorption systems using nanofluids. Some of the key parameters that need to be considered in the computational models for CO2 absorption using nanofluids include the concentration and size of the nanoparticles, the properties of the base fluid, the temperature and pressure of the system, and the mass transfer coefficient between the gas and liquid phases. Until now, many modeling studies have been accomplished on CO2 absorption (J.-z. Jiang, Liu, and Sun 2017; Ansarian and Beiki 2022; Sodeifian and Niazi 2021). Jamali et al. (Jamali and Azari 2023), reviewed the numerical simulation of CO2 absorption columns using the computational fluid dynamics (CFD) strategy and investigated the application of different nanoparticles in various amine-based solutions and the effect of different packings in the packed bed absorption columns. Rashidi et al. (Rashidi and Mamivand 2022), examined the effect of NPs volume fraction, nanofluid flow rate, and temperature on the mass transfer coefficient for CO2 absorption by Al2O3-water nanofluid and concluded that the increase of nanoparticle concentration, temperature, and fluid flow rate favors the mass transfer coefficient. Generally, the modeling of carbon dioxide absorption can suffer from a few weaknesses such as assumptions, incomplete data, simplifications, and model validation, which may lead to inaccurate predictions or results.
In this study, SiO2 nanoparticles were first synthesized, followed by the doping of Co onto them. Next, their performance on CO2 absorption was investigated at 20 °C, which was a low and challenging temperature. In this regard, several concentrations of nanoparticles and volumes of the nanofluid were prepared and finally, the nanoparticle dispersion stability was evaluated by measuring the total CO2 absorption over a period of ten days. It also tried to predict the absorption rate and mass transfer coefficient by presenting a new comprehensive correlation. To the best of our knowledge, this is the first investigation of the absorption rate, molar flux, mass transfer coefficient, and diffusivity coefficient of CO2 into a Co/SiO2 water-based nanofluid in a single bubble column through a set of comprehensive experiments and precious correlation.
Experiments
Materials and instrumentations
Tetra epoxy silane (TEOS, 95%) and Bis(cyclopentadienyl)cobalt(II) (Cobaltocene, 98%) were purchased from Merck Company, Germany, and used to synthesize Co/SiO2 NPs. Deionized water was used to prepare and dilute nanofluids. All chemical materials were used as received without further purification.
To assess the size distribution of dry and dispersed nanoparticles in deionized water, the transmission electron microcopy (TEM) and dynamic light scattering (DLS) were, respectively, performed. The size of nanoparticles and their agglomeration were characterized using TEM images, taken from Hitachi, 9000 NA, Japan (Andrade et al. 2012). To do TEM tests, a dispersed suspension of NPs in ethanol (0.001 wt%) was sonicated using an ultrasonic bath Parsonic 30 S-400 W, 28 kHz, for 20 min, followed by placing on the graphite surface. Then, to remove ethanol from the samples, the samples were placed into a vacuum oven for 4 h. DLS measurements were performed by Malvern, Zeta Sizer Nano ZS, United Kingdom (Xu, Zhang, and Song 2003; Pham, Fullston, and Sagoe-Crentsil 2007). The zeta potential (ξ-potential) tests were accomplished using ELSZ-2000 (Otsuka Electronics Co., Osaka, Japan) to measure the stability and surficial electrostatic charges of NPs (Darvanjooghi and Esfahany 2016). ξ-potential accounts for the electrostatic charges of NPs, leading to repulsive forces among dispersed particles. NPs’ stability is known based on the positive and negative high ξ-potential, whereas low ξ-potentials indicate the tendency of NPS to agglomeration (Davoodi et al. 2016). A mass flow controller instrument (MFC, Brooks model, 1-888-554-flow, USA) was employed to inject CO2 into the nanofluids through the absorption setup. A CO2 sensor (Testo 535, Germany) was used to measure CO2 concentration in the outlet gases. To prepare nanofluids, a certain amount of the synthesized Co/SiO2 NPs were measured using a precise electric balance (TR 120 SNOWREX, Taiwan) and added to the water. The pH of the solutions was measured using a pH meter (PCE-PHD 1, UK). To prevent agglomeration of NPs, an ultrasonic processor (QSONICA-Q700, NY, USA) was utilized. A mechanical ball-mill (YKM-2 L, China) was used to grind the clustered NPs. A magnetic stirrer (IKA-10,038, Germany) was used to stirrer the solutions.
Methods
Synthesis of Co/SiO2 NPs
The SiO2 nanoparticles were synthesized through the chemical vapor deposition (CVD) method based on the methodology exposed by Dev et al. (Dev et al. 2021). Tetra epoxy silane was selected as the precursor of SiO2 nanoparticles. To do so, first, 0.05 g of this reagent was completely dispersed in 50 mL of acetone. Then, to promote CO2 absorption of SiO2 NPs, 12.0 wt% of cobaltocene was added to the acetone medium. The prepared solution was then transferred to the tubing furnace (800 °C) with a nitrogen flow rate of 10.0 mL min− 1. This process led to Co/SiO2 NPs with purple color.
Preparation of nanofluid
First, the nanoparticles were placed (4 h) in a ball mill device for the separation of the accumulated NPs. Then, nanofluids were prepared by dispersing a specific amount of the synthesized Co/SiO2 NPs in 80 mL of deionized water. A vast range of NPs concentrations ranging from 0.001 to 0. 1 wt% were examined in this study. Next, the prepared suspensions were stirred at 800 rpm for 5 h. Finally, the sonication process dispersed NPs in the nanofluid through three series of 20 min. the cycle time and amplitude of the sonication process were set on 0.5 s and 70%, respectively.
Experimental setup
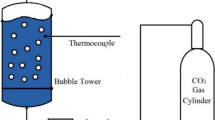
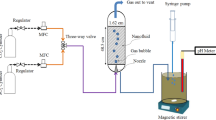
The experimental setup was consisted of a bubble absorber column, filled with the synthesized Co/SiO2 NPs loaded in the nanofluid. A specific volume of N2 and CO2 was sequentially injected into the nanofluid in the absorption column. Figure 1 illustrates the schematic diagram of the bubble absorber column which consists of a 170 mm height and 60 mm diameter Poly(methyl methacrylate) tube utilized as a semi-batch device to examine CO2 absorption by means of the nanofluid. To control the rate of feed gases, two regulators were placed in the outlet of the CO2 and N2 accumulators. CO2 was injected into nanofluids in the absorber column with the constant flow rate of 80 standard cubic centimeters per minute (SCCM) in each experiment. The gas flow rates were measured by two mass flow controllers (MFC) at the inlets of the absorber column. A gas diffuser was embedded at the bottom of the absorber column to produce a minimum bubble size ranged from 6.9 to 7 mm. The rising time of the bubbles was almost 2.3 s. The concentration of CO2 is accurately measured and recorded by the CO2 sensor at the outlet of the absorber column in equal time intervals. The temperature of the absorbent was automatically monitored and controlled during the absorption process using a set of thermocouples and thermostats.
Experimental procedure
To measure the CO2 absorption in the prepared nanofluid, first, pure N2 was injected into the absorber column for 1 min. Next, 80 mL of the prepared nanofluid was injected to the absorber column. The temperature of the absorber column was adjusted to 19 °C. N2 was reinjected again for 1 min to purge CO2 from the nanofluid. CO2 was injected into the nanofluid at 80 Sccm. CO2 concentration in the outlet gas stream was recorded every 3 s for 27 min.
Results and discussion
Nanofluid characterization
Figure 2 (a & b) illustrate the TEM (a) and DLS (b) images of Co/SiO2 NPs used for the nanofluid preparation. It was found that the average size of the synthesized nanostructures was 70 nm with a semi-spherical morphology without any special agglomeration (Shi et al. 2008).
The average size of NPs measured by the DLS test was equal to that of the TEM test, affirming no considerable agglomeration during NPs dispersion in the base fluid. Therefore, results confirmed that the method used in this study for NPs dispersion led to a well-dispersion of NPs.
Zeta-potential analysis was accomplished to evaluate the NPs’ stability in the nanofluid (áO’Brien 1990). Since the value of the ξ-Potential indicates the magnitude of the electrostatic repulsion among particles with similar charges and the synthesized Co/SiO2 NPs had a high ξ-Potential value of − 98.7 mV. Since the ξ-Potential of the synthesized NPs was lower than − 45 mV, it was confirmed that the synthesized NPs benefited from high stability (Faraji et al. 2010).
Absorption
Maximum absorption
Nanofluids were prepared with different NPs concentrations including 0.001, 0.01, 0.02, 0.05, and 0.1wt.% of NPs. The CO2 absorption was measured over a 27 min period. The experiments were repeated three times at each volume fraction of the synthesized NPs and the standard deviations were shown as the error bars. Figure 3 (a & b) exhibits the average CO2 molar flux into the Co/SiO2 nanofluid. Based on the results presented in Fig. 3 (a & b), the average CO2 molar flux increased with increasing Co/SiO2 NPs from 0.001 to 0.02 wt% while the molar flux decreased for higher NPs loadings (0.02 to 0.1 wt%). It can be concluded that the absorption molar flux of CO2 has a maximum value of 0.02 wt% of the synthesized NPs. Besides, it was found that Co/SiO2 NPs intensified the micro-convections and improved mass transfer rate in comparison with base fluid, thus, the initial increase in the absorption of CO2 would be reasonable with the aforesaid NPs mass fraction. However, increasing the number of NPs causes the viscosity of nanofluids to increase, thereby dominating the micro-convection impacts of NPs together with reducing the CO2 absorption within the nanofluid (Esmaeili Faraj et al. 2014; Darvanjooghi and Esfahany 2016).
Furthermore, Fig. 4 affirms the higher amount of CO2 absorption in nanofluids with different mass loadings of NPs than in deionized water. It was found that the maximum CO2 absorption enhancement in comparison to pure water is 28% at the nanofluid including 0.02 wt% of Co/SiO2 NPs.
Probing the rate of mass transfer
To find out the effect of absorbent volume on the CO2 absorption rate, 4 different volumes (80, 100, 120, and 150 mL) of the nanofluid were prepared while the concentration of the synthesized NPs was kept at the optimum mass fraction of 0.02 wt% at the temperature of 25 °C. Findings (Fig. 5) revealed that the absorption rate and mass transfer flux decreased with the enhancement in the volume loading of nanofluid. It can be attributed to the high height of absorbent in the vessel at a higher volume of nanofluid. Due to the forced and natural convection of the nanofluid under the specified geometry of the vessel, there is a wider diffusion regime at a higher height of absorbent than the smaller one. Since the Brownian motion of NPs and CO2/water mixing flow are two important factors affecting CO2 absorption, it was found that the effect of nanoparticles becomes weaker by forced or natural convection in a good mixing condition. As a result, the effect of the Brownian motion of NPs is in the reverse relationship with the height of absorbent, which is in good agreement with other findings (Samadi, Haghshenasfard, and Moheb 2014).
Absorption stability
The stability of nanofluid suspension is an important parameter, in determining the success of the absorption process. NPs suspended in the adsorbent are under the influence of the intermolecular repulsive force caused by the Coulomb force and the attractive force originating from the van der Waals force. [3]. Under this condition, nanoparticles may agglomerate, grow in size, and form greater clusters. It is highly likely that heavy clusters sediment due to gravitational force. Since the main reason for the mass transfer enhancement of nanofluid is the presence of NPs, their sedimentation causes the absorption efficiency to reduce. In this study, to evaluate the stability of Co/SiO2 nanofluid, a nanofluid was prepared based on the optimum condition (80 mL of nanofluid, 0.02 wt% of NPs, and 20 °C of absorbent temperature). After a 5 min sonication of the nanofluid, its stability (the rate of CO2 absorption over time) was tested over 10 days. Results were shown in Fig. 6. Since the rate of CO2 absorption versus time decreases with a completely uniform slope, which is related to the saturation of the nanofluid with CO2, no agglomeration process has occurred inside the nanofluid for 10 days. Therefore, it can be concluded that the prepared Co/SiO2 nanofluid is an acceptably stable absorbent.
Mass transfer coefficient
To calculate the mass transfer coefficient, different volumes of CO2 gas (20, 25, 30, 35, 40, 45, and 50 mL) were separately injected into the absorber column and then CO2 concentration and its molar flux were measured. Figure 7 illustrates the average molar flux versus the dissolved concentration of CO2 in the liquid bulk. Results confirmed the reduction of average molar flux by increasing CO2 bulk concentration, attributing to the decrease of mass transfer driving force. Besides, this observation followed a linear behavior. To evaluate this linear trend, the principal mass transfer equation (Eq. 1) was utilized and the experimental values were fitted to it.
where Kl represents the mass transfer coefficient (m.s− 1) at the liquid phase. CCO2 (mol.m− 3) and C*CO2 (mol.m− 3) show the bulk and gas-liquid interface concentration of CO2, respectively. It is worth mentioning that the observed CO2 concentration in the interface was accounted for by extrapolating the line fitted on the obtained experimental data. It raises from the assumption of linear pattern for gas concentration and molar flux. Results (Fig. 7) showed that the suggested model was acceptably fitted to the experimental data with an R2 value of 0.9855, confirming the high accuracy of the regression and low deviation of the model from the experimental data.
It can be concluded that the dashed line diagram (vertical plot) represents the observed concentration of CO2 at the liquid-bubble interface. Besides, the average molar flux diagram versus the bulk concentration of CO2 (diagonal plot) shows the gas absorption operating line. According to the slope of the operating line in Fig. 7, the relative mass transfer coefficient for CO2 absorption (Kl) using Co/SiO2 nanofluid was found to be 1.953 * 10− 4 (m.s− 1), which was 1.89 times more than that for the water alone (relative mass transfer coefficient). The CO2 absorption using nanofluid indicated higher values for relative gas concentration and relative mass transfer coefficient at the liquid-bubble interface.
Diffusivity Coefficient
Generally, the diffusivity of gases into a liquid has a significant impact on both the rate of gas absorption and the magnitude of the mass transfer coefficient. In this study, it was tried to benefit from Eq. 2 to obtain CO2 diffusivity into the Co/SiO2 nanofluid. Equation 2 presents raising a single bubble within a fluid according to Dankwert`s theory (Esmaeili-Faraj and Nasr Esfahany 2016; Zhao et al. 2003).
where the main factors of the model affecting the rate of mass transfer are the diffusivity of gas within a liquid (D), the thickness of the diffusion layer (δ), the radius of bubbles (r0), and the rate of surface renewal (s). Nave represents the CO2 molar flux (mol.m− 2s− 1) while CCO2 and CCO2,i are the CO2 concentration through the liquid bulk and at the interface of liquid-bubble (mol.m− 3), respectively.
Comparing Eqs. 1 and 2 results in Eq. 3, showing the mass transfer coefficient of a gas within a liquid based on a single bubble model:
In this study, Eq. 3 was used to estimate the CO2 diffusivity through the nanofluid. Based on Darvanjooghi et al. report, impressive factors of Eq. 3 like D, s, and δ violently depend on the size of NPs in the nanofluid (Darvanjooghi, Esfahany, and Esmaeili-Faraj 2018). They mentioned that the range of NPs` size was between 50 and 70 nm, the rate of surface renewal (s) was 6.85 mm.s− 1, and the thickness of the diffusion layer was 0.201 mm. In this study, on the one hand, the average size of nanoparticles was 70 nm, and on the other hand, the values of s and δ were assumed to be constant during the CO2 absorption, depending on only the mean diameter of NPs. Besides, the mass transfer coefficient of CO2 within the nanofluid was previously calculated. Therefore, Eq. 3 can be simplified to Eq. 4 as follows:
where s and δ were considered to be 6.85 and 0.201, respectively (Darvanjooghi, Esfahany, and Esmaeili-Faraj 2018). Equation 4 was solved according to the Newton-Raphson method as follows:
where \(\frac{{\partial F\left( {s,\delta ,{D_n}} \right)}}{{\partial {D_n}}}\) can be calculated based on the partial derivation of Eq. 4. Besides, the initial value of D0 was considered to be 10− 10.
Results illustrated that the diffusion values of CO2 into Co/SiO2 nanofluid and base fluid were 5.86 * 10− 9 and 2.12 * 10− 9 m2.s− 1, respectively.
Previous studies confirmed that only Brownian micro-convection and grazing effect are predominate mechanism for gas absorption into a nanofluid (Darvanjooghi, Esfahany, and Esmaeili-Faraj 2018; Esmaeili-Faraj and Nasr Esfahany 2016; Ashrafmansouri and Nasr Esfahany 2016; Ullah et al. 2023; Koo and Kleinstreuer 2005). In this study, it was found that Brownian mechanism serves an important role for the CO2 absorption into Co/SiO2 nanofluid. It is because CO2 molecules have not a strong polar structure and asymmetric molecular configuration to generate considerable molecular charges (O = C = O) to be absorbed on surface charge of nanoparticles. Therefore, increasing volume of nanofluid is not in the favor of more CO2 absorption because of lower micro-convections. It is worth mentioning that since Co/SiO2 NPs have a high magnitude of surface charge (Darvanjooghi and Esfahany 2016), attributing to the silanol bond formation (Si-O-H), the grazing effect could be another affecting mechanism with a low-intensity.
Correlation
Equation 6 calculates the mass transfer of a single bubble in a fluid (Vasconcelos, Orvalho, and Alves 2002). Equation 6 has been accepted as a precious correlation to predict gas absorptions into a vast range of liquids using a single bubble absorber column (Calderbank and Lochiel 1964).
To use Eq. 6 for the estimation of Sh number for the CO2 absorption into a nanofluid, some other physical properties are needed to obtain like kinematic viscosity, dynamic viscosity, and density of Co/SiO2 nanofluid (Mishra et al. 2014). These physical properties can be calculated according to Eq. 7 to 9 as follow:
where φ is the volume fraction of Co/SiO2 NPs in the base fluid and can be calculated from Eq. 10. µnanofluid and µbasefluid are dynamic viscosities of the nanofluid and base fluid, respectively. ρp and ρbasefluid present the bulk density of NPs (2.196 kg.m− 3) and density of the base fluid (1000 kg.m− 3), respectively.
Re, Sh, and Sc numbers were calculated from Eq. 11 to 13, respectively.
In these Equations, Ub is the rising velocity of the bubbles in the absorber column, which was considered to be 0.21 m.s− 1. Besides, db means the diameter of the bubbles, which was measured at almost 7 mm.
Table 1 shows the physical properties of Co2 absorption into Co/SiO2 nanofluid and base fluid.
Based on the summarized values in Table 1 and Eq. 11, there were no significant changes in Re number in the nanofluid or base fluid within the absorption i.e., νnanofluid ≈ νbasefluid. Therefore, it can be concluded that Re did not have a significant effect on the Sc number and followed a relative function according to below:
m and K were accounted for using a two-dimensional regression analysis upon the experimental data illustrated in Fig. 8. Based on the results, Eq. 15 was introduced for the physical parameters with R2 = 0.99. the following Equation can predict the Sh number for CO2 absorption into a nanofluid at Reb~1300.
Conclusions
In this research, Co-doped SiO2 nanoparticles were synthesized and used to enhance CO2 absorption in a single bubble column at 20 °C and 1 atm. Results confirmed that the prepared nanofluid had high stability with the ξ-potential lower than − 45 mV. TEM and DLS analyses represented the average size of the synthesized NPs was 70 nm. The results also confirmed that the NPs’ weight% and the volume of the nanofluid served important roles in the CO2 absorption rate in such a way that the optimum condition was achieved for 80 mL of the nanofluid including 0.02 wt% of NPs. Increasing NPs concentration from 0.001 to 0.02 favors CO2 absorption while the higher increase caused the absorption rate to decrease. Besides, CO2 molecules showed a better absorption rate in lower volumes of nanofluids. Indeed, it was found that although both the grazing effect and the Brownian motion of NPs served a crucial role in increasing CO2 absorption, the Brownian motion of NPs was the predominant mechanism. Moreover, mass transfer parameters affecting CO2 diffusivity into the Co/SiO2 nanofluid like Sherwood (Sh) and Schmidt (Sc) numbers were calculated. Finally, a new correlation was introduced to predict the Sh number over the Sc number in a gas-nanofluid column (Re ~ 1300) with a high accuracy of R2 = 0.99.
References
Åhlén M, Zhou Y, Hedbom D, Cho HS, Strømme M, Terasaki O, and Ocean Cheung (2023). Selective captureseparation of potent greenhouse gases with gallium-and vanadium-based metal-organic frameworks
Al-Absi, Akram A, Axelle Domin M, Mohamedali AM, Benneker, Nader Mahinpey (2023) CO2 capture using in-situ polymerized amines into pore-expanded-SBA-15: performance evaluation, kinetics, and adsorption isotherms. Fuel 333:126401
Amaris C, Bourouis M, Manel, Vallès (2014) Passive intensification of the ammonia absorption process with NH3/LiNO3 using carbon nanotubes and advanced surfaces in a tubular bubble absorber. Energy 68:519–528
Andrade ÂngelaL, José D, Fabris JD, Ardisson, Manuel A, Valente, José MFF (2012) “Effect of tetramethylammonium hydroxide on nucleation, surface modification and growth of magnetic nanoparticles.“ Journal of Nanomaterials 2012
Ansarian O, Beiki H (2022) Nanofluids application to promote CO2 absorption inside a bubble column: ANFIS and experimental study. Int J Environ Sci Technol 19(10):9979–9990
áO’Brien RW (1990) Electroacoustic studies of moderately concentrated colloidal suspensions. Faraday Discuss Chem Soc 90:301–312
Ashrafmansouri S-S, Mohsen Nasr Esfahany (2016) Mass transfer into/from nanofluid drops in a spray liquid‐liquid extraction column. AIChE J 62(3):852–860
Ban ZH, Keong LK, Azmi Mohd Shariff (2014) Physical absorption of CO2 capture: a review. Adv Mater Res 917:134–143
Calderbank PH, Lochiel AC (1964) Mass transfer coefficients, velocities and shapes of carbon dioxide bubbles in free rise through distilled water. Chem Eng Sci 19(7):485–503
Darvanjooghi MHosseinK, Mohsen Nasr Esfahany (2016) Experimental investigation of the effect of nanoparticle size on thermal conductivity of in-situ prepared silica–ethanol nanofluid. Int Commun Heat Mass Transfer 77:148–154
Darvanjooghi MH, Karimi MN, Esfahany, Seyyed Hamid Esmaeili-Faraj (2018) Investigation of the effects of nanoparticle size on CO2 absorption by silica-water nanofluid. Sep Purif Technol 195:208–215
Davoodi S, Mohammadreza M, Sadeghi M, Naghsh, Ahmad Moheb (2016) Olefin–paraffin separation performance of polyimide Matrimid®/silica nanocomposite membranes. RSC Adv 6(28):23746–23759
Dev A, Sardoiwala MN, Karmakar S (2021) Silica nanoparticles:: methods of fabrication and multidisciplinary applications. Functionalized Nanomaterials II. CRC Press, pp 189–206
Dutcher B, Maohong Fan, and, Armistead G, Russell (2015) Amine-based CO2 capture technology development from the beginning of 2013 a review. ACS Appl Mater Interfaces 7(4):2137–2148
Esmaeili Faraj S, Hamid MN, Esfahany M, Jafari-Asl, Nasrin Etesami (2014) Hydrogen sulfide bubble absorption enhancement in water-based nanofluids. Ind Eng Chem Res 53(43):16851–16858
Esmaeili-Faraj S, Hamid, Mohsen Nasr Esfahany (2016) Absorption of hydrogen sulfide and carbon dioxide in water based nanofluids. Ind Eng Chem Res 55(16):4682–4690
Fang M, Yi N, Di W, Wang T, Qinhui Wang (2020) Emission and control of flue gas pollutants in CO2 chemical absorption system–A review. Int J Greenhouse Gas Control 93:102904
Faraji M, Yamini Y, Rezaee MJJotICS (2010) Magnetic nanoparticles: synthesis, stabilization, functionalization, characterization, and applications. J Iran Chem Soc 7:1–37
Fu H, Xue K, Li Z, Zhang H, Gao D, Chen H (2023) Study on the performance of CO2 capture from flue gas with ceramic and PTFE membrane contactors. Energy 263:125677
Gautam A, Monoj Kumar Mondal (2023) Review of recent trends and various techniques for CO2 capture: special emphasis on biphasic amine solvents. Fuel 334:126616
Giorgetta MA, Johann Jungclaus CH, Reick S, Legutke J, Bader M, Böttinger V, Brovkin T, Crueger M, Esch, Fieg K (2013) Climate and carbon cycle changes from 1850 to 2100 in MPI-ESM simulations for the coupled model Intercomparison Project phase 5. J Adv Model Earth Syst 5(3):572–597
Hamalová K, Neubertová V, Vostiňáková M, V Fíla, and Z Kolská (2023) Amine-doped PEBA membrane for CO2 capture. Mater Lett 333:133695
He T, Liu Z, Son H, Gundersen T, Wensheng Lin (2023) Comparative analysis of cryogenic distillation and chemical absorption for carbon capture in integrated natural gas liquefaction processes. J Clean Prod 383:135264
Huhe FNU, Jaelynne King, Steven SCC (2023) Amine-based sorbents for CO2 capture from air and flue gas—a short review and perspective. Res Chem Intermed : 1–27
Hussin F, Aroua MK, Saidur R, Zaim Nor Rashid Zainol Nor Rashid (2023) Nanofluids for CO2 capture. Nanomaterials for Carbon Dioxide capture and Conversion Technologies. Elsevier, pp 89–135
Jamali M, Ahmad Azari (2023) A review on computational Fluid Dynamics Simulations of Industrial Amine Absorber Columns for CO2 capture. ChemBioEng Reviews 10(1):6–21
Jiang J, Zhao B, Cao M, Wang S, Zhuo Y (2013) Chemical absorption kinetics in MEA solution with nano-particles. Energy Procedia 37:518–524
Jiang Jia-zong, Liu L, Bao-min Sun (2017) Model study of CO2 absorption in aqueous amine solution enhanced by nanoparticles. Int J Greenhouse Gas Control 60:51–58
Jung W, Lee J, Jong Suk Lee (2023) New facile process evaluation for membrane-based CO2 capture: apparent selectivity model. Chem Eng J 460:141624
Kim Wun-gwi, Kang HU, Jung Kang-min, and Sung Hyun Kim (2008) Synthesis of silica nanofluid and application to CO2 absorption. Sep Sci Technol 43(11–12):3036–3055
Kim Y, Lee J, Cho H, Kim J (2023) Novel cryogenic carbon dioxide capture and storage process using LNG cold energy in a natural gas combined cycle power plant. Chem Eng J 456:140980
Koo J, and Clement Kleinstreuer (2005) Impact analysis of nanoparticle motion mechanisms on the thermal conductivity of nanofluids. Int Commun Heat Mass Transfer 32(9):1111–1118
Koytsoumpa E, Ioanna C, Bergins, Emmanouil Kakaras (2018) The CO2 economy: review of CO2 capture and reuse technologies. J Supercrit Fluids 132:3–16
Lashgarinejad A, Hosseini SS, Irani V, Mohammad H, Ghasemi R, Mohammadpour, Ahmad Tavasoli (2023) Enhancement of CO2 absorption and heat transfer properties using amine functionalized magnetic graphene oxide/MDEA nanofluid. J Iran Chem Soc : 1–14
Lee JW, Yong Tae Kang (2013) CO2 absorption enhancement by Al2O3 nanoparticles in NaCl aqueous solution. Energy 53:206–211
Lee JS, Lee JW, Yong Tae Kang (2015) CO2 absorption/regeneration enhancement in DI water with suspended nanoparticles for energy conversion application. Appl Energy 143:119–129
Lee J, Won IT, Pineda JH, Lee, Yong Tae Kang (2016) Combined CO2 absorption/regeneration performance enhancement by using nanoabsorbents. Appl Energy 178:164–176
Meng Y, Chen L, Yang X, Yang H, Mao Z, Chen S, and Yu Hou (2023) Spontaneous desublimation of carbon dioxide in turbo-expander applied for cryogenic carbon capture. Int Commun Heat Mass Transfer 140:106528
Mishra P, Chandra S, Mukherjee SK, Nayak, Arabind Panda (2014) A brief review on viscosity of nanofluids. Int nano Lett 4:109–120
Mota-Martinez, Maria T, Jason P, Hallett, Niall Mac Dowell (2017) Solvent selection and design for CO 2 capture–how we might have been missing the point. Sustainable Energy & Fuels 1(10):2078–2090
Ochedi FO, Yu JYuH, Liu Y, Hussain A (2021) Carbon dioxide capture using liquid absorption methods: a review. Environ Chem Lett 19:77–109
Pang C, Wu W, Sheng W, Zhang H, Yong Tae Kang (2012) Mass transfer enhancement by binary nanofluids (NH3/H2O + ag nanoparticles) for bubble absorption process. Int J Refrig 35(8):2240–2247
Pham KN, Damian, Fullston, Sagoe-Crentsil K (2007) Surface charge modification of nano-sized silica colloid. Aust J Chem 60(9):662–666
Pineda I, Torres JW, Lee I, Jung, Yong Tae Kang (2012) CO2 absorption enhancement by methanol-based Al2O3 and SiO2 nanofluids in a tray column absorber. Int J Refrig 35(5):1402–1409
Rashidi H, and Sajad Mamivand (2022) Experimental and numerical mass transfer study of carbon dioxide absorption using Al2O3/water nanofluid in wetted wall column. Energy 238:121670
Raynal L, Bouillon P-A, Gomez A, Broutin P (2011) From MEA to demixing solvents and future steps, a roadmap for lowering the cost of post-combustion carbon capture. Chem Eng J 171(3):742–752
Rozaiddin M, Aishah S, Kok Keong Lau (2022) “A review on enhancing solvent regeneration in CO2 absorption process using nanoparticles.“ Sustainability 14 (8): 4750
Samadi Z, Haghshenasfard M, Ahmad Moheb (2014) CO2 absorption using nanofluids in a wetted-wall column with external magnetic field. Chem Eng Technol 37(3):462–470
Sharif M, Wu HFanX, Yu Y, Zhang T, Zhang Z (2023) Assessment of novel solvent system for CO2 capture applications. Fuel 337:127218
Shi H, Liu F, Yang L, Han E (2008) Characterization of protective performance of epoxy reinforced with nanometer-sized TiO2 and SiO2. Prog Org Coat 62(4):359–368
Skjervold VT, Giorgia Mondino L, Riboldi, Lars ON (2023) “Investigation of control strategies for adsorption-based CO2 capture from a thermal power plant under variable load operation.“ Energy: 126728
Small R, Justin J, Bacmeister D, Bailey A, Baker S, Bishop F, Bryan J, Caron J, Dennis P, Gent, Hsiao-ming Hsu (2014) A new synoptic scale resolving global climate simulation using the Community Earth System Model. J Adv Model Earth Syst 6(4):1065–1094
Sodeifian G, and Zahra Niazi (2021) Prediction of CO2 absorption by nanofluids using artificial neural network modeling. Int Commun Heat Mass Transfer 123:105193
Tavakoli A, Rahimi K, Saghandali F, Scott J, Emma Lovell (2022) Nanofluid preparation, stability and performance for CO2 absorption and desorption enhancement: a review. J Environ Manage 313:114955
Ullah H, Shoaib M, Khan RA, Nisar KS, Muhammad Asif Zahoor Raja, and, Islam S (2023) “Soft computing paradigm for heat and mass transfer characteristics of nanofluid in magnetohydrodynamic (MHD) boundary layer over a vertical cone under the convective boundary condition.“ International Journal of Modelling and Simulation: 1–25
Ünveren E, Erdal Bahar Özmen Monkul, Şerife Sarıoğlan, Nesrin Karademir, and Erdoğan Alper. 2017. Solid amine sorbents for CO2 capture by chemical adsorption: a review. Petroleum 3 (1): 37–50
Vasconcelos JMT, Sandra P, Orvalho, Sebastião S, Alves (2002) Gas–liquid mass transfer to single bubbles: effect of surface contamination. AIChE J 48(6):1145–1154
Wang M, Joel AS, Ramshaw C, Eimer D, Nuhu MM (2015) Process intensification for post-combustion CO2 capture with chemical absorption: a critical review. Appl Energy 158:275–291
Wu Y, Xu J, Mumford K, Stevens GW, Fei W, Wang Y (2020) Recent advances in carbon dioxide capture and utilization with amines and ionic liquids. Green Chem Eng 1(1):16–32
Xu G, Zhang J, Guangzhi Song (2003) Effect of complexation on the zeta potential of silica powder. Powder Technol 134(3):218–222
Zarei F, Keshavarz P “Enhanced Co2 Absorption and Reduced Regeneration Energy Consumption Using Modified Magnetic Nanoparticles.“ Available at SSRN 4376289
Zhang Z, Cai J, Chen F, Li H, Zhang W, Wenjie Qi (2018) Progress in enhancement of CO2 absorption by nanofluids: a mini review of mechanisms and current status. Renewable Energy 118:527–535
Zhang Z, Tohid N, Borhani, Abdul GO (2020) Status and perspective of CO2 absorption process. Energy 205:118057
Zhao B, Wang J, Yang W, Jin Y (2003) Gas–liquid mass transfer in slurry bubble systems: I. Mathematical modeling based on a single bubble mechanism. Chem Eng J 96(1–3):23–27
Acknowledgments
The authors are grateful to Shiraz University for supporting this research.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Competing interests
This research received no external funding.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Heidari, S., Esmaeilzadeh, F., Rafati, R. et al. Experimental and modeling of CO2 absorption in a bubble column using a water-based nanofluid containing co-doped SiO2 nanoparticles. Model. Earth Syst. Environ. 10, 3229–3241 (2024). https://doi.org/10.1007/s40808-023-01869-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40808-023-01869-1