Abstract
The Abakaliki area has been renowned for lead–zinc mining and hard rock quarrying, and these activities generate acid mine drainages (AMDs) and chemical dissolution of minerals into the water, respectively. This research is aimed at assessing the influence of AMDs and other dissolved elements on water quality for domestic and irrigation uses in the area. Thirty-five water samples were collected and analyzed for physicochemical parameters, including: pH, total dissolved solid, electrical conductivity, major cation and anion and heavy metals using atomic absorption spectrophotometric methods. Mathematical calculations were used to deduce irrigation parameters. Result indicates that pH of water samples is slightly basic to acidic, and EC ranges from 5.28 to 1492 µS/cm. Only samples around Nigercem, Nkalagu and lead–zinc mines at Enyigba were above WHO permissible limit for drinking water. The concentrations of Na+, K+, Ca2+, Mg2+ and HCO3− were within WHO set standard for drinking water, while Cl− and SO42− were above the WHO set standard. Irrigation parameters showed soluble sodium percentage between 8.33 and 100.00, sodium percentage ranges between 2.77 and 300.00%, Kelly ratio ranges from 0.03 to 3.00, magnesium absorption ratio ranges between 16. 92 and 123.5, total hardness ranges between 3.00 and 125.0, residual sodium bicarbonate ranges between − 23.84 and 0.11, and potential salinity ranges from 2.93 to 14.77 within the study area. Abandoned mine water in the area is fairly suitable for irrigation uses, but unsuitable for domestic uses. This is due to high chemical activities taking place in the mine ponds, and these 17 ponds have been abandoned for over 2 decades. Deductions from Soltan classification revealed that 98% of groundwater falls within deep meteoric water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Allen et al. (1996), Choubey (1991), Gupta (1999), Singh et al. (2018), Singh (1998), Tiwary (2001), Ezeh et al. (2016) and Obasi and Akudinobi (2019b) have shown that mining activities threaten the quality and quantity of water resources in many parts of the world. This is because mining processes consume, divert and can seriously pollute the environment including soil and water resources, as there are often times no proper water management plans at most mine sites, including the present study area. Water is often discharged without any treatment. This may pollute the natural surface drainage and other water resources (Singh et al. 2007; Obasi et al. 2018a, b). In hydrogeological studies, groundwater quality is of prime and fundamental importance. The physicochemical and bacterial attributes determine the usefulness or otherwise of groundwater for various uses, viz. domestic, municipal, industrial, agricultural and mining. In hydrogeochemistry, groundwater quality study is divided into two categories: (1) evaluating geochemical processing influencing groundwater chemistry and (2) assessment of groundwater for its uses, including domestic, industrial, animal husbandry and irrigational uses. Most times, groundwater deterioration does not occur when mining is carried out in the hilly region, but deteriorates when mining activities are carried out below the earth surface; at this stage, it easily penetrates the water table of the region, thus resulting in groundwater seepage problems (Njenga 2004; Mishra and Patel 2001). The condition gets worse when the mine goes deeper. Most often, the study area is faced with groundwater seepage problems, either in a running mine or in an open mine, which requires an in-depth hydrogeological study of the mining area.
The study area is richly endowed with mineral deposits (such as galena, chalcopyrite, siderite and pyrite) and economic raw materials (such as limestone, pyroclastic and baked shale) (Obasi and Akudinobi 2019a). These minerals and economic raw materials are considered very useful for construction purposes, cement manufacture and industrial developments. The quarry and mine sites covered in this study are located at Nkalagu, Enyigba, Setraco, Amasiri and Nkaliki. Petters (1991) stated that at Nkalagu quarry, the Nigerian Cement Company (NIGERCEM) quarried up to 6 m thick of limestone. At Amaeze, Setraco has quarried for sandstone for over 2 decades. At Umuoghara, the limestone and baked shale quarry has continued to expand for about 3 decades, while the pyroclastic quarry at Ezzagu (Sharon) extends over 20 km of land and this has been exploited for over 5 decades. On the other hand, the Enyigba area is the hub of lead–zinc mine in southern Nigeria (Fig. 1a, b). The presence of these mineral deposits and the activities within these areas has led to a drastic increase in population, especially around the active mines and quarries; this population increase is believed to have altered the ecosystem and environment and also affect the water resources of the area (Obasi et al. 2018a, b; Eyankware et al. 2017). Emphasis is placed on groundwater because of its availability all year round. Aghazadeh and Mogadam (2004) and Hossien (2004) further stated that hydrochemical evaluation of groundwater systems is usually based on the availability of a large amount of information concerning its chemistry. Its chemistry depends on a number of factors, such as general geology, degree of chemical weathering of the various rock types, quality of recharge water and inputs from sources other than water–rock interaction. Such factors and their interactions result in a complex groundwater quality. Previous researches carried out within various parts of Ebonyi State showed that major sources of pollution affecting the water quality for domestic and irrigation uses are wastes from rice mill at Rice Mill market located in Abakaliki, waste from mechanical village, also located close to Rice mill, and wastes from mining activities, and these wastes are channeled into the main river that cut across the entire state via its tributaries (Obasi et al. 2015; Eyankware et al. 2016). Eyankware (2016) and Eyankware et al. (2017), 2018a, b further stated the rock–water interaction is major process influencing groundwater chemistry within certain parts of Ebonyi State, especially within the Asu River Group. Other factors such as precipitation, evaporation and crystallization have little or no effect on groundwater chemistry compared to rock–water interaction (rock dominance). Although various researches have been carried out in selected areas of Ebonyi State, to assess the quality of water for domestic and irrigation uses in Ebonyi State (Eyankware 2016; Eyankware et al. 2017, 2018a, b; Obasi et al. 2018a, b; Obasi and Akudinobi 2019b; Ede and Nnabo 2015; Ojobo and Nnabo 2015; Okogbue and Ukpai 2013; Moses and Ruth 2015, Amadi et al. 2010), it is worthy to note that a comparative study has not been carried out to evaluate the impact of mining activities from abandoned and active mines, on water quality for domestic and irrigation uses. Hence, this study aims at evaluating the effect of active and abandoned mines on water resources, for domestic and irrigation uses.
The study area
Location, accessibility, climate and physiography
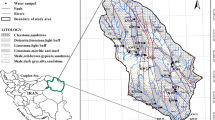
The study area is located in Ebonyi State, Nigeria. It lies between latitude 5°45̕ N–6°45̕ N and longitude 7°25̕E–8°20̕ E comprising Nkalagu, Amaeze, Umuoghara and Enyigba. The area is accessible through various networks of roads as shown in Fig. 2. There are two marked seasons in the study area: the dry and the wet seasons. The wet season begins in March and ends in October, while the dry season begins in October and ends in February. Temperature in the dry season ranges between 25 and 29°C and 16° and 28°C during the rainy season. The average monthly rainfall ranges between 3.1 mm in January and 270 mm in July. The average annual rainfall varies from 1750 to 2250 mm. The climate of the area favors the dispersion of the resultant pollutants from the mining and quarry activities. This high amount of rainfall results in surface runoff that transports the pollutants and also assists percolation. The study area is part of the rainforest region of southeastern Nigeria. It has a humid climate and evergreen vegetation. The vegetation cover is composed of very dense trees and underground creepers. These trees are mostly tall, with buttress roots, while the vegetation is controlled by many factors, including the drainage, topography, geology and rainfall. The area has been described as part of the low land rainforest region (Igbozuruike 1975). The drainage system of the study area is dendritic with the Ebonyi River as the major river that drains the area. Other tributaries such as the Ikwo, Iyiodu and Ngada rivers control the drainage with the underlying lithology.
Geology and tectonics
The study area lies within the Lower Benue Trough, Nigeria. The Trough resulted from the breakup of the west Gondwanaland to produce the African and South American plates, and the opening of the South Atlantic Ocean during the Cretaceous (Albian). The Trough could be defined as an “Intracratonic rift system” (Uma 1998) or an “intercontinental Cretaceous basin about 1000 km in length stretching in a NE–SW and resting unconformably upon the Precambrian Basement (Benkhelil 1987). The Abakaliki Trough merged during the Santonian tectonic phase when, at the same time, the Anambra Basin began to subside. In the axial part of the Abakaliki Trough, a syn-sedimentary magmatic activity was recorded in the Asu River Group (Albian) which subjected the area to infillings due to tectonic movement which were recorded in the sedimentation slumping, syn-sedimentary faulting and shearing (Okogbue 2013). A strong tectonic episode during the Santonian affected the Albian, Cenomanian and Turonian sediments producing numerous folds, faults and fractures in the area. Sediments affected by this pervasive deformation were intruded by numerous mafic to intermediate intrusions and cal-alkaline lavas and tuffs. The Abakaliki Anticlinorium is characterized by intensive deformations including tight folding with associated cleavage resulting from 330° to 150° trending compression of Santonian age.
Locally, the area is underlain by the Asu River Group (Albian) and the Eze-Aku Formation (Turonian). The Albian transgression was responsible for the deposition of very thick fissile, dark gray shales, siltstones and subordinate limestones. The shales in most places lie unconformably on the Basement Complex (Reyment 1965). The thickness is up to 2000 m (Reyment 1965; Nwachukwu 1972). These sediments are rich in ammonites as well as foraminifera, radiolarian and pollens (Reyment 1965). The development of the thick Eze-Aku Shale Formation composed of gray flaggy calcareous shales and sandstone with interbedded limestone lenses, evidenced the Turonian transgression. The formation grades laterally into the Amasiri Sandstone near Afikpo and Markurdi sandstone around Markurdi (Reyment 1965). The formation varies in thickness up to 1200 m, and it is overlain by about 900 m of bluish gray, bedded shale with some fine-grained (Agbani Sandstones) (Fig. 3).
Materials and methods
Sample collection and preparation
Thirty-five representative water samples at various quarry and mine sites in Amasiri (Setraco), Nkalagu (Nigercem), Enyigba, Umuoghara and Nkaliki quarry sites were systematically collected according to APHA (1999), BIS (2012) and CGWB (2007) (Figs. 1, 2). Samples of abandoned mine pits were only accessed. The water samples were stored in a plastic1-liter container for detailed chemical analysis. These containers were washed thoroughly with distilled water and dried before being filled with the water samples. To obtain a composite sample, waters were collected after pond waters were cleared of visible wastes before sample collection. Filtration of water samples was done in the field using 0.45-µm-diameter disposable filters to ensure the removal of suspended solids before storage in prepared bottles. Acidification of samples was done with 1.0 mL of conc. HNO3 using new syringes. This is necessary to prevent sorption. The samples were stored in ice-packed containers to maintain the transportation temperature. Accordingly, before the sampling, sample bottles and beakers were washed thoroughly and soaked in distilled water acidified with 1.0 mL of HNO3 for 3 days. They were also rinsed with dilute HNO3 and vigorously rinsed at least three times with the water sources at the point of water collection.
Laboratory and analytical methods
Methods of laboratory analysis for physicochemical parameters and analytical calculation methods for irrigation indices are presented in Tables 1 and 2.
The accuracy of geochemical analysis was determined by calculating the ionic balance, which was within (1:1 ± 0.01%) as plotted using Surfer 10 software package. Irrigation parameters were determined by calculating the relations below in (meq/L). The suitability of groundwater for irrigation was evaluated by comparing the water samples with various water quality standards for irrigation.
Analytical check/ionic balancing
The correctness of the chemical results was assessed using the relationship between the anions and the cations in the analyzed samples as expressed in meq/L. The equations according to Hounslaw (1995), Domenico and Schwartz (1990) are represented as:
The above equation gave a cation–anion ratio of 1:1 ± 0.01, which confirms that the geochemical analysis was accurate.
The cation–anion balance was also assessed using electrical neutrality equation which requires that the sum of positive ions must be equal to sum of negative ions in solution expressed in meq/L.
Results and discussion
Result of physical and chemical parameters is presented in Table 3 in meq/L.
Evaluation of groundwater quality for drinking purpose
pH
Hem (1985) stated that pH of water is a very important indication of its quality and provides important information regarding its geochemical equilibrium. The pH of the analyzed samples varies from 5.1 to 8.4 with a mean value of 6.51 (Table 4). Although pH has no direct effect on human health, all biochemical reactions are sensitive to variation of pH (Subba Rao and Krishna Rao 1991). From Table 4, most of the sample locations fall within the acidic pH range except locations EAO/05, 14, 23, 28 and 29. However, over 85% falls within the safe range of water consumption as stipulated by WHO (1984) (Fig. 4).
Sodium and potassium (Na+ and K+)
The concentration of sodium within the study area varies between 0.02 and 2.29 mg/L with an average value of 0.84 mg/L. Maximum permissible limit for sodium in water is 200 mg/L as per WHO (2011) standard. Prasanth et al. (2012) and Ramesh and Elango (2011) hinted that if the concentration of sodium is above permissible level, it could lead to salty taste and health hazards such as blood pressure, kidney stones, arteriosclerosis, edema and hyperosmolarity. The concentration of potassium ranges between 0.39 and 37.14 mg/L; it is within WHO drinking water set standard.
Calcium and magnesium (Ca2+ and Mg2+)
The maximum permissible limit for calcium in water is 75 mg/L WHO (2011). Result indicates that the concentration of calcium within the study area ranges between 0.4 and 16.3 mg/L with an average value of 2.93 mg/L. In sedimentary terrain like that of study area, calcium occurs as carbonates in limestone rocks of the area, especially around Nkalagu, where there are large deposits of limestone and are cementing material in sandstone in the Amasiri area (Hem 1985). Annapoorna and Janardhana (2015) further stated that calcium infiltrates into groundwater through ion exchange processes. Magnesium is commonly found in ferromagnesian minerals in carbonates sedimentary rocks. The standard limit for magnesium in water as stipulated by WHO (2011) is 200 mg/L. The magnesium concentration ranges between 0.06 and 5.1 mg/L with an average of 1.61 mg/L (Fig. 5).
Chloride (Cl−)
The desirable level for chloride in water is 250 mg/L. Cl− ion varies between 49.27 and 267.29 mg/L with an average value of 138.24 mg/L. Samples EAO/22 and 29 (around Enyigba) were above WHO permissible limit for drinking water quality. This could be attributed to dissolution of sulfide minerals on the ores which occurs in the area.
Sulfate (SO42−)
The desirable level for sulfate in groundwater is 200 mg/L. Result shows that the value of SO42− ranges between 88.85 and 340.53 mg/L with an average value of 204.39 mg/L. This is high level of water pollution and is attributed to the process of AMDs which occurs in the area (Figs. 6, 7).
Pie chart showing TDS concentration against (WHO 2011) TDS (mg/L) in percentage (%)
Bicarbonate (HCO3−)
The value of bicarbonate (HCO3−) in the water samples varies from 0.00 to 14. 3 mg/L with an average value of 5.24 mg/L (Table 3). The content of HCO3− has no known adverse health effects, and all groundwater samples lie within the desirable limit of 300 mg/L (WHO 1990) (Table 5).
Electrical conductivity (Ec)
Ec is related to the concentration of salts dissolved in water. Result from Table 3 shows that the value of Ec ranges between 5.28 and 1492 µS/cm, and the entire samples were within WHO permissible limit except at sampling location EAO/10, 14, 16, 18, 29 and 32 that were slightly above the permissible limit for drinking water quality. The high values of Ec can be attributed to high chemical activities going on in the abandon mines (Table 6).
Evaluation of water quality for irrigation
For effective evaluation of water quality for irrigation, the computed values of irrigation parameters are shown in Table 7.
Soluble sodium percentage (SSP)
Iqbal et al. (2012) and Omo-Irabor et al. (2018) stated that high sodium concentration decreases permeability of soil and destroys the soil structure, thus affecting the drainage of the area which leads to a reduction in crop production and plant growth. SSP was calculated using Richards (1969) and Todd (1980). The value of SSP ranges from 8.33 to 100.00 (Table 7). From Fig. 8, samples EAO/01, 03, 04, 06, 07, 10, 11, 12, 14, 17, 18, 21, 22, 23, 25, 27, 28, 30, 32, 33 and 35 (samples from Amasiri and Nkalagu) fall within the suitable category and hence it is considered suitable for irrigation, while samples EAO/02, 05, 08, 09, 15, 16, 19, 20, 24, 26, 29, 31 and 34 fall within the unsuitable category, implying that the samples are unsuitable for irrigation.
Magnesium adsorption ratio (MAR)
At equilibrium of Ca and Mg in groundwater, MAR makes the soil more alkaline, thereby reducing the soil quality and crop yield (Paliwal 1972; Haritash et al. 2014). The MAR was calculated using Eq. 4 (Table 2). More magnesium in water will adversely affect crop yield as the soil becomes more alkaline. Value below 50 is considered acceptable limit of MAR (Ayers and Westcot 1985). The value of MAR in the study area ranges between 16.92 and 123.5. Samples EAO/01, 02, 08, 09, 10, 16, 21, 23, 26, 28, 30 and 33 (Table 7) and 11 were observed to within the set limit and hence they are considered to be suitable for irrigation, while samples EAO/03, 04, 05, 05, 06, 07, 11, 12, 13, 14, 17, 18, 19, 20, 22, 24, 25, 27, 31, 32, 34 and 35 are considered unsuitable for irrigation
Sodium percentage (Na%)
Sodium is considered as one of the important elements in determining the suitability of water for irrigation because of its effect on soil (when present in exchangeable form), as it causes adverse physicochemical changes in the soil, particularly to the soil structure. A high salt concentration in the water leads to the formation of saline soil, and the higher concentration of sodium leads to development of alkali soil (Omo-Irabor et al. 2018). Irrigation water could be a source of excess sodium in the soil solution; hence, it should be evaluated for its hazard (Sayyed and Wagh 2011). From Fig. 9 and Table 7, samples EAO/01, 03, 04, 06, 09, 10, 11, 12, 18, 20, 21, 22, 23, 25, 26, 27, 33 and 35 are within the excellent category. This implies that the listed samples are considered suitable for irrigation, while samples EAO/03, 05, 13, 14, 16, 17, 19, 24, 27, 30 and 31 fall within the good category (Table 11); hence, they are considered suitable for irrigation. Sample EAO/34 is within the doubtful category. Samples EAO/08, 29 and 32 are within the unsuitable category; hence, they are considered unsuitable for irrigation.
Rating of water samples on the basis of electrical conductivity and sodium percent (after Wilcox 1955)
Sodium adsorption ratio (SAR)
SAR is most generally used in the assessment of suitability of water for irrigation. The SAR measures the samples in terms of the relative concentration of sodium ions to the sum of calcium and magnesium ions in the water (Ramesh and Bhuvana 2012). Richards (1954) stated that sodium concentration in water affects and leads to the deterioration of the soil properties, thus reducing its permeability. The value of SAR for the study area ranges between 0.07 and 6.00, implying that the samples are suitable for irrigation.
Hanson et al. (1999) stated that water samples with higher salinity are needed for soil structure. Figure 10b shows the impact of irrigation water on the soil properties. Sawid and Issa (2015) stated that SAR of irrigation water represents the tendency of sodium ions to be adsorbed at ion exchange within the soil matrix at the expense of calcium and magnesium; this results in the dispersion of soil particles, which could reduce the soil infiltration capacity. Additionally, reduction in soil permeability caused as a result of high SAR values of irrigation water that can be minimized by the high salinity values (Ec of water). With similar SAR values, samples with higher salinity are better suited for the soil structure (Hanson et al. 1999). The SAR versus EC plot (Fig. 10b) shows the impact of irrigation water on the soil properties, and it revealed that samples EAO/07, 09, 10, 13, 16, 17, 18, 19, 24, 27, 29, 31 and 32 are within no reduction infiltration category, while the rest samples are within slightly to moderately reduction infiltration.
Potential salinity (PS)
Potential salinity was introduced as an important parameter for assessing the suitability of water for irrigation uses. It is defined as the chloride concentration plus half of the sulfate concentration (Ogunfowokan et al. 2013). The suitability of water for irrigation is not dependent on the concentration of soluble salts in the water (Doneen 1964; Doneen 1961). However, low solubility salts are precipitated in the soils, thus accumulating with each successive irrigation, whereas the concentration of highly soluble salts increases the salinity of the soil (Doneen 1964). According to Doneen (1964), PS value less than 3 meq/L is suitable for irrigation purposes. The value of PS in the study area ranges between 0.07 and 1.87 meq/l with a mean value of 0.54 meq/L (Table 5). Based on the view of PS, the water is fit for irrigation.
Residual sodium carbonate (RSC)
According to U.S. Salinity Laboratory, when RSC value is < 1.25 meq/L, it is considered suitable for irrigation; values between 1.25 and 2.5 meq/L are of marginal quality and value > 2.5 meq/L is considered unsuitable for irrigation. The values of RSC for the study area range between 0.11 and − 23.84 meq/L with an average value of 1.33 meq/L (Tables 8 and 11).
Electrical conductivity (Ec)
Ahmed et al. (2002) stated that the most influential water quality guideline on crop productivity is the water salinity hazard which is measured by electrical conductivity (Ec). The primary effect of high Ec water on crop productivity is the inability of the plant to compete with ions in the soil solution for water. This often leads to physiological drought. From Table 11, samples EAO/04, 05, 11, 12, 20, 24 and 25 are within excellent category, while sample locations EAO/03, 06, 08, 17, 21, 23, 26, 28, 30, 31,, 31, 33, 34 and 35 are within good category; hence, they are considered suitable for irrigation (Tables 9 and 11). Samples EAO/01, 02, 07, 09, 10, 13, 14, 15, 16, 18, 19, 29 and 32 are within the doubtful category and are considered unsuitable for irrigation. The presence of dissolved organic constituents in water samples could be attributed to the high level of Ec (Fig. 11).
Total hardness (TH)
Sawyer and McCarty (1967) stated that when the value of TH is less than 75, then water is said to be soft, and value between 75 and 150 is classified as moderately hard, while value within 150–300 is classified as hard, and lastly, value greater than 300 is regarded as very hard. From Table 10, samples EAO/01, 03, 04, 05, 06, 07, 08, 09, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34 and 35 are within the soft category, while EAO/02 and 22 are classified as moderately hard (Table 10, Fig. 12).
Geochemical diagram/classification of groundwater
Hydrochemical diagrams are aimed at interpretation of evolutionary trends with reference to groundwater systems, and this interpretation helps in better understanding of the groundwater geochemistry. Chadba plot (1999) and other hydrochemical cross-plots (Figs. 13a–c and 14) have been emphasized for better understanding of the groundwater system in the area (Table 11).
Factors controlling groundwater chemistry was evaluated using cross-plot such as Ca2+ + Mg2+ (SO42− + HCO3−) versus Na+ + Cl−, Na+ + K+ versus total cation and Na+ versus Cl−. When ion exchange is the dominant process that affects the hydrochemistry of groundwater, the relationship between (Ca2++Mg2+) − (SO42− + HCO3−) versus (Na–Cl).
should be a straight line (Herman 1978). From Fig. 13a, it is observed that (Ca2+ + Mg2+) − (SO42− + HCO3−) versus Na+ + Cl− were in equal line of 1:1, implying that ion exchange is a major process influencing groundwater chemistry within the study area. Figure 13b shows that the points are on the 1:1 equal line, indicating contributions sodium and potassium are from weathering process. Figure 13c shows that in groundwater the Na versus Cl (Fig. 13c) plot indicates that most of the samples are slightly below the equal line. The high concentration of Na can be attributed to carbonate weathering. Na versus Cl can be used to identify the evaporation process in groundwater.
Chadba plot indicates that all water samples (EAO/01 to 35) of the entire area fall within Ca2+–Mg2+–Cl− water type with permanent hardness. This water type is attributed to the evolutionary dissolution of calcium and magnesium from the calcic-rich rocks (shales, limestone and calcareous sandstones) in the area.
Classification of groundwater samples of the area
Groundwater samples were classified based on Cl−, SO42− and HCO3− concentrations (Soltan 1999). Three major classes of groundwater exist in the area, namely sulfate water type, chloride water type and bicarbonate water type.
Base-exchange indices (r1) The base-exchange indices were estimated using Eq. 11a and b to further classify groundwater (Soltan 1999) (Eq. 12a; Table 2), where r1 is the base-exchange index and Na+, Cl− and SO42− concentrations are expressed in meq/L. If r1< 1, the groundwater sources are of Na+–SO42− type, while r1 > 1 indicates the sources are of Na+–HCO3− type.
Meteoric genesis indices (r2) The groundwater sources can also be classified based on meteoric genesis index and can be computed using Eq. 12b (Table 2) (Soltan 1999). If r2 < 1, the groundwater source is of deep meteoric water percolation type, while r2 > 1 indicates that it is of shallow meteoric genesis indices (r2). Groundwater within the study area fell within the category of deep meteoric water (Table 12), implying that the groundwater is greatly influenced by precipitation process. This is line with previous studies carried out by Eyankware et al. (2018a, b) and Eyankware (2017) which stated that groundwater fell within deep meteoric water type.
Conclusion
Hydrochemical characterization of abandoned mine waters for domestic and irrigation uses in Abakaliki, SE Nigeria, was carried out. The recent drive of economic diversion of the government into agriculture has made the abandoned mine waters an option to sustain crop production. This study is expedient to investigate the chemical characterization of these waters due to the chemical dissolutions and AMDs which are generated in the mine areas. The study area comprised Setraco quarry (Amasiri), Nigercem (Nkalagu), Abakaliki quarry cluster (Umuoghara) and Ezzagu pyroclastic mines. Thirty-five water samples were collected and analyzed for physicochemical parameters and heavy metals using atomic absorption spectrophotometric methods. Mathematical calculations were used to deduce irrigation parameters. From this research, it was observed that 90% pH was within acidic range, while samples EAO/05 (Amasiri), 14 (Nkalagu), 19 (Setraco), 23, 28 and 29 (Enyigba) are within basic, physicochemical parameters; Na+, K+, SO42−, NO3− and Ca2+ were within WHO permissible limit at various sampling points, while magnesium and chloride concentration were above WHO drinking water quality standard at various mine and quarry sites. Results from ion exchanges cross-plots reveal that weathering process was the major process that influences geochemical makeup of groundwater. As for irrigation indices such as MAR, SAR, KR, TH and RSC were found below the permissible limit within the study area, while indices such as SSP, Na% and PS were above the set standard as various sampling points within Amasiri, Nkalagu and Umuoghara mines, respectively. Mine pond waters were not highly affected because groundwater in the area exists in fracture shales; this water bearing unit has low porosity and does not allow free flow of contaminants in all directions.
References
Aghazadeh N, Mogadam AA (2004) Evaluation effect of geological formation on groundwater quality in the Harzandat plain aquifer. Symp Geosci Iran 22:392–395
Ahmed SS, Mazumder QH, Jahan CS, Ahmed M, Islam S (2002) Hydrochemistry and classification of groundwater, Rajshahi City Corporation area, Bangladesh. J Geol Soc India 60:411
Allen SK, Allen JM, Lucas S (1996) Concentration of contaminants in surface water samples collected in west-central Indiana impacted by acid mine drainage. Environ Geol 27:34–37
Amadi AN, Olasehinde PI, Yisa J (2010) Characterization of groundwater chemistry in the coastal plain–sand aquifer of Owerri using factor analysis. Int J Phys Sci 5(8):1306–1314
Annapoorna H, Janardhana MR (2015) Assessment of groundwater quality for drinking purpose in rural areas surrounding a defunct copper mine. Aquat Procedia 4:685–692. https://doi.org/10.1016/j.aqpro.2015.02.088
APHA (1999) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington DC
Ayers RS, Westcot DW (1985) Water quality for agriculture. FAO Irrig Drain Pap 29(1):1–130
Benkhelil J (1987) Structural frame and deformation in the benue trough of Nigeria. Bull Centres Rech Explor- Prod. Elf – Aquitaine 11:160–161
Bureau of Indian Standards (BIS) (2012) Indian standard drinking water specification (second revision) BIS 10500:2012, New Delhi
Central Ground Water Board (CGWB) (2007) Groundwater brochure of Puducherry region UT of Puducherry, pp 1–27. http://www.cgwb.gov.in/District_Profile/Puduchery/Puducherry.pdf
Chadha DK (1999) A proposed new diagram for geochemical classification of natural waters and interpretation of chemical data. Hydrogeol J 7:431–439
Choubey VD (1991) Hydrological and environmental impact of coal mining, Jharia coalfield, India. Environ Geol 17:185–194
Domenico PA, Schwartz FW (1990) Physical and chemical hydrology. Wiley, New York, p 410
Doneen LD (1961) The influence of crop and soil on percolating waters. Proceeding of Ground Water Recharge Conference, California, USA
Doneen LD (1964) Water quality for agriculture. Department of Irrigation, University of California, Davis, p 48
Eaton FM (1950) Significance of carbonates in irrigation waters. Soil Sci 39:123–133
Ede CO, Nnabo PN (2015) Hydrogeochemical evaluation of groundwater quality of Abakaliki and its environs, Southeastern Nigeria. Int J Innov Sci Res 13(2):689–696
Eyankware MO (2016) hydrochemical appraisal of groundwater for irrigation purpose: a case study of Ekaeru Inyimagu and its adjoining area, Ebonyi State, Nigeria. Indian J Sci 23(88):924–994
Eyankware MO (2017) Hydrogeochemical evaluation of groundwater for irrigation purposes in mining areas of Umuoghara Near, Abakaliki, SE, Nigeria. Sci Technol 3(9):1–19
Eyankware MO, Obasi PN, Akakuru OC (2016) Use of hydrochemical approach in evaluation of water quality around the vicinity of Mkpuma Ekwaoku mining district, Ebonyi State, SE. Nigeria for Irrigation Purpose. Indian J Sci 23(88):881–895
Eyankware MO, Akudinobi BEB, Akakuru OC (2017) Quality assessment and hydrochemical charactertics of groundwater in Ughelli and its adjoining area for irrigation purpose, Niger Delta Region. Nigeria. Indian J Sci 3(10):120–134
Eyankware MO, Nnajieze VS, Aleke CG (2018a) Geochemical assessment of water quality for irrigation purpose, in abandoned limestone quarry pit at Nkalagu area, Southern Benue Trough Nigeria. Environ Earth Sci 77:66. https://doi.org/10.1007/s12665-018-7232-x
Eyankware MO, Ogwah C, Okeke GC (2018b) Geochemical evaluation of groundwater origin using source rock deduction and hydrochemical facies at Umuoghara Mining Area, Lower Benue Trough, SE Nigeria. Int Res J Earth Sci 6(10):1–11
Ezeh VO, Eyankware MO, Irabor OO, Nnabo PN (2016) Hydrochemical evaluation of water resources in Umuoghara and its environs, near Abakaliki, South Eastern Nigeria. Int J Sci Healthc Res 1(2):23–31
Freeze RA, Cherry JA (1979) Groundwater. Prentice Hall, Englewood Cliffs
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170(3962):1088–1090
Gupta DC (1999) Environmental aspects of selected trace elements associated with coal and natural waters of Pench valley coalfield of India and their impact on human health. Inter J Coal Geol. 40:133–149
Hanson B, Grattan SR, Fulton A (1999) Agricultural Salinity and Drainage. University of California Irrigation Program, University of California, Davis
Haritash AK, Gaur S, Garg S (2014) Assessment of water quality and suitability analysis of river Ganga in Rishikesh. Appl Water Sci India. https://doi.org/10.1007/s13201-014-0235-1
Hem JD (1985) Study and interpretation of the chemical characteristics of natural water, 2nd edn. US Geol Surv Water Supply Paper 2254:363
Hem JD (1991) Study and interpretation of the chemical characteristics of natural water: USGS Professional Paper Book 2254. Scientific Publishers, Jodhpur
Herman B (1978) Groundwater quality. Groundwater hydrology. McGraw-Hill, Kohgakusha Ltd., Tokyo, pp 339–375
Hossien MT (2004) Hydrochemical evaluation of groundwater in the Blue Nile Basin, eastern Sudan, using conventional and multivariate techniques. Hydrogeol J 12:144–158
Hounslaw W (1995) Water quality data: analysis and interpretation. CRC Press, Boca Raton, pp 71–127
Igbozuruike MU (1975) Vegetation types. In: Oformata GEK (ed) Nigeria in maps, eastern state. Ethiope Publ, Benin, pp 30–31
Iqbal H, Inam A, Bakhtiyar Y, Inam A (2012) Effluent quality parameters for safe use in agriculture. In: Water quality, soil and managing irrigation of crops
Kelly WP (1963) Use of saline irrigation water. Soil Sci 95(4):385–391
Moses OE, Ruth OE (2015) Environmental degradation on land in Enyigba with reference to Artisan Lead-Zinc miner in south eastern Nigeria. J Multidiscip Sci Res 3(3):32–34
Mishra PC, Patel RK (2001) Study of the pollution load in the drinking water of Rairangpur, a small tribal dominated town of North Orissa. Indian J Environ Ecoplan 5(2):293–298
Njenga JW (2004) Comparative studies of water chemistry of four tropical lakes in Kenya and India. Asian J Water Environ Pollut 1:87–97
Nwachukwu SO (1972) The tectonic evolution of the southern portion of the Benue Trough, Nigeria. Geol Mag 109(05):411–419
Obasi PN, Akudinobi BEB (2019a) Heavy metals occurrence, assessment and distribution in water resources in the lead–zinc mining areas of Abakaliki, southeastern Nigeria. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-019-02489-y
Obasi PN, Akudinobi BEB (2019b) Pollution status of arable soil and stream sediment in the mining areas of Abakaliki, Lower Benue Trough. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-019-02337-z
Obasi PN, Eyankware MO, Akudinobi BBE, Nweke MO (2015) Hydrochemical investigation of water resources around Mkpuma Ekwaoku mining district, Ebonyi State Southeastern Nigeria. Afr J Geo-sci Res 3(3):01–07
Obasi PN, Obini N, Ani CC, Okolo CM (2018a) Evaluation of hydrochemical attributes of the Amachara mining Area, lower Benue trough. Int J Sci Eng Sci 2(1):60–65
Obasi PN, Esom NE, Okolo CM, Edene EN (2018b) Assessment of water pollution status in the mining area of Ameka, South Eastern Nigeria using Metal Pollution Index. Int J Sci Eng Sci 2(1):66–73
Ogunfowokan AO, Obisanya JF, Ogunkoya OO (2013) Salinity and sodium hazards of three streams of different agricultural land use systems in Ile-Ife. Nigeria. Applied Water Science 3(1):19–28. https://doi.org/10.1007/s13201-012-0053-2
Ojobo JR, Nnabo PN (2015) Levels of heavy metals on groundwater in Abakaliki and its environs, southeastern Nigeria. Int J Innov Sci Res 12(2):444–452
Okogbue CO, Ukpai SN (2013) Geochemical evaluation of groundwater quality in Abakaliki Area, Southeast Nigeria. Jordan J Earth Environ Sci 5(1):1–8
Omo-Irabor OO, Eyankware MO, Ogwah C (2018) Integration of hydrogeochemical analytical methods and irrigation parameters in the evaluation of groundwater quality at Ibinta, Southern Benue Trough Nigeria. FUPRE J Sci Ind Res 2(1):38–49
Paliwal KV (1972) Irrigation with saline water. IARI Monograph no 2 (new series), New Delhi, p 198
Petters SW (1991) Regional geology of Africa, vol 40. Lecture notes in earth sciences series. Springer, Berlin, Heidelberg
Prasanth SS, Magesh NS, Jitheshlal KV, Chandrasekar N, Gangadhar K (2012) Evaluation of groundwater quality and its suitability for drinking and agricultural use in the coastal stretch of Alappuzha District, Kerala, India. Appl Water Sci 2(3):165–175
Raghunath IIM (1987) Groundwater, 2nd edn. Wiley, New Delhi, pp 344–369
Ramesh K, Bhuvana JP (2012) Hydrochemical characteristics of groundwater for domestic and irrigation purposes in Periyakulam taluk of Theni district, Tamil Nadu. Int Res J Environ Sci 1(1):19–27
Ramesh K, Elango L (2011) Groundwater quality and its suitability for domestic and agricultural use in Tondiar river basin. Environ Monit Assess. https://doi.org/10.1007/s10661-011-2231-3
Reyment RA (1965) Aspects of geology of Nigeria. Ibadan University Press, Ibadan, p 145
Richards LA (1954) Diagnosis and improvement of saline and alkaline soils. Department of Agriculture, U.S
Richards LA (1969) Diagnosis and improvement of saline and alkali soils. In: United States salinity Laboratory Staff Agricultural Handbook No. 60. The United States Government Printing Office, Washington DC
Sawid FA, Issa AA (2015) Assessment of groundwater quality for drinking and irrigation purposes, Martubah plain, eastern Libya. Trans Ecol Environ 196:311–325
Sawyer CN, McCarty PL (1967) Chemistry for sanitary engineers, 2nd edn. McGraw-Hill, New York, p 518
Sayyed MRG, Wagh GS (2011) An assessment of groundwater quality for agricultural use: a case study from solid waste disposal site SE of Pune, India. Proc Int Acad Ecol Environ Sci 1(3–4):195–201
Singh G (1998) Impact of coal mining on mine water quality. Int J Mine Water 7:45–59
Singh AK, Mondal GC, Kumar S, Singh TB, Tewary BK, Sinha A (2007) Major ion chemistry, weathering processes and water quality assessment in upper catchment of Damodar River basin, India. Environ Geol 54:745–758
Singh PK, Dahiphale P, Yadav KK, Singh M (2018) Groundwater, water science and technology library, 76. https://doi.org/10.1007/978-981-10-5789-2_1
Soltan ME (1999) Evaluation of groundwater quality in Dakhla Oasis (Egyptian Western Desert). Eviron Monit Assess 57:157–168
Subba Rao N, Krishna Rao G (1991) Groundwater quality in Visakhapatnam urban area, Andhra Pradesh. Indian J Environ Health 33(1):25–30
Tiwary RK (2001) Environmental impact of coal mining on water regime and its management. Water Air Soil Pollut 132:185–199
Todd DK (1980) Groundwater hydrology, 2nd edn. Wiley, New York, p 516p
Uma KO (1998) The brine fields of the Benue Trough, Nigeria: a comparative study of geomorphic, tectonic and hydrochemical properties. J Afr Earth Sci 26:261–275
WHO (1971) International standards for drinking water. World Health Organisation, Geneva
WHO (1983) Guidelines for drinking water quality. World Health Organisation, Geneva, p 186
WHO (1984) Guidelines for irrigation water quality, vol 1. Recommendations, Geneva, p 130
WHO (1990) Environmental health criteria 81: vanadium [R]. World Health Organization, Geneva, pp 1–35
WHO (2011). Guideline for drinking water quality recommendations, 4th edn, vol 1. World Health Organization, pp 308–399
Wilcox LV (1955) Classifcation and use of irrigation water. USDA, Circular, Washington, DC, p 96
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Eyankware, M.O., Obasi, P.N., Omo-Irabor, O.O. et al. Hydrochemical characterization of abandoned quarry and mine water for domestic and irrigation uses in Abakaliki, southeast Nigeria. Model. Earth Syst. Environ. 6, 2465–2485 (2020). https://doi.org/10.1007/s40808-020-00827-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40808-020-00827-5