Abstract
This study monitored the concentration and distribution of the oxides of Cr, Ni, Cu, Zn and As in soils overlained by different lithological materials in southeast Nigeria. A 30 × 30 m spatial resolution of digital elevation model guided the selected eight soil profile pits dug for the study. A total of n = 27 soil samples were collected from the eight soil profile pits. Samples were air dried, and milled into 3–4 µm powder and analyzed using X-ray fluorescence. The result revealed that the oxides were irregularly distributed vertically, with the least heavy metal oxides obtained in the Ap horizons. Nevertheless, Cr2O3 was the most dominant and potentially toxic alongside NiO in Ishibori. Also, As2O3 was potentially toxic in most of the studied soils, with those in Ishibori as the most prone. Using multiple linear regression, the prediction was within the acceptable range (R2 > 0.50) except for As. For the prediction function of Cr, fine and coarse sand were negatively correlated with Cr, while CEC was positively correlated with Cu and Zn.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil health is the capacity of a specific kind of soil to function as a living system within a natural or influenced ecosystem and land-use boundaries to sustain plant and animal productivity, enhance water or air quality, and support human health habitation. Such soils maintain a diverse community of soil organisms that help regulate soil functions such as clean air, water, bountiful crops and forests, productive grazing areas and beautiful landscapes, and food security. Healthy soils must ensure co-existence between soil organisms and crops for healthy and optimum production. The terms soil health and soil quality are functionally synonymous and often interchangeably used within scientific communities. However, scientists prefer soil quality, while farmers prefer soil health (Harris and Bezdicek 1994). The use of soil health by scientists is on the rise as it implies a connection with soil biology.
Oxides of Cr, Ni, Cu, Zn and As are collectively called first main transition series. They are oxides of potentially toxic metals (Tóth et al. 2016). However, Cu, Ni and Zn are beneficial and essential for plant growth and development (Loftleidir 2005). Ions of these elements are regarded as micronutrients, especially when their concentrations are found within acceptable or threshold values for tolerance by crops and harmless to animals. Consequently, concentrations exceeding acceptable limits in the environment cause toxicity. This explains the guideline value. The content of heavy metals in the parent rocks translates to its natural content in the soils overlying them. However, heavy metals content in the surface soils is jointly influenced by soil-forming processes and pollution (McGrath 1995). Previous studies (Opaluwa et al. 2012; Oluyemi et al. 2008) had focused on bioaccumulation as the resultant impact of low amounts of metals in soils. Such metals originate mainly from their mobile components in dumpsites, quarries, oil-polluted areas or from parent materials to farmlands through leaching, runoffs and eventual uptake by crops.
Heavy metals occur naturally in the ecosystem and vary in concentration with location (Opaluwa et al. 2012), especially when influenced by anthropogenic sources or natural processes (Vare 2006; Singh et al. 2011). Such influence may negatively impact the environment through the food chain, underground sea-page, and biodegradability. An important natural source of contamination is geologic bedrock or substratum (Tchounwou et al. 2012).
Elements of the first main transition series are of important plant nutritional significance. Chromium, Cu and As (Duruibe et al. 2007; Shaheen et al. 2016), and Ni and Zn are of particular concern because of known detrimental health effects on humans through food consumption. For instance, a low concentration of Ni can stimulate growth in higher plants (Welch 1981), while a high concentration may result in Zn and Fe deficiency (Anderson et al. 1973).
Soil is a vital resource for sustaining a quality environment and food supply. Its fitness for agricultural purposes must be held in high esteem, especially when blanket and blind fertilizer application is practiced in agricultural areas. Unreported investigations on oxides of heavy metals in soils derived from diverse lithology in the study area complicate identifying potential problems resulting from agricultural practices. As crucial as heavy metals and their oxides are to the environment, there have not been many available studies on the concentration and contribution of metal oxides in the soil of Sub-Saharan Africa under different lithological materials.
The present research aims to evaluate the concentration, vertical distribution and potential risk of oxides of Cr, Ni, Cu, Zn, and As in farmlands by simple comparison with standard limits. The study also investigated the relationship between metals oxides and soil properties via a multivariate statistical method.
Materials and methods
Location, geology, and climate of the study area
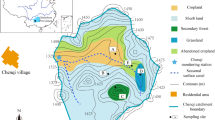
The research was conducted in Cross River State (5o32ʹ & 4o27ʹ N, 7o50ʹ & 9o28ʹ E), Southeast Nigeria. The selected study sites were Ishibori (06°39ʹ17ʹʹ N, 08°47'51ʹʹ E), Agoi Ibami (05°43ʹ27ʹʹ N, 08°10ʹ37.2ʹʹ E), and Mfamosing (05° 04ʹ'41.8ʹʹ N; 08° 27ʹ49.8ʹʹ E). The Sedimentary lithology of Cretaceous and Tertiary ages in the area is associated with shale, siltstone, and fine-grained sandstone (Ofem et al. 2020). They are common in the Ikom depression (Mamfe Rift) and Calabar flank.
Tropical humid climate with distinct wet and dry seasons characterize the study areas. The Ishibori area is moist sub-humid and characterized by southern guinea savannah. At the same time, Agoi Ibami and Mfamosing are mainly moist, humid to per humid and are found in the tropical rainforest. Rainfall varies between 1251.4 and 3347.8 mm/annum in Ishibori, while a range of 1760.3–3770.8 mm/annum is typical of Agoi Ibami and Mfamosing. The temperature of 22.96-33.75 °C characterizes Ishibori, while 22.56–31.95 °C was reported for Agoi Ibami and Mfamosing (Sambo et al. 2016a). Mean relative humidity in Ishibori was reported as 72.14 %, with a mean evaporation rate of 2.24 mm/day and a range of 1.8–2.8 mm/day. In Agoi Ibami, a mean value of 81.71 % was reported for relative humidity, while the evaporation rate had a mean of 3.61 mm/day with a range of 2.9–5.0 mm/day. In Mfamosing, 7.92–80.75 % and 84.99 % were obtained as range and mean, respectively, for relative humidity, while the evaporation rate had a range of 0.9-3.0 mm/day (Sambo et al. 2016b).
Field and laboratory procedures
Google satellite imagery, geology maps and digital elevation models (DEMs) (at 30 m × 30 m spatial resolution) of Ishibori, Agoi Ibami and Mfamosing in Southeast Nigeria were obtained. DEMs were obtained from United States Geological Survey Explorer SRTM 1 (Shuttle Radar Topography Mission). The elevation ranges of the study areas were delineated from high to low, and one soil profile pit was sited along each slope range. Soils over shale-limestone-sandstone formation (SLS), limestone-sandsone formation (LS) and alluvium (ALV) were studied across locations. Consequently, IH1P1, AI1P1 and MF2P1 (SLS), AI2P2 and MF1P1 (LS), and IH2P2, AI3P1 and MF3P2 (ALV) were randomly sited and dug to represent the eight (8) soil units (Fig. 1). A total of 27 composite soil samples were used for the study.
Composite soil samples were obtained from pedogenic horizons (bottom to top) and processed for the determination of bulk density (undisturbed core method), particle size distribution (Bouyoucos hydrometer), soil pH (H2O), organic carbon (Walkley–Black modified acid–dichromate) and cation exchange capacity by neutral NH4OAc using the standard procedures (Soil Survey Staff 2014). An automatic mill was used to pulverize and homogenize air-dry soil samples into a fine powder (3–4 µm). The powder samples were analyzed with the 2019 version of XRF Delta Premium Spectrometer (Tejnecky et al. 2015). The oxides were converted to their elemental forms using a standard conversion excel sheet. This was done for easy rating of the oxides.
Modeling approach
Multiple linear regression (MLR) was performed in the StatView environment to model the prediction of heavy metals using mineral particle sizes, organic carbon and CEC. The MLR model is expressed thus:
where metal is the response variable, represented by Cr, Ni, Cu, Zn or As. \(x_{1}\), \(x_{2}\)… xk are predictor variables; coarse sand (CS), fine sand (FS), silt, clay, organic carbon (OC) and CEC. ∊ is the residual term of the model and βo is regression intercept, while βx represent regression coefficients.
Test for significance
Ho: β1 = βk = 0; Ha: βj ≠ 0; for at least one j.
Fvalue (calculated) > Fstat; reject null hypothesis.
Model adequacy
Root mean square error (RMSE), and coefficient of determination (R2) were used to evaluate the performance of the models. A lower value of RMSE is preferred, while values of R2 closer to 1 are preferred such that; R2 ≥ 0.75 is the best and R2 ≥ 0.50 – 0.75 is acceptable prediction (Li et al. 2016).
Statistical analysis
Statistical analyses and model computations were performed using StatView version 5.0.1.
Results
General soil characteristics
The general soil characteristics are presented in Table 1. The soil particle size distribution is dominated by the sand fraction (fine sand dominated the total sand fraction). Soil bulk density was less than 1.6 g/cm3 and presented soils which will encourage root proliferation, air and water movement and without hard-pans. Soil pH ranged between 5.1 and 7.2 in the entire soils with the highest mean value of 6.2 occurring in IH1P1, AI2P2, and MF3P2. Soil pH values indicated strongly acid and neutral reactions. Low soil pH values (4.5–5.5) indicate the presence of significant amounts of exchangeable Al3+, which becomes insoluble when silica is soluble and leachable at higher pH. Organic C was rated low (< 10 g/kg) to very high (> 20.0 g/kg) in the entire soils. Organic C in soils over alluvium was quite high compared to the corresponding soils in the uplands of similar locations. This indicates the dominance of accumulation over the decomposition of organic matter in poorly drained soils. Cation exchange capacity (NH4OAc) varied with soil depth and lithology and varied from means of 7.7 in AI2P2 and 44.5 cmol/kg in MF2P1. The values were low (< 12 cmol/kg), moderate (12–25 cmol/kg) or high (> 25 cmol/kg). High CEC indicates the high capacity of the soils to retain cations.
Concentration and distribution of the metals
Oxides of the metals obtained by XRF as well as their metal equivalents are presented in Table 2. The distribution of the oxides in soil profiles is presented in Fig. 2 and indicates irregular distribution with soil depth. However, NiO in IH1P1 and IH2P2 showed a regular decrease with soil depth. The results showed that the minimum mean concentration of Cr2O3 was 33.2 in AI3P1 and maximum value of 176.4 mg/kg was obtained in the Ap horizon of IH1P1 (Fig. 2). Mean of 11.0 was recorded in MF3P2 and 86.0 mg/kg in IH2P2, while NiO recorded means of 11.0 and 86.0 mg/kg in MF3P2 and IH2P2, respectively. The concentration of CuO was lowest in AI3P3 with an average value of 9.3 and highest in the Ap horizon of IH2P2 with an average concentration of 35.4 mg/kg. ZnO concentration ranged from mean of 17.0 in AI3P1 to 99.0 mg/kg in MF2P1. Amongst the oxides, As2O3 recorded the least values with means of 4.7 in MF3P2 and 10.6 mg/kg in IH1P1. The oxides in the studied soils indicate irregular trends with soil depth without clear and consistent deposition at any particular depth.
Prediction of the metals via a linear regression (MLR)
The multiple linear regression functions describing the relationships between the Cr, Ni, Cu Zn and As with soil properties are presented in Table 3. The result revealed that r2 for Cr equation was 0.65 (Eq. 2) and could explain Cr variation in the study area, with coarse sand and fine sand being represented as the most important variables in the prediction. Ni model produced r2 = 0.66 with organic carbon being the only important variable. Furthermore, the r2 values for Cu, Zn and As are 0.61, 0.50 and 0.46, respectively with decreasing preference for acceptance. All the models produced acceptable functions except for As which yielded r2 value of less than 0.5. Such values are unacceptable for prediction (Li et al. 2016).
Discussion
Concentration and potential risk of the metals
Chromium oxide
Values of Cr oxide in IH1P1, MF2P1 (SLS) and IH2P2 (ALV) exceeded the world-wide average of 42 mg/kg (Kabata-Pendias and Pendias 1999), as well as the threshold limit of 100 mg/kg. The values were within the guideline value of < 200 mg/kg (MEF 2007) in IH1P1 (SLS) and IH2P2 (ALV). The values in the affected soils also exceeded acceptable limit of 100 mg/kg (Stêpniewska et al. 2001). Soils overlying SLS may have impacted the adjoining low elevation soils of ALV (IH2P2) with Cr2O3 via leaching and surface runoff as shales have been identified as hosts to Cr (Frank et al. 2020). The distribution of Cr2O3 is quite erratic in the soil profiles as it varied between horizons and lithology and may be attributed to the sedimentary lithology. Cr2O3 concentration classifies the soils as potential ecological threats. Such elevated concentrations may be toxic to plants, especially if it emanates from industrial wastes or spills (Avudainayagam et al. 2003). Rates exceeding 3000 mg/kg have resulted in toxicity and yield reduction (Naidu et al. 2000) and inhibit the activities of soil microbes (Megharaj et al. 1999) thereby facilitating the accumulation of organic matter.
Nickel oxide
The concentration of NiO in the soils was less than 50 mg/kg recommended as threshold value (MEF 2007). However, in IH1P1 (SLS) and IH2P2 (ALV) where concentrations were between the threshold and guideline values, NiO may be a potential threat to the soil system. By the world-wide average (WWA) of 18 mg/kg (Kabata-Pendias and Pendias 1999), values of NiO in IH1P1, IH2P2 and MF2P1 were in excess and may be a threat to the ecosystem. However, AI1P1 (SLS) and AI2P2 (LS) in the Yakurr area may require Ni fertilizers, especially if N-fixing crops are advocated. In the soils over ALV, the below threshold levels may induce essential biochemical and physiological reactions in plants (Nagajyoti et al. 2010). The findings of this study are in agreement with earlier studies that reported concentrations of less than 86–100 mg/kg (McGrath 1995). Ni concentration in the soils may be traced to the parent material, especially its low content in the alluvial soils (Cempel and Nikel 2006) or the use of phosphate fertilizers in the agrarian area. The direct relationship between NiO, and organic C and CEC in the Ni function indicates its increasing concentration with an increase in these parameters. Similar findings have earlier been reported (Suavé et al. 2000; Tye et al. 2004). NiO varied irregularly between soil horizons in the sedimentary lithologies and negates the uniform distribution of Ni (Cempel and Nikel 2006).
Copper oxide
CuO concentration in the studied soils was below the threshold value of 100 mg/kg (MEF 2007). However, values in IH1P1, MF2P2 (SLS), AI2P2 (LS) and IH2P2 (ALV) exceeded the world-wide average of 14 mg/kg (Kabata-Pendias and Pendias, 1999) and may be a threat to the environment. Low levels of Cu2+ may enhance photosynthesis (Mahmood and Islam, 2006), though the critical values for the toxicity of Cu vary from one crop to another. For instance, 25 mg/kg is pegged for cabbage leaf (Hara et al. 1976), while 17–21 mg/kg has been reported for lettuce leaf (Davis and Beckett, 1978). In any case, the studied soils were within acceptable range for lettuce and cabbage production, however, values in MF2P1, IH1P1 (SLS) and IH2P2 (ALV) were above the range. Consequently, Cu toxicity is a potential threat to IH1P1. Its positive correlation with organic C and CEC and the studied oxides imply interdependence. High organic matter favours high CEC and heavy metal sorption in soils (Kabata-Pendias 2007), while excess Cu in soils is toxic to plants and humans, and affect photosynthesis, pigment synthesis, protein metabolism, membrane integrity (Yang et al. 1995) and decreases shoot fresh weight (Yang et al. 2002). Copper oxide seems regular with higher concentrations in the B horizons.
Zinc oxide
ZnO concentration in the studied soils is within acceptable threshold limits of < 200 mg/kg (MEF 2007) and less than the WWA of 62 mg/kg (Kabata-Pendias and Pendias, 1999) except in MF2P1. Natural background concentration of total Zn in soils is 10–300 mg/kg (WHO 2001), while in the Netherlands, the critical limits of Zn in soils is 720 mg/kg (NMHPPE, 1991). Values obtained in this study were either within or less than the above limits, and the soils are said to be safe in terms of Zn contamination. Furthermore, a direct positive correlation between Zn and CEC as shown in the Zn function (Eq. 5) insinuates its adsorption and bonding to the soil exchange complex when the surface area of the soils is enhanced. Soils high in organic matter have higher adsorptive capacity and bonding for Zn (Chukwuma et al. 2010). Similarly, Zn availability in soils is affected by organic matter, CEC, and clay (Okafor and Opuene 2007; Nabulo et al. 2008). The somewhat low total Zn in the soils may have been due to its interaction with organic ligands. Zinc oxide also seems to be more concentrated in the transition B and C horizons but partly irregularly distributed with soil depth.
Arsenic oxide
The concentration of As2O3 was in excess of the threshold value of 5 mg/kg, but less than the guideline value of 50 mg/kg (MEF, 2007), and above the WWA of 4.7 mg/kg (Kabata-Pendias and Pendias 1999) in IH1P1, IH2P2, AI1P1, AI2P2 and MF2P2. Such values are most likely to pose a threat to the ecology. However, in AI3P1, MF1P1 and MF3P2 values were less than the threshold value and tolerable for crop production. These values are within soil natural levels of 1–40 mg/kg (Zandsalimi et al. 2011). The levels may be increased by the use of pesticides and fertilizer application (Nagajyoti et al. 2010). Natural background concentration of As2O3 in agricultural soils was also stated as 5 mg/kg (Mandal and Suzuki, 2002), while the EU recommended a maximum of 20 mg/kg (Leonard, 1991). Values of As2O3 in all the studied soils were equal to or above the limits, an indication that aeolian addition, pesticide or fertilizer application may have increased its concentration in the soils. The values are less than 10 mg/kg reported in Finland (Makela-Kurtto et al. 2007) and 3.2 mg/kg in Sweden (Eriksson et al. 1997). In the present study, As2O3 has a direct positive relationship with clay, and a poor correlation with organic matter. Values below threshold limits are likely to induce essential biochemical and physiological reactions in plants.
Relationship between metals oxides and soil properties via a MLR
Increasing toxicity of Zn as a result of the addition of Cu is described as the most consistent effect on plant growth (Luo and Rimmer 1995). The amount of Zn and Cu adsorbed depends on organic matter, clay minerals, metal oxides, and hydroxides (Ross 1994). Increasing soil CEC by raising soil organic matter and the surface area of clay minerals, invariably increases Zn and Cu concentration. The R2 values of Cu and Zn indicate that the models explain 0.61 and 0.50 of the variability, respectively.
Important correlations did not exist between arsenic and any of the predictor variables and contradicted previous studies (Martín et al. 2011; Romero-Freire et al. 2014). According to these authors, the solubility and toxicity of As is controlled by soil properties. The low adjusted R2 value indicates that any additional input variables are not likely to add value to the model. This negates the earlier presentation of high adjusted R2 (> 0.50) for Cr, Ni, and Cu, indicating that additional input variables will add value to the model.
The p- values of the ANOVA tables suggests a good fit model for predicting Cr, Ni, Zn and Cu compared to the mean value. Similarly, MLR models predicting Cr, Ni, Zn and Cu had R2 values within 0.50 and 0.75 and are within the acceptable limits described by Li et al. (2016).
Conclusion
The oxides were irregularly distributed spatially and vertically with soil depth. The Ap horizons have the least value of all the oxides except in soils influenced by shale-limestone-sandstone (SLS) lithology, which had a reverse or irregular trend. Nevertheless, Cr2O3 was the predominant and potentially toxic oxide alongside NiO in soils underlain by SLS. Furthermore, the arsenic oxide was potentially toxic in most of the studied soils, with soils over SLS emerging as the most prone. This has placed the soils on the watch list for Cr2O3, NiO and As2O3 toxicity. The prediction functions were all important except that of As2O3. For the prediction function of Cr, fine and coarse sand were negatively correlated with Cr, while CEC was positively correlated with Cu and Zn. Similarly, organic carbon was positively correlated with Ni.
Highlights of the study
-
Arsenic oxide was potentially toxic, while NiO and Cr2O3 were a threat in the soils influenced by shale-limestone-sandstone formation in Ishibori.
-
Positive and significant relationship was obtained from the Cu and Zn functions against CEC, while fine and coarse sand correlated negatively with Cr. Organic C correlated positively with only Ni (p < 0.05).
References
Anderson JG, Mayer DR, Mayer RK (1973) Heavy metal toxicity levels of nickel, cobalt and chromium in the soil and plants associated with visual symptoms and variation in growth of an oat crop. Aust J Agric Res 24:557–571
Avudainayagam S, Megharaj M, Owens G, Kookana RS, Chittleborough D, Naidu R (2003) Chemistry of chromium in soils with emphasis on tannery waste sites. Rev Environ Contam Toxicol 178:53–91
Cempel M, Nikel G (2006) Nickel: a review of its sources and environmental toxicology. Pol J Environ Stud 15:375–382
Chukwuma MC, Eshett ET, Onweremadu EU, Okon MA (2010) Zinc availability in relation to selected soil properties in a crude oil polluted eutric tropofluvent. Int J Environ Sci Tech 7(2):261–270
Davis RD, Beckett PHT (1978) Upper critical levels of toxic elements in plants: II. Critical levels of copper in young barley, wheat, rape, lettuce, and ryegrass, and of nickel & zinc in young barley and ryegrass. New Phytol 80:23–32
Duruibe JO, Ogwuegbu MO, Egwurugwu JN (2007) Heavy metal pollution and human biotoxic effects. Int J Phys Sci 2:112–118
Eriksson J, Andersson A, Andersson R (1997) Current status of Swedish arable soils. Naturvårdsverket Rapport 4778. Uppsala: Naturvårdsverket, p 59 (ISBN 91-620-4778-7)
Frank AB, Klaebe RM, Lohr S, Xu L, Frei R (2020) Chromium Isotop Composition of organic-rich Marine Sediments and their Mineral phases and implications for using black Shales as a Paleoredox archive. Geochim Cosmochim Acta 270:338–359
Hara T, Sonoda Y, Iwai I (1976) Growth response of cabbage plants to transition elements under water culture conditions. II. Cobalt, nickel, copper, zinc, and molybdenum. Soil Sci Plant Nutr 22:317–325
Harris RF, Bezdicek DF (1994) Descriptive aspects of soil quality/health. In: Doran JW, Coleman DC, Bezdicek DF, Stewart BA (eds) Defining soil quality for a sustainable environment. SSSA Special Publication Number 35, Wisconsin, pp 23–36
Kabata-Pendias A (2007) Trace Elements in Soils and Plants, 3rd edn. CRC Press, Boca Raton, FL, USA
Kabata-Pendias A, Pendias H (1999) Biogeochemistry of trace elements, 2nd edn. Wyd, Nauk PWN, Warsaw, p 400 (Po)
Leonard A (1991) Arsenic. In: Meriam E (ed) Metals and their compounds in the environment. VCH, Weinheim, pp 751–762
Li L, Lu J, Wang S, Ma Y, Wei Q, Li X, Cong R, Ren T (2016) Methods for estimating leaf nitrogen concentration of winter oilseed rape (Brassica napus L.) using in situ leaf spectroscopy. Ind Crops Prod 91:194–204
Loftleidir H (2005) Essential trace elements for plants, animals and humans
Luo Y, Rimmer DL (1995) Zinc-copper interaction affecting plant growth on a metal-contaminated soil. Environ Pollut 88(1):79–83. https://doi.org/10.1016/0269-7491(95)91050-U
Mahmood T, Islam KR (2006) Response of rice seedlings to copper toxicity and acidity. J Plant Nutr 29:943–957. https://doi.org/10.1080/01904160600651704
Makela-Kurtto R, Eurola M, Justen A, Backman B, Luoma S, Karttunen V, Ruskeeniemi T (2007) Arsenic and other Elements in Agro-systems in Finland and particularly in the Pirkanmaaa region. MTT Agrifood Research, Finland Geological Survey of Finland, Pp. 119
Mandal BK, Suzuki KT (2002) Arsenic round the world : a review. Talanta 58(1):201–235
Martín F, Simón M, Arco E, Romero A, Dorronsoro C (2011) Arsenic behaviour in polluted soils after remediation activities. In: Hernandez-Soriano MC (ed) Soil health and land use management. InTech, Rijeka, pp 201–216
McGrath SP (1995) Nickel. In: Alloway BJ (ed) Heavy metals in soils. Blackie Academic & Professional, London
Megharaj M, Singleton I, Kookana R, Naidu R (1999) Persistence and effects of fenamiphos on native algal populations and enzymatic activities in soil. Soil Biol Biochem 31:1549–1553
Ministry of the Environment, Finland (MEF) (2007) Government Decree on the Assessment of Soil Contamination and Remediation Needs (214/2007, March 1, 2007).
Nabulo G, Oryem-Origa H, Nasinyama GW, Cole D (2008) Assessment of Zn, Cu, Pb and Ni contamination in wetland soils and plants in the Lake Victoria Basin. Int J Environ Sci Technol 5:1. https://doi.org/10.1007/BF03325998
Nagajyoti PC, Lee KD, Sreekanth TVM (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8:199–216. https://doi.org/10.1007/s10311-010-0297-8
Naidu R, Smith LH, Mowat D, Kookana RS (2000) Soil-plant transfer of chromium from tannery waste sludge: results from a glass-house study. In: Naidu R, Willet IR, Mahimairaja S, Kookana R, Ramasamy K (eds) Towards Better Management of Soils Contaminated with Tannery Waste. ACIAR (Australian Centre for International Agricultural Research) Proceedings, no 88. Canberra, Australia, pp 133–143
NMHPPE (1991) Environmental quality standards for soil and water. Netherlands Ministry of Housing, Physical Planning and Environment, Leidschendam
Ofem KI, Asadu CLA, Ezeaku PI, Kingsley J, Eyong MO, Katerina V, Václav T, Karel N, Ondrej D, Vít P (2020) Genesis and classification of soils over limestone formations in a Tropical Humid Region. Asian J Sci Res 13:228–243
Okafor EC, Opuene K (2007) Preliminary assessment of trace metals and polycyclic aromatic hydrocarbons in the sediments. Int J Environ Sci Tech 4(2):233–240
Oluyemi EA, Feuyit G, Oyekunle JA, Ogunfowokan AO (2008) Seasonal variations in heavy metal concent. Int J Environ Sci Technol 2(5):89–96
Opaluwa OD, Aremu MO, Ogbo LO, Abiola KA, Odiba IE, Abubakar MM, Nweze NO (2012) Heavy metal concentrations in soils, plant leaves and crops grown around dump sites in Lafia Metropolis, Nasarawa State, Nigeria. Adv Appl Sci Res 3(2):780–784
Romero-Freire A, Sierra-Aragón M, Ortiz-Bernad I, Martín-Peinado FJ (2014) Toxicity of arsenic in relation to soil properties: implications to regulatory purposes. J Soils Sediments 14:968–979. https://doi.org/10.1007/s11368-014-0845-0
Ross SM (ed) (1994) Toxic metals in soil-plant systems. Wiley, New York
Sambo EE, Ufoegbune GC, Eruola AO, Ojekunle OZ (2016a) Impact of rainfall variability on flooding of rivers in Cross River Basin, Nigeria. In: Nigerian Meteorological Society (NMETS), "Climate Variability and Change: Impact, Science, Innovation and Policy" at Federal College of Education Osiele, Abeokuta. 21–24 November, 2016
Sambo EE, Ufoegbune GC, Eruola AO, Ojekunle, OZ (2016b) Pattern of Climate Change in Cross River Basin of Nigeria: Implication to Agriculture and Food Security Nigerian Meteorological Society (NMETS), "Climate Variability and Change: Impact, Science, Innovation and Policy" at Federal College of Education Osiele, Abeokuta. 21–24 November, 2016
Shaheen N, Irfan NM, Khan IN, Islam S, Islam MS, Ahmed MK (2016) Presence of heavy metals in fruits and vegetables: Health risk implications in Bangladesh. Chemosphere 152:431–438
Singh R, Gautam N, Mishra A, Gupta R (2011) Heavy metals and living systems: an over view. Indian J Pharmacol 43:246. https://doi.org/10.4103/0253-7613.81505
Soil Survey Staff (SSS) 2014 Kellogg Soil Survey Laboratory Methods Manual. Soil Survey Investigations Report No. 42, Version 5.0. R. Burt and Soil Survey Staff (ed.). U.S Department of Agriculture, Natural Resources Conservation Service. P. 1001.
Stêpniewska Z, Bucior K, De Boodt M (2001) Chromium and its forms in soils in the proximity of the old tannery waste lagoon. Int Agrophys 15:121–124
Suavé S, Hendershot W, Allen HE (2000) Solid- solution partitioning of metals in contaminated soils: Dependence on pH, total metal burden, and organic matter. Environ Sci Technol 34(7):1125–1131
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. In: Molecular, clinical and environmental toxicology. Springer, Basel, pp 133–164,https://doi.org/10.1007/978-3-7643-8340-4_6
Tejnecky V, Samonil P, Grygar TM, Vasat R, Ash C, Drahota P, Sebek O, Nemecek K, Drabek O (2015) Transformation of iron forms during pedogenesis after tree uprooting in a natural beech-dominated forest. CATENA 132:12–20
Tóth G, Hermann T, Da Silva MR, Montanarella L (2016) Heavy metals in agricultural soils of the European Union with implications for food safety. Environ Int 88:299–309
Tye AM, Young SD, Crout N, Zhang H (2004) Speciation and solubility of Cu, Ni and Pb in contaminated soils. Eur J Soil Sci 55(3):579–590
Vare L (2006) Anthropogenic inputs of heavy metals to the Kongsfjord area. Geophys Res Abstr 8:06079
Welch RM (1981) The biological significance of Nickel. J Plant Nutr 3:345–356
World Health Organization (WHO) (2001) Environmental health criteria 221; Zinc. WHO Library Cataloguing-in-Publication Data
Yang X, Baligar DC, Martens DC, Clark RB (1995) Influx, transport, and accumulation of cadmium in plant species grown at different Cd (II) activities. Environ Sci Health B30(4):569–583
Yang X, Long X, Ni W, Ye Z, He Z, Stoffella PJ, Calvert DV (2002) Assessing copper thresholds for phytotoxicity and potential dietary toxicity in selected vegetable crops. J Environ Sci Health Part B-Pestic Food Contam Agric Wastes B37(6):625–635
Zandsalimi S, Karimi N, Kohandel A (2011) Arsenic in soil, vegetation and water of a contaminated region. Int J Environ Sci Tech 8(2):331–338
Acknowledgements
The contributions of Laboratory Staff of Soil Science and Soil Protection at Czech University of Life Sciences, Czech Republic is acknowledged.
Funding
This research was funded by Tertiary Education Trust Fund (TETFUND/DAST&D/UNIV/CALABAR/ASTD/2017/VOL.1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interests
There are no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ofem, K.I., Asadu, C.L.A., Ezeaku, P.I. et al. Profile distribution and soil health implication of some oxides in agrarian soils overlying geologic formations in Southeast Nigeria. Model. Earth Syst. Environ. 8, 1923–1932 (2022). https://doi.org/10.1007/s40808-021-01188-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40808-021-01188-3