Abstract
Electrochemical Micromachining is a unique kind of non-traditional machining practice for the production of micro and nano features. This paper aims to measure and assess the performance and geometrical features of ECMM on SS304 alloy by influencing the effect of different types of electrolytes viz.; Passivating, Non-Passivating and Composite electrolyte (CPE). Material removal rate (MRR), circularity, conicity, and overcut are considered as the performance and geometrical features. Effects of input parameters are correlated to the performance of output responses. The results showed the composite electrolyte used to provide improved performance in MRR and geometric features in evaluation with passive and non-passive electrolytes. This paper deals with the modes Operandi of optimization with Technique for Order Preference by Similarity Ideal Solution approach (TOPSIS) for the multiresponse characteristics involved in ECMM process based on the Multi-Criteria Decision Making Methodology (MCDM). The results clearly specified voltage of 7 V, the feed rate of 0.5 mm/min and duty cycle of 0.7 with CPE as electrolyte displaying the optimal conditions and the parameter setting involved for the improvement of performance and hole geometrical characteristics in ECMM process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Electrochemical Micromachining is only one of its kind of the unconventional machining technique utilized in the machining of soft and hard materials. Industries are in need of very minute features in micro range and stress free materials after machining. ECMM is based on the anodic dissolution of a metallic workpiece by a conductive tool. It can efficiently produce micro-sensors, micro-actuators, micro-accelerometers, micro-reactors, micro-pumps, food industry devices and medical devices. Achieving maximum MRR along with good geometric features and the best surface finish is most advantageous [1, 2], as it will provide some major advantages such as no heat-affected zone or deformation in the products. The manufacturing industry’s use of technology and applications of pure electrochemical machining is high as in the cases of aerospace, automotive, defense, food processing, and medical applications, etc. [3]. Electrolytes play a vital role in micromachining. Material removal occurs through applied electric current and electrolyte reaction with no thermal stress developed on the workpiece. The absence of physical interaction between the workpiece and tool results in no thermal interaction in both the electrodes. Since chemical erosion mechanism is involved when the electrolyte hits the workpiece, there are changes develop in the surface texture, surface integrity and micro-cracks are commonly observed along with the grain interfaces [4].
SS304 alloy has excellent corrosion resistance that leads to its use in various fields that include automobiles, aerospace, nuclear reactors, missiles, turbines, defense, and engineering. Machining requirements of micro-holes and grooves with a high aspect ratio are on the increase due to the need for precision and miniaturized parts to meet application demands. Hence, there is significant research value to studying the micro machining parameters for SS304 alloy [5]. Machining circular micro holes of diameter 500 μm on SS304 alloy is done by using different types of electrolytes in which the optimized parameter setting of 700 kHz, 600 ns and 21 V using HSS Tool with 0.4 mol/L dilute H2SO4 as the electrolyte creates a minimum taper angle [6]. Attempts have been made to produce micro tapered holes. Tapered holes are produced in nickel plate using the ECMM process with 0.5 mol/L NaNO3 as an electrolyte is used in fuel jet nozzles [7]. Pure water is used as the electrolyte in PW-ECM process for making eco-friendly micromachining in creating precise micro holes, trilateral cavities as well as square cavities in stainless steel, which have proven stability and reliability of the machine and its process [8]. Citric acid is also used as an electrolyte, for drilling micro-holes on the SS304 workpiece and the use of citric acid in industries has been demonstrated to be safe and eco-friendly [9]. Mineral water is used as an electrolyte in ultra-short pulsed power supply ECM for the production of the micro pin to avail added benefits such as economy, system control, and reduced environmental impact when compared with conventional electrolytes [10]. Reduced amount of sludges and clogging of the electrolyte is noticed when acidified sodium chloride is used as an electrolyte in electrochemical machining. Salt electrolytes such as sodium chlorate, sodium chloride, sodium nitrate are well-known to generate huge quantity of byproducts during machining [11]. The NaCl electrolyte is non-toxic, inexpensive and its conductivity is stable over a broad range of pH value, which increases its conductivity with the increase in temperature. The NaCl electrolyte also generates stray machining, corrosive environment and produces a large volume of sludge due to its high throwing power.
The effect of the electrochemical dissolution behavior of SS304 alloy in sodium nitrate electrolyte solution with the low current density has been investigated, and qualitative analysis has been made for the dissolved specimen through scanning electron microscopy and X-Ray diffraction. The solid black byproducts formed while machining has been examined for their composition [12]. Comparison has been made between ECMM process with acidified NaNO3 electrolyte (sulfuric acid of 0.05 m/L added to NaNO3) solution and NaNO3 electrolyte solution and the performance of machining stainless steel with voltage, pulse on time, electrolyte concentration for the material removal rate and overcut was experimentally investigated [13]. The usage of sodium nitrate is meant to control and reduce stray machining considering its lower throwing power. In electrochemical machining of HCHCR Steel (High Carbon High Chromium Die Steel) where nano copper particles are made to suspend in NaCl electrolyte, which ultimately improves MRR and surface roughness and prevents spike formation in IEG that results in high current density [14].
Some researchers have done an investigation of the ECMM process with composite electrolytes. Addition of complexing agent to electrolytes was taken up for machining SS304 alloy and for efficient dissolution and elimination of electrolytic products. Composite electrolytes (a mixture of two or more electrolytes) contain complexing agents such as ligands and neutral salts. A study of different electrolytes was made for machining in the ECMM process and among them, an optimized electrolyte with a mixture of 1.0 mol/L NaNO3 and 0.01 mol/L sodium citrate was taken as a basic electrolyte for the study of MRR and inlet diameter of the micro hole with respect to feed rate [15]. Seven different types of electrolytes were studied and optimized for anodic dissolution of titanium alloy in ECMM process for finding a suitable machining electrolyte in which mixed electrolytes afford individual properties of all the electrolytes involved in machining, and ethylene glycol-based mixed electrolyte showed a better performance with respect to MRR, circularity, conicity and overcut with respect to the duty ratio [16]. NaCl-containing ethylene glycol solution is used to machine micro holes in titanium because of its intrinsic electrochemical property when compared with the water-based electrolyte, excellent surface integrity, acceptable shape accuracy, smooth surface, small average diameter and small taper angle that is obtained [17]. UV rays were employed to heat the electrolyte to enhance the performance of EMM to understand the heated electrolyte effect on process parameters. Electrolyte heated with UV rays was shown to be more suitable for higher MMR where accuracy is not critical [18]. The effect of electrolyte pressure on shape accuracy for producing a reverse tapered hole is studied by both numerical simulation and experimental tests predicted that increase in electrolyte pressure is beneficial in removing insoluble electrolytic products, improving the shape accuracy and machine stability [19]. In recent times, manufacturing industries is looking for the optimized approach for improving the quality of products and performance of the process i.e. the performance which ensures the better cut quality of the product under optimum conditions. These conditions were met by decision-making methodologies [20]. Polyethylene glycol is a polyether compound that varies according to its molecular weight with numerous applications from modern manufacturing to pharma industries as a lubricating coating for diverse surface applications in both aqueous and non-aqueous environments. It is almost white, waxy or paraffin-like in appearance with low toxicity and less systemic absorption [21]. In this work, polyethylene glycol is mixed with sodium nitrate called CPE. The qualitative models for the cathodic reactions are proposed to determine the influence of the adhered products on the electrochemical characteristics of the wires. The morphologies of blackened wires after machining were examined by SEM, while the compositions of the electrolysis products adsorbed on the wire were investigated by XRD and XPS [22]. SiC abrasives is mixed with Nacl electrolyte of varied concenterations to find the machininability of the composite material aluminum boron carbide to find out the MRR, Ra and overcut [23]. A review and particular study on drilling of high aspect ratio micro holes were carried out to find the effects of various key factors [24]. A detailed study on ANOVA is carried out for analsying the effect of contribution of various factors involved in machining [25] and same technique is incorporated in this work.
This study relates to the measurement of qualities of the drilled hole through MRR, circularity, conicity, and overcut in SS304 alloy by the combined effect of TOPSIS approach with SIMOS weighting criteria method by multiresponse optimization of ECMM process parameters. Hence it is the need of the hour to develop ECMM by entrainment of efficient universally accepted electrolyte. A review of the relevant literature reveals the total absence of any work carried out in the ECMM process through CPE as an electrolyte for machining SS304 alloy. In this study, an effort has been made to machine SS304 alloy using three different electrolytes, namely, NaNO3, NaCl and CPE, including discussion of their dissolution mechanisms.
Materials and Methods
Experimental Setup
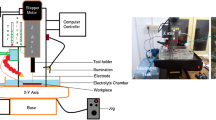
The micromachine used in this study is TTECM-10 electrochemical machine setup. The schematic of the machine is shown in Fig. 1. In this study, the workpiece material chosen was SS304 grade alloy of a rectangular plate of length 50 mm, breadth 50 mm, and thickness of about 400 μm. The tool material used was a hollow electrolytic copper tool which was in the cylindrical form with a significant diameter of 300 μm. For this experiments, polarity was chosen as the tool as a cathode to that of the workpiece as a anode. The specifications of the machine are given in Table 1. Recently, the use of various combinations of materials and alloys has been seen in ECMM research.
Electrolytes
The Electrolytes used in this study were NaCl, NaNO3, and CPE. CPE is the mixture of PEG 6000 and NaNO3. PEG was purchased from Aldrich. Salt forms of NaCl; NaNO3 and CPE were mixed with 1 l of distilled water. The experiments were carried with 0.3 M of NaCl, 0.2 M of NaNO3 and 0.2 M of CPE. All the electrolytes mentioned were aqueous-based. The selection of the electrolytes was done on the basis of the material depending on the roughness range of the work to be carried out.
Design of Experiments
Details of the machining conditions for SS304 alloy and the selected number of input level values of the related parameters are given in Table 2. In view of the fact, this is a beginning to explore CPE as the electrolyte, limited parameters and levels are used. A pilot study was made in the experiments on the effect of different process variables of input parameters with their well-fitted factors for the chosen electrolytes. A detailed study was made through a pilot work for the interactions. Differences between these inputs from others were studied considering that these values were found appropriate for the machining conditions, and the experiments were done. The response parameters such as MRR, circularity, conicity and overcut were used for the assessment of the performance of the ECMM process by altering the levels of the input factors. The other ECMM process parameters were kept at a constant level. These include pH, electrolyte concentration, electrolyte flushing, etc.
Experimental Procedure
Minitab software was used in designing of the experiments through the use of commercial Taguchi method. An L27 orthogonal array was developed for the performance of the experimental runs. The workpiece and tool were dipped in acetone for about half an hour to eliminate the presence of impurities on the surface. Current passage through the tool and workpiece was checked using an ammeter for confirming the given potential passes to both electrodes for every experiment. Graphs indicating MRR, circularity, conicity and overcut were plotted on the basis of experimental results. In the graph, X-axis denotes the number of the experimental run as each electrolyte was made to run a total of nine experiments as per design of experiments mentioned in Table 3.
Multi-Criteria Decision Making Methodologies
In MCDM’s, TOPSIS method was found to be highly successful in the decision-making methodologies since it involves easy computational steps, a reduced amount of computational time and the result values are very close to the best solution. TOPSIS performs better than other multiresponse optimization methods.
For the ECMM process, MRR was chosen as ‘larger the better’ performance characteristics, while other responses were chosen as ‘smaller the better’ performance characteristics. The output responses such as circularity, conicity and overcut should be the minimum as it is collectively considered the geometrical parameters so, it is chosen as such a characteristic. ECMM process comprises multiresponse characteristics, and hence satisfactory MCDM techniques were seen as needed for the selection of the optimal process parameters. On the other hand, only some researchers have shown using these methods for the selection of the process parameters and the manufacturing process. Hence, in this study, an attempt has been made to explore the ECMM process parameters, by altering the level of voltage (V), duty cycle, feed rate with three different electrolytes to measure the output responses that include MRR, circularity, conicity, and overcut which were taken collectively as a multi-objective problem for getting the best results among all the chosen alternatives.
TOPSIS method requires exact input data from the multi criteria problems for assigning weights to the entire criterion, which use the relative significance of multi-criteria for the output responses that are taken as actual problems in industries. SIMOS weighing procedure was used for providing subjective input weights to each factor. In this method, the output responses will be positioned from the least to the most significant factor. Differentiation involved in decision making for the responses from the least to the most significant in MRR, circularity, conicity and overcut was done according to the significance of the output responses. The decision model for the selection of optimal process parameters in ECMM was interpreted and chosen as shown in Fig. 2. The decision model shows the basis of selection of optimal process parameters and also the interaction of input parameters with respect to output responses.
The hole machined in the SS304 workpiece was 300 μm in diameter with three alternate electrolytes viz.; NaCl, NaNO3, and CPE as per design of experiments. The dissolved SS304 alloy specimen using NaCl, NaNO3, and CPE as electrolytes is shown in Figs. 3, 4 and 5. A separate tool was used for each L9 experiments. The hole diameters were measured using VMS and represented by the unique value of their individual exact diameters. The workpiece was weighed before and after machining. Metal removal rates were found by measuring the weight difference using an electronic weighing balance. The measurement of MRR is based on the weight difference of the workpiece before and after machining. The theoretical calculation of circularity, conicity and overcut is explained. Circularity is calculated by the difference of maximum circumscribing circle at entry and maximum inscribing circle at the exit of the micro hole. Conicity is the three-dimensional measure of the error of the machined hole. Overcut is measured by the difference between the tool diameter and average diameter of the hole produced in the workpiece. VMS images of the entry and exit side of the machined hole using NaCl, NaNO3, and CPE as electrolytes are shown in Figs. 3, 4 and 5 respectively.
The experimental outcomes for the output responses that include MRR, circularity, conicity and overcut for NaCl, NaNO3 and CPE electrolytes are shown in Table 3.
Multiresponse Optimisation
Optimization Steps Using TOPSIS Approach
In this work, SIMOS procedure was carried out and individual input weights to the output responses were specified. Equal weights were provided for all the output responses and computational steps of the method followed were summarized below. The weighting criterion was considered and well planned as shown below:
TOPSIS was selected from among the other optimization techniques such as Grey relational analysis, ELECTRE I, II, and FUZZY TOPSIS. In this study, design set of criteria (output responses) is ranked by decision-makers based on their significance, from least to the most important i.e. MRR is kept as least important while geometric features such as overcut, conicity, and circularity are reserved as most important. So, the weights provided for overcut, conicity, and circularity are same while MRR is given the least weightage. The optimization steps of TOPSIS were designed and calculated. The steps involved in the procedure are detailed below.
- Step 1.
Normalized values are taken by eliminating the units of all criteria, and selecting the alternatives.
Such that.
- i:
number of experimental runs,
- j:
number of output responses,
- xij:
normalized value of ith experimental run linked with jth output response
Table 4 represents the normalized performance matrix (rij) calculated with the help of eq. 1.
-
Step 2.
The weighted normalized matrix (wij) was determined using the product of normalized value and the weighted values. Equation 2 given below was used for the calculation.
Computational steps of SIMOS weighting procedure to the response parameters from least to the most important is considered and displayed in Table 5.
-
Step 3.
Measurements of the best alternative performance (S+) and the worst alternative performance (S−) for each output response which is an ideal alternative were made using eq. 3 considered when jth criterion included better performance where “S+” denoted a positive unique solution.
Similarly “S− “values were measured when jth criterion included the worst performance whereas “S−“denoted a negative unique solution
- Step 4.
This step involved the measurement of the performance of each criterion. “D+i” represents the values from S+ values which was chosen as the best distance, and “D-i” represents the values from S− values which was chosen as the worst alternative distance. D+i and D-i values are calculated by means of the following eq. 4 and 5.
Where, i = 1, 2, 3 ….27.
- Step 5.
The values of closeness coefficient (Ci) for each alternative were measured using eq. 6.
Preference rank was setup using the “Ci” value and arranged according to the best alternative, related to the unique solution. Table 6. Shows the performance, closeness coefficient value, and rank of each alternative under the best and worst conditions.
TOPSIS method was used in order to find the optimal level of the machining parameters. Average closeness coefficient values were determined. Predicted response values (ɣpredicted) were evaluated for finding the optimal level of ECMM process machining parameters using eq. 7.
Table 7 is the mean response table for the corresponding closeness coefficients. Experimental run 22 showed rank 1 and hence the same set of optimized process parameters was found in the mean response table, without the requirement of any confirmation test. The confirmation test is meant to run the experiment with the optimal conditions brought out from the optimization method. The closeness coefficient value of each level of the machining parameter in the L27 orthogonal array was used for getting their mean values. These values denote the optimal level of process parameters by every factor level. Amongst all the values of 27 closeness coefficients, experimental number 22 showed the maximum closeness coefficient value. Hence it was measured and taken as the best multi-response characteristic for machining. Experiment number 22 showed higher closeness coefficient value so the related process parameters are chosen as the optimal parameter setting level from the considered multi-criteria output responses.
Results and Discussions
Based on the experimental outcomes, different output response factors namely MRR, circularity, conicity and overcut of the machined holes were measured. The most reliable hole was chosen for the three electrolytes and SEM image was taken to study the micro hole characteristics. The proper power supply was needed to control the geometrical features. Micro holes and geometrical features machined with different electrolytes were evaluated on the basis of the entry and exit diameters of the micro hole. Graphs representing the trends of MRR, circularity, conicity and overcut were plotted to denote evaluation of the performance of all electrolytes and the input parameters involved in machining. The effect of voltage, duty cycle and feed rate with respect to output responses was studied with the objective of providing improvement to the quality of micro holes produced. The effect of aqueous-based electrolytes in the dissolution performance of SS304 alloy was also studied with the help of SEM image based on the results of TOPSIS optimization. Formation of oxide layers in and around the geometry of the micro holes was noticed, this was due to the nature of the electrolytes. The effects of input parameters and also the performance of aqueous-based electrolytes during ECMM of SS304 alloy are discussed. Figure 6 is the interaction plot to study the effect of input process parameters. In order to that, the confidence level is chosen as 95% so the significance level is 5%. The significance and interaction of the chosen input parameters namely electrolyte, voltage, feed rate and duty cycle can be clearly studied with the plot.
Effect of the Process Parameters in the Machining of SS304 Alloy
Machinability of SS304 alloy using ECMM depends on the chosen input parameters chosen namely, electrolyes, voltage, duty cycle, and feed rate. Input parameters play a vital role in the machining of SS304 alloy. In this study, the MRR obtained with CPE electrolyte was seen as the maximum amongst all the electrolytes. This is in agreement with multi-response optimization results. The higher metal dissolution takes place with a voltage of 7 V, the feed rate of 0.5 mm/min and duty cycle of 0.7 with CPE as the electrolyte. An increase in the output response parameters was seen with an increase in voltage, feed rate, and duty cycle.
Effect of Aqueous-based NaCl Electrolyte in Machining SS304 Alloy and Hole Morphology
Experimental run 1–9 denotes the machining of SS304 alloy using NaCl electrolyte in which non-uniform material loss in the workpiece surface occurs. Aqueous NaCl electrolyte solution contains chloride ions which react with SS304 alloy that artificially corrodes the workpiece by creating its hydroxides and then it dissolves. Figure 7 depicts the SEM image of the hole machined using NaCl as an electrolyte, the hole machined is seen as irregular in shape and size. Termination of machining due to the short circuit was observed as the micro tool progressed towards workpiece and touched the workpiece surface. Formation of brown-colored sludges around the micro tool in the machining zone was observed. The color of the electrolyte changed to yellow with the use of NaCl as the electrolyte. The surface finish of the NaCl electrolyte etched sample was improper. This was considered as important as it was directly involved with the mechanical strength of the material.
The dissolution of SS304 alloy is known to be uncontrolled at the entry and exit of the micro hole with irregular etching leading to high pitting on the surface area of the workpiece. Some evidence of localized attack such as micro pits was detected in and around the hole profile. This dissolution behavior was the result of the presence of a large number of aggressive Cl− ions which cause improper etching. The presence or absence of chloride ions determines the extent of passive film breakdown. Pitting marks and formation of brown oxides were noticeable on the entry as well as on the inner surface wall of the micro hole. Moreover, when stainless steels are exposed to chloride ions, they destroy the protective passive film irregularly and turn into the vulnerable electrolyte to attack SS304 alloy surface severely and the attack occurs in a localized manner. So for machining SS304 alloy, NaCl is found to be not suitable as it does not provide the best hole profile with better geometrical aspects.
Effect of Aqueous-based NaNO3 Electrolyte in Machining SS304 Alloy and Hole Morphology
Experimental runs 9–18 relate to the machining of SS304 alloy using NaNO3 electrolyte in which uniform material loss in workpiece surface occurs. Figure 8 depicts the SEM image of the hole machined using NaNO3 electrolyte, the hole machined doesnot undergo vigorous electrochemical attack in and around the surface. The SEM image shows improvement in hole profile by the micro-hole formed compared to the micro-hole produced with sodium chloride electrolyte. The current density in the entrance of the hole led to poor surface quality and on the inner surface wall of the micro hole.
Selective metal dissolution that begins in parallel with the formation of bubbles was observed in nitrate solutions. Oxygen evolution reaction in the tool is attributed to N2 formation by the reduction reaction of nitrate ions. There was the occurrence of selective material removal with elapse of time, and more black products covered and accumulated on the workpiece surface. Hydroxides are formed and nucleated near the dissolving metal surface. Oxide layers with black color were formed. This was primarily a combination of carbides and oxides which required a study for their composition. With the passage of time, loose black products became thicker and were eventually moved by the electrolyte leaving some traces of debris along the inner surface as shown in Fig. 9. Workpiece surface was observed with pitting marks of smaller dimensions, revealing consistent dissolution with controlled hole shape at the entry and exit sides. So, for machining SS304 alloy, NaNO3 electrolyte was found suitable but a very small quantity of black products that emerge on the surface of the substrate. The micro hole confirmed the development of a shiny ring pattern near the hole entry with some confined pitting marks, which was evident in the SEM image. However, microscopic roughness is observed near the surface of the inner wall.
Effect of Aqueous-based CPE Electrolyte in Machining SS304 Alloy and Hole Morphology
Experimental runs 18–27 denote the machining of SS304 alloy using CPE as the electrolyte in which there was the occurrence of uniform material loss in the workpiece surface. Figure 10 shows the SEM image of the hole machined using CPE electrolyte, and there was no electrochemical attack seen in and around the surface of the hole machined. The micro hole did not confirm the development of a shiny ring pattern near the hole entry when compared to the hole machined in NaCl and NaNO3 electrolyte. The effect of using CPE electrolyte caused a reduction in the effect of pitting marks near the micro hole entry. The usage of a composite electrolyte caused the occurrence of very low current density at the edge of the tool leading to the creation of machining products in small number.
The outcome of CPE electrolyte was the creation of the bubble in small number which completely eradicated the short circuit difficulty seen during machining for the optimal combinations of process parameters resulting in lower etching rate. It also caused a reduction in the formation of metal hydroxides providing encouraging results by preventing regular short circuit as it reduced the contact of oxygen throughout the machining. Metal dissolution occurred primarily as a result of the electrochemical processes involving anodic oxidation and simultaneous cathodic reduction, in parallel with both ionic and electrical conductions between the anode and the cathode. CPE electrolyte acted as an ionic conductor that could be transferred directly to the metal surface as a result of the solid electrochemical reactions taking place at the surface of the workpiece which made a solid-solid micro contact with the ions of nitrates and the target metal surface. The addition of PEG was meant to maintain the ionic conductivity towards the workpiece and also provide smooth corners around the micro-hole formed as it has the capability to attach directly into the porous film of the metal surface as shown in Fig. 11. Addition of NaNO3 was required to ensure complete protection and free from pitting surface. CPE has low toxicity and is highly cost-effective than current mixed electrolytes. The advantage of using CPE is the formation of fewer gas bubbles.
Effect of Voltage on the Machining Performance of SS304 Alloy
The nature of voltage distribution has an influence on the profile of the micro hole. The amount of the voltage supplied defines the potential difference and also the geometrical features. Figure 12 shows the material removal rate trend graph of SS304 alloy using different electrolytes, in which CPE electrolyte gives larger MRR when compared to other electrolytes, despite NaCl and NaNO3 electrolyte providing better MRR but not better than CPE electrolyte. It clearly shows CPE electrolyte providing the best metal removal rate among all the electrolytes. There was an increase in MRR with the increase in voltage as machining current also increased. A drastic increase in MRR was seen in the case of CPE electrolyte when compared to other electrolytes. Multiresponse optimization results indicated experimental run 22 provided larger MRR, low circularity, conicity and overcut. In NaCl electrolyte, the increase in contamination of the electrolyte caused the deposition followed by the occurrence of irregular dissolution in the material. In NaNO3 electrolyte, MRR was also maintained at low current density as the current efficiency was still higher with the feed rate of the tool. The presence of some undissolved particles was observed. Machining voltage of 7 V was applied between tool and workpiece for CPE electrolyte, creating a potential difference between them. Transfer of ions and electrons occurred and material from the surface of the material was removed using the basic mechanism of artificially corroding the workpiece surface. The pulse voltage applied was the average machining voltage that provided even current density distribution and also minimized the variation in the electrical conductivity in the machining region. When voltage increased from 6 V to 7 V, the potential difference between cathode and anode increased the electrolytic ionization as it increased the current density, where joule heat was generated which was not maximum and tending the MRR to increase. This is in agreement with the fact that when the voltage increases, the current density increases in IEG thus increasing the MRR.
Circularity, conicity and overcut are also to be taken care while producing a micro hole. Considering the geometrical features, they directly increase with the increase in voltage supply. While using NaCl electrolyte, the increase in machining voltage caused a decrease in the current flow and localization effect. So inaccurate machining occurred leading to circularity, conicity, and overcut phenomenon. At a higher voltage, H2 bubbles broke down and uncontrolled material removal occurred from the workpiece as a consequence providing a steep increase in circularity, conicity and overcut. So overcut increases more rapidly at a higher voltage range. A smaller localization effect, caused an increase in the flow of the stray current actually increases, sequentially causes more material removal from the more current density affected area of the workpiece paving the way to circularity, conicity and overcut. But in this CPE electrolyte, there was an increase in localization effect which provided controlled material removal leading to less circularity, conicity and overcut. Due to smaller bubble formation that did not hinder electrolyte providing better geometrical features. Figure 13 is the circularity trend graph of the three electrolytes used in which, except in the experimental level 4 and 5 of CPE electrolyte, the circularity is decreased with the performance of NaCl and NaNO3 electrolyte. Increase in the circularity and conicity for CPE electrolyte was seen for the setting voltage of 7 V, the feed rate of 0.6 mm/min, and duty cycle of 0.8 which was the maximum setting level of feed rate, duty ratio and an average value of voltage signifies the setting level is not preferable for CPE electrolyte. Hence, the duty cycle and voltage are preferable for improving the geometrical properties at a low feed rate.
Figure 14 is the conicity trend graph of the used three electrolytes in which the conicity value of CPE electrolyte lies intermediate with the values compared among NaCl and NaNO3 electrolyte. Conicity increases for the experimental level 9 of CPE electrolyte which is up to 2.31 in which the setting level is about 8 V, 0.7 mm/min and duty cycle of 0.7. Maximum voltage is also not suitable for the improvement of geometrical features.
Figure 15 is the overcut trend graph of the three electrolytes used in which there is a big decrease in the overcut of CPE electrolyte with the values compared between NaCl and NaNO3 electrolytes. Overcut should be less in the case of all geometrical parameters as the expected micro hole diameter should not be more than the tool diameter. The micro hole produced was seen as very good in the case of CPE electrolyte as no hole was more than the diameter of 301 μm due to the intrinsic electrochemical property of the CPE when compared to the other electrolytes. The transfer of ions is narrowed down to the possible nearest anodic surface. But the overcut trend in the other electrolytes was more indicating the better suitability of CPE as mixed type electrolyte for producing a micro hole with a small overcut.
Effect of Feed Rate on Machining Performance of SS304 Alloy
Feed rate is one vital input factors to be considered while producing a micro-hole. Throughout the machining, the micro tool was in the active movement towards the stable workpiece. Increasing the feed rate in between the workpiece and tool results in high current density as conducting path length decreases thus enhancing the rapid anodic dissolution. In this work, the tool remaining at a particular location at a lower feed rate increased the charge flow rate. When NaCl electrolyte was used, an increase in feed rate caused the occurrence of variations in local conductivity creating a non – uniform current density with a tendency to create non-uniform dissolution of metal, constituting the main reason for the sidewall erosion resulting in a larger overcut. The intensity of the current was higher at the tip of the tool than at the other areas, leading to poor dimensional uniformity. At a higher feed rate, the tool did not remain in a particular location for a longer cycle thereby causing a decrease in machining time and overcut and providing good geometrical features and MRR. Increasing the feed rate during the use of CPE electrolyte caused easy interactions of the tool and the workpiece. Hence, with a decrease in machining time, the material that got eroded on the side walls was less. This ultimately caused a reduction in circularity, conicity followed by the overcut. Production of good geometrical features and a higher material removal rate by CPE electrolyte was observed. This was due to the input feed to the micro tool, that is, the workpiece dissolution of the micro-hole from the entrance to exit being higher, hence a low diversity between the entry and exit diameters of the micro-hole. In addition to that, the applied feed rate made the tool stay for a certain time duration in the workpiece. The effect of circularity, conicity and overcut can be reduced through a selection of the best possible tool feed rate. Higher feed rate specifically above 0.9 mm/min caused frequent contact of anode and cathode leading to short circuit and made the machining process unstable. ECMM can produce better experimental results with the recommended optimal feed rate of 0.3 to 0.7 mm/min. In CPE electrolyte, when the feed rate was increased, the micro hole formed was more precise than that of the hole formed in other electrolytes with increased feed rate.
Effect of Duty Cycle on Machining Performance of SS304 Alloy
Duty cycle plays a significant role in machining performance. MRR and geometrical features can also be improved with the applied duty cycle. With the increase in the duty cycle, MRR increased gradually up to a certain value, and then circularity, conicity and overcut also increased. Applied pulse on time with low current density, led to a substantial improvement in machining accuracy and geometrical aspects. Improvement in the machining performance was achieved with the proper pulse over a duration and applied pulse intervals between them. Pulse off time should be properly supplied for improving MRR considering that longer pulse off time ensures the entry of new electrolyte in the IEG which washes away debris. In CPE electrolyte, the formation of debris was very small, so continuous refreshment of electrolyte helped enhancement of the surface quality and geometrical features. MRR showed a tendency to increase in higher pulse cycle which was actually an increase in pulse on time. Higher duty cycle above 0.8 led to a declined rate of MRR and improper geometrical characteristics due to the complication in the electrolyte exchange mechanism. ECMM can produce better experimental results with the recommended duty cycle in the range of 0.4 to 0.7. At a duty cycle of 0.7, maximum material removal can be observed in all pulse period conditions i.e. at this condition pulse on time is considerably more than the pulse off time. Shorter pulse period with smaller duty cycle is needed to obtain good surface and machining accuracy. During pulse power, the sludges are formed as a result of dissolution adhered in the IEG was obviously pulled off by the CPE electrolyte as the viscosity is also very high during the pulse-off time, and fresh electrolyte come into contact for the next cycle in the machining gap. As the duty cycle changed from 0.6 to 0.7, the depth of the micro-hole was decreased providing proper conicity. A localized material removal should not happen in this case as it does not form a circular micro hole. Hence power pulse given should be with a certain amount of pulse off time to control the depth of the micro-hole. So, material removal occurred during the pulse-on time with controlled etching to produce a micro-hole with good geometrical aspects and better surface finish thereby increasing the efficiency of machining.
Analysis of Variance
Table 8 represents the ANOVA table showing the contribution factor for the machining process which shows electrolyte as the predominant input process parameter in machining SS304 alloy. ANOVA table depicts that totally around 39.93% of the contribution is provided by the electrolyte.
Conclusions
In this study, TOPSIS method was carried out for the ECMM process, for optimizing the input and output process parameters for their multiresponse characteristics. Ranks were generated for the output response parameters in ECMM of SS304 alloy based on the SIMOS weighting criteria method. Conclusions are drawn as:
Higher MRR and lower circularity, conicity and overcut are achieved and identified based on the combinations of the ECMM process parameters and their levels.
For the multiresponse optimization of ECMM process parameter, experiment number 22 has been identified as conferring maximum closeness coefficient value whose experimental setting are follows: Electrolyte as CPE, voltage of 7v, the duty cycle of 0.7, and feed rate of 0.5 mm/min.
Combinations of the input process parameter in the ECMM process suggested as the optimum levels are identified on the basis of the response values of closeness coefficients. The conclusions drawn from the observations in the ECMM experiments is that the TOPSIS method is successful for solving multiresponse problems.
For all the chosen output responses such as MRR, circularity, conicity and overcut of the machined micro hole, The CPE electrolyte generate superior results among the choice of electrolytes. This is due to the reason, mixed type of electrolytes offers shared advantages of all the electrolytes added.
Electrolytes are the most dominant parameters in reducing the surface quality of the machined workpiece. Better surface finish is achievable in CPE.
Geometrical features namely, circularity, conicity and overcut are affected mainly by duty ratio, feed rate followed by voltage.
This research work has shown the possibility of using CPE as an electrolyte in the ECMM process. It gives high MRR and reduced circularity, conicity and overcut compared to the commercially used electrolyte and CPE emanates less smoke and fewer odors.
Electrolytes with higher ionic conductivity are expensive and need import but CPE can be prepared easily. It is economically viable (import substitute) and commercially feasible. Use of CPE electrolyte helps increase in productivity.
Various types of electrolytes with higher ionic conductivity are available in the market. A further study of the purpose of using it as an electrolyte to offer all their properties as an advantage and which can be used commercially in industries can be taken up.
Abbreviations
- ECMM:
-
Electrochemical micromachining
- MRR:
-
Material removal rate
- CPE:
-
Composite electrolyte
- PEG:
-
Poly ethylene glycol
- DOE:
-
Design of experiments
- TOPSIS:
-
Technique for order preference by similarity ideal solution
- IEG:
-
Inter electrode gap
- VMS:
-
Video measuring system
- ANOVA:
-
Analysis of variance
- SEM :
-
Scanning electron microscope
References
Chen C, Li J, Zhan S, Yu Z, Xu W (2016) Study of microgroove machining by micro ECM. Proc CIRP 42:418–422
McGeough JA (1988) Advanced methods of machining. Chapman and Hall, London
Lin GM, Cai HZ (2012) Electrochemical machining technology and its latest applications. Adv. Mater.Res 472-475:875–878
leese RJ, Ivanov A (2016) Electrochemical micromachining: an Introduction. Adv Mech Eng 8(1):1–13
Gupta AK, Krishnamurthy HN, Singh Y, Prasad KM, Singh SK (2013) Development of constitutive models for dynamic models for dynamic strain aging regime in austenitic stainless steel 304. Mater Des 45:616–627
Das AK, Saha P (2013) Machining of circular micro holes by electrochemical micro-machining process. Int J Adv Manuf 1:314–319
Yong L, Ruiqin H (2013) Micro electrochemical machining for tapered holed of fuel jet nozzles. Proc CIRP 6:395–400
Bao H, Xu J, Ying L (2008) Aviation- oriented micromachining technology- micro- ECM in pure water. Chin J Aeronaut 21:455–461
Ryu SH (2009) Micro fabrication by electrochemical process in citric acid electrolyte. J Mater Process Technol 209:2831–2837
Yang Y, WataruNatsu WZ (2011) Realization of ecofriendly electrochemical micromachining using mineral water as an electrolyte. Precis Eng 35:204–213
Sharma S, Jain VK, Shekar R (2002) Electrochemical drilling of Inconel super alloy with acidified sodium chloride. Int J Adv Manuf Technol 19:492–500
Wang D, Zhu Z, Wang N, Zhu D, Wang H (2015) Investigation of the electrochemical dissolution behavior of inconel 718 and 304 stainless steel at low current density in NaNO3 solution. Electrochim Acta 156:301–307
Thanigaivelan R, Arunachalam RM, Karthikeyan B, Loganathan P (2013) Electrochemical micromachining of stainless steel with acidified sodium nitrate electrolyte. Proc CIRP 6:351–355
Sekar T, Arularasu M, Sathiyamoorthy V (2016) Investigations on the effects of Nano- fluid in ECM of die steel. Measurement 83:38–43
Gudong L, Yong L, Kong Q, Hao T (2016) Selection and optimization of electrolyte for micro electrochemical machining on stainless steel 304. Proc CIRP 42:412–417
Anasane SS, Bhattacharyya B (2016) Experimental investigation on suitability of electrolytes for electrochemical micromachining of titanium. Int J Adv Manuf Technol 86:2147–2160
Liu W, Zhang H, Luo Z, Zhao C, Ao S, Gao F, Sun Y (2018) Electrochemical micromachining on titanium using the NaCl-containing ethylene glycol electrolyte. J Mater Process Tech 255:784–794
Soundarrajan M, Thanigaivelan R (2018) Investigation on electrochemical micromachining (ECMM) of copper inorganic material using UV heated electrolyte. Russ J Appl Chem 91(11):1805–1813 ISSN 1070-4272
Liu G, Li Y, Kong Q, Yu L (2018) Impact analysis of electrolyte pressure on shape accuracy of micro holes in ECM with hollow electrodes. Proc CIRP 68:420–425
Yuvaraj N, Pradeep Kumar M (2014) Multiresponse optimization of abrasive water jet cutting process parameters using TOPSIS approach. Mater Manuf Process: 882–889. https://doi.org/10.1080/10426914.20.
Kassiff G, Ben Shalom A (1971) Experimental relationship between swell pressure and suction. Geotechnique 21:245–255
Gao C, Ningsong Q, Ding B, Shen Y (2019) An insight into cathodic reactions during wire electrochemical micromachining in aqueous hydrochloric acid solution. Electrochim Acta 295:67–74
Sankar M, Gnanavelbabu A, Rajkumar K, Thushal NA (2017) Electrolytic concentration effect on the abrasive assisted-electrochemical machining of an aluminum–boron carbide composite. Mater Manuf Process 32(6):687–692. https://doi.org/10.1080/10426914.2016.1244840
Rahman Z, Das AK, Chattopadhyaya S (2017) Microhole drilling through electrochemical processes: a review. Mater Manuf Process 33(13):1379–1405. https://doi.org/10.1080/10426914.2017.1401721
Sadagopan P, Mouliprasanth B (2017) Investigation on the influence of different types of dielectrics in electrical discharge machining. Int J Adv Manuf Technol 92:277. https://doi.org/10.1007/s00170-017-0039-1.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mouliprasanth, B., Hariharan, P. Measurement of Performance and Geometrical Features in Electrochemical Micromachining of SS304 Alloy. Exp Tech 44, 259–273 (2020). https://doi.org/10.1007/s40799-019-00350-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40799-019-00350-y