Abstract
Purpose of Review
Stem cells respond to local paracrine signals; more recently, however, systemic hormones have also emerged as key regulators of stem cells. This review explores the role of steroid hormones in stem cells, using the Drosophila germline stem cell as a centerpiece for discussion.
Recent Findings
Stem cells sense and respond directly and indirectly to steroid hormones, which regulate diverse sets of target genes via interactions with nuclear hormone receptors. Hormone-regulated networks likely integrate the actions of multiple systemic signals to adjust the activity of stem cell lineages in response to changes in physiological status.
Summary
Hormones are inextricably linked to animal physiology and can control stem cells and their local niches. Elucidating the molecular mechanisms of hormone signaling in stem cells is essential for our understanding of the fundamental underpinnings of stem cell biology and for informing new therapeutic interventions against cancers or for regenerative medicine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Physiological homeostasis requires hormones, which are circulating chemical messengers that ensure that cells with diverse functions in tissues throughout the body work in a coordinated manner to maintain organismal health [1, 2]. Hormones are produced by specialized cells in highly innervated glandular tissues, such as the pituitary and thyroid, and function as long-distance signals to communicate changes in physiological conditions, including altered carbohydrate and lipid metabolism, growth, sexual maturation, and stress. Hormones affect every organ system in the body; it is therefore not surprising that tissue-resident stem cells, which are the sources of new cells within established tissues, can sense and respond to circulating hormones as do terminally differentiated cells [3–7]. In particular, steroid hormones, known as vital modulators of organismal growth, developmental transitions, and secondary sex characteristics, have recently achieved greater prominence as new roles for them in the regulation of stem cell function have emerged (Table 1) [3, 4]. Elucidating the fundamental molecular mechanisms of how hormonal signals are sensed and interpreted by stem cells, in their native environment, is critical for the development of future therapeutic strategies for regenerative medicine and cancer treatment.
In this review, we highlight new discoveries about the role of steroid hormones in the control of tissue-resident stem cells. We focus on the Drosophila female germline stem cell (GSC), a model system that illustrates how stem cells respond to steroid hormones in a physiological context. We summarize the major themes of how diverse steroid hormones regulate stem cell fate and the differentiation of stem cell progeny, using examples from Drosophila and mammalian stem cell lineages to suggest key areas for future study. Finally, we discuss the implications of steroid hormone control of stem cell fate and function for human diseases and potential regenerative medicine applications.
Tissue-Resident Stem Cells: an Essential Source of Cells for Tissue Homeostasis and Regeneration
Most adult tissues require the activity of stem cells for homeostasis and proper function. Tissue-resident stem cells have two defining characteristics: they self-renew, maintaining a stem cell pool throughout the life of the organism, and they generate daughter cells that can differentiate into one or more distinct terminal fates [26]. These properties ensure that tissue integrity and cellular diversity are maintained in the face of normal cellular turnover, tissue remodeling, or damage. Adult stem cells are lineage-restricted, such that they only generate daughter cells specific to their tissue of residence. For example, mammalian hematopoietic stem cells replenish all of the mature cells in the blood cell lineage [27], while intestinal stem cells give rise to the absorptive and secretory cell types that compose the intestine [28]. Stem cells have also been identified in tissues with less frequent cellular turnover, such as the brain [29], or, conversely, that undergo dramatic remodeling during adult life, such as the mammary epithelium [4]. Given their central roles in tissue homeostasis, stem cells must be tightly regulated to prevent tissue overgrowth or atrophy.
A major challenge in the field of stem cell biology is to understand at the molecular level the mechanisms by which stem cells maintain their defining properties and adjust their activity in the context of intact organisms. Over the years, a variety of model stem cell systems ranging from invertebrates to mammals have emerged, largely due to advances in lineage tracing that enable stem cell identification. Of these, the fruit fly, Drosophila melanogaster, stands out as a pioneer for many key experimental demonstrations underlying current models for the maintenance of stem cell identity and function [30]. Like mammals, Drosophila have multiple tissue-resident stem cell populations that sustain the production of differentiated cells. The ease with which Drosophila is reared; the wealth of available genetic tools for cell-specific gene manipulation; the amenable cell biology of their stem cell-supported tissues; and the remarkable evolutionary conservation of molecular, cellular, and physiological mechanisms make them a powerful model organism for stem cell research.
The Drosophila Female Germline Stem Cell: a Model System for Studying Stem Cell Regulation by Steroid Hormone Signaling
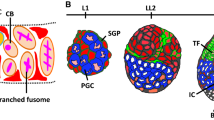
The Drosophila female GSC system has been a major experimental model for the elucidation of the cellular and molecular basis of stem cell niches and for exploring how whole-body physiology can impact stem cell lineages. Female GSCs give rise to the cellular precursors for Drosophila oocytes [31, 32]. GSCs are housed in a structure called the germarium (Fig. 1a, b) at the anterior tip of each of the 14 to 16 ovarioles that comprise the Drosophila ovary (Fig. 1c). GSCs reside in a somatic niche composed of terminal filament cells, cap cells, and a subset of escort cells (Fig. 1a). The niche produces bone morphogenetic protein (BMP) signals that are necessary for GSC self-renewal [32]. GSCs are physically attached to cap cells via E-cadherin and divide asymmetrically to create a posteriorly displaced cystoblast, the daughter cell destined for differentiation, while retaining the other daughter as a GSC in the niche. The cystoblast divides four more times with incomplete cytokinesis. One of the cells of the resulting 16-cell cyst becomes the oocyte, while the other 15 become nurse cells that support oocyte development and produce factors required by the early embryo [31]. Somatic follicle cells derived from follicle stem cells (FSCs) surround each germline cyst to form an egg chamber or follicle that subsequently leaves the germarium (Fig. 1a). The anatomy of the Drosophila ovary, coupled to the availability of sophisticated genetic and cell biological tools, greatly facilitates the analysis of GSCs and their descendants. Specifically, GSCs and their progeny exist in a predominantly linear arrangement, providing in a single ovariole an orderly time course of their recent developmental history (Fig. 1c). Drosophila female GSCs therefore remain one of the best models for the study of stem cell function and regulation at the single cell level in vivo. In particular, definitive evidence that stem cells are controlled by multiple endocrine factors, including steroid hormones, tied to the physiological status of the organism was obtained in the Drosophila GSC system [32].
The steroid hormone ecdysone controls multiple steps of Drosophila oogenesis. Female germline stem cells (GSCs) reside in a germarium (a, b) at the anterior tip of each of the 14–16 ovarioles (c) that compose the Drosophila ovary. a GSCs are anchored to adjacent cap cells that, along with terminal filament (TF) cells, send signals to maintain GSCs in a self-renewing fate. GSCs divide to form daughter cells (cystoblasts, CB), which divide four additional times to form 16-cell germline cysts composed of nurse cells (nc) and an oocyte (oo). Escort cells (gray) signal to germ cells to promote differentiation. Follicle stem cells (FSCs) divide to form prefollicle cells, which surround the 16-cell germline cyst, giving rise to a follicle that leaves the germarium. Prefollicle cells give rise to a variety of specialized follicle cells (fc; red) that form an epithelial monolayer around each cyst. b Confocal micrograph of a germarium immunolabeled with anti-Vasa (green; labels all germ cells), anti-Hts (red; labels early germline-specific organelles called fusomes and follicle cell membranes), anti-Lamin C (red; labels the nuclear envelope of cap cells), and DAPI (blue; labels all nuclei). Dashed lines outline GSCs (white) and cystoblasts (yellow). Scale bar, 10 μm. c Summary of ecdysone-regulated processes in the ovary. Ecdysone is produced by older follicles and stimulates the EcR/Usp complex expressed throughout the ovary. Activation of the complex results in a variety of cellular responses mediated by distinct ecdysone-responsive transcription factors, such as E74, E75, E78, and Br
Steroid Hormone Signaling Is Essential for GSC Function and Development of Their Progeny
The best characterized steroid hormone in Drosophila is ecdysone, which is structurally similar and functionally analogous to the mammalian sex steroids estrogen and progesterone [33, 34••]. Ecdysone signals by binding to the ecdysone receptor, a heterodimeric complex of two conserved nuclear hormone receptors encoded by ecdysone receptor (EcR) and ultraspiracle (usp) [35]. Binding of ecdysone to its receptor transcriptionally activates a wide variety of target genes, including transcription factors that direct cell-specific target gene regulation [36]. Drosophila synthesize ecdysone from dietary plant-based cholesterols through a multi-step enzymatic process [37]. During development, ecdysone is synthesized in an endocrine organ called the ring gland and regulates many processes, including organismal growth, ovarian cell differentiation, and ovary size [10, 36, 38, 39, 40••], whereas in adults, it is produced predominantly in late-stage follicles in the ovary [37, 41, 42].
In adult females, ecdysone is an essential regulator of female metabolism and reproduction [34••, 42, 43••, 44••]. For example, during copulation, males transfer sex peptide, a seminal fluid peptide that stimulates a variety of neurons to induce post-mating changes in females, including up-regulation of ecdysone synthesis in the ovary [43••, 45–47]. Ecdysone acts in the female central nervous system to promote a metabolic state supportive of oogenesis [34••]. Specifically, knockdown of ecdysone signaling in neurons reduces feeding rates in female flies, as well as decreases whole-body triglyceride and glycogen storage, leading to decreased egg production [34••].
Ecdysone signaling also has multiple roles during oogenesis to ensure efficient egg production under favorable conditions (Fig. 1c) [42, 44••, 48–50]. EcR, usp, and the ecdysone target genes broad (br), ecdysone-induced protein 74EF (E74), ecdysone-induced protein 75B (E75), and ecdysone-induced protein 78C (E78) are all required for oogenesis [48, 49, 51••, 52–54]. Ecdysone signaling mediates the increase in GSC proliferation induced by the first mating of young females [43••] and is necessary for sustained GSC proliferation and self-renewal [8]. Ecdysone signaling is also necessary for the differentiation of germ cells and the individualization of germline cysts into discrete follicles [9••, 10, 11, 12••]. As oogenesis proceeds, ecdysone signaling is required for the growth and survival of germline cysts at two important developmental stages. Germline cysts harboring mutations in EcR, E74, E75, or E78 frequently degenerate at stage 4/5 [48, 49, 51••], and E75 is thought to control a nutritional checkpoint at stage 8/9 that permits reabsorption of follicles in starved females [55]. Ecdysone signaling promotes vitellogenesis by upregulating yolk protein expression in follicle cells [56] and stimulating lipid accumulation in oocytes [34••]. For the latter, activation of EcR and E75 results in the transcriptional activation of genes involved in lipid metabolism, including the transcription factor SREBP [34••]. Taken together, these data demonstrate that ecdysone signaling controls a wide variety of cellular functions in the ovary.
Ecdysone regulation of the GSC lineage involves direct and indirect mechanisms. Proliferation and self-renewal are impaired in usp and E74 mutant GSCs, indicating that ecdysone signals are directly received by GSCs to control proliferation and maintenance [8]. Ecdysone also stimulates GSCs indirectly through neighboring somatic cells. Knockdown of EcR, usp, E75, or the EcR co-activator tai in escort cells and early follicle cells results in decreased GSC number and proliferation [10, 11, 43••]; however, it is unclear whether a requirement for ecdysone signaling during GSC establishment may contribute to these effects. Of note, the regulation of GSC function by ecdysone is largely independent of systemic insulin signaling, despite the fact that neural insulin signals are also necessary for GSC proliferation and, indirectly, for their maintenance [8, 43••, 57].
Recent evidence suggests that ecdysone signaling is also required in the follicle cell lineage. A genetic screen designed to find ecdysone-responsive genes required for oogenesis identified several genes that modulate the fate and proliferative capacity of FSCs [12••]. FSCs give rise to the follicle cell lineage, which is required for proper germline development and ultimately forms the eggshell [31]. Ecdysone signaling is well known to regulate follicle cell function at later stages of oogenesis [52–54, 58, 59••, 60, 61••, 62], but its roles in early somatic cell differentiation remain largely undescribed. While our data suggest that ecdysone signaling controls FSC function [12••], it remains to be investigated whether this occurs through similar molecular mechanisms as those controlling GSCs. The screen identified a mixture of downstream targets, some common to GSCs and FSCs and some with separate effects, suggesting that ecdysone signaling may control the two stem cell populations largely independently. Moreover, ecdysone signaling in escort cells and in the FSC lineage indirectly regulates the differentiation of GSC descendants. Reduction of EcR or E75 in escort cells and early follicle cells blocks germ cell differentiation and results in defects in cyst encapsulation by follicle cells [9••, 10, 11]. Similarly, ecdysone signaling in the Drosophila testes regulates the proliferation and self-renewal of cyst stem cells, indirectly affecting the maintenance of GSCs [13••]. The differential downstream activation of steroid hormone targets in distinct cell types appears to be a critical determinant of stem cell fate and daughter cell differentiation.
Regulatory Themes and Future Directions
The mechanisms of ecdysone signaling in Drosophila are well characterized and largely functionally conserved relative to those of mammalian steroid hormones [34••, 36, 63]. Studies on ecdysone signaling in the Drosophila GSC lineage can therefore serve as a guide for the investigation of the role of other steroid hormones in mammalian stem cells. Based on our current knowledge of how ecdysone controls GSCs, we can identify three major challenges for future research.
Identification of Steroid Hormone Target Cells
One of the most challenging problems continues to be the identification of relevant cellular players. Which stem cells receive steroid hormone signals directly? What other cell types relay hormonal signals to stem cells? What signal does each cell type receive, and how are direct and indirect signals integrated to control stem cells and their progeny? For example, steroid hormone signaling appears to regulate Drosophila GSCs through both cell autonomous and non-autonomous mechanisms. Ecdysone acts directly on GSCs to modulate BMP signaling in GSCs via a functional interaction with the ATPase-dependent chromatin remodeling complex nucleosome remodeling factor (NURF), to control their self-renewal [8]. Ecdysone signaling also indirectly controls GSC maintenance by acting on adjacent somatic cells; however, the molecular mechanisms have yet to be described [10, 11, 43••]. Recent evidence suggests that mouse hematopoietic stem cells directly respond to estrogen via estrogen receptor alpha [17•]. In contrast, mammary stem cells lack estrogen and progesterone receptors but are exquisitely sensitive to steroid hormone signaling [64, 65]. In this case, progesterone and estrogen signal to adjacent luminal epithelial cells, stimulating the production of Wnt4 and RANKL (a signaling molecule upstream of NFκB), which in turn signal to mammary stem cells to promote self-renewal and proliferation [15, 16]. Estrogen and androgen also regulate muscle satellite cells, indirectly promoting their self-renewal and long-term proliferation by upregulating Notch/Delta signaling in myofibrils [18•]. Thus, in the Drosophila GSC, the muscle satellite cell, and the mammary stem cell, steroid hormone signaling interacts with local paracrine signals to control stem cell function. This makes the identification of steroid-responsive cells challenging, as traditional gene knock-out approaches for steroid receptors may yield complex phenotypes due to effects in both stem cells and support cells. It is more accurate to envision stem cells as responsive to a complex signaling environment, encompassing both paracrine and endocrine signals that interact among themselves, rather than each signal functioning independently. Given the complexity of steroid hormone signaling, and the extensive cross-talk between stem cells and other surrounding cells, identifying the key cellular players in tissues maintained by stem cells will be a cell type- and tissue-specific proposition.
Elucidation of the Effects of Other Nuclear Hormone Receptors on Stem Cells
Steroid hormone receptors, such as EcR and Usp in Drosophila, belong to the evolutionarily conserved nuclear hormone receptor superfamily of transcription factors [63, 66, 67]. Nuclear hormone receptors share a common structure with two major domains: a ligand binding domain and a highly conserved DNA binding domain. This unique structure, coupled with their ability to bind a variety of distinct small molecules, including fatty acids, vitamins, and nitric oxide, allows nuclear hormone receptors to directly affect gene expression in response to the concentration of their ligands in the target cell [66–69]. They are thus widely required in both Drosophila and mammals for development, reproduction, and metabolism [67]. Although nuclear hormone receptor function has been characterized in many adult tissues, we have only recently come to appreciate the importance of this diverse superfamily in the control of tissue-resident stem cells [3]. For example, Beyaz and colleagues identified peroxisome proliferator-activated receptor-δ (PPAR-δ) and its heterodimeric binding partners liver/retinoid X receptor (LXR/RXR) as mediators of fatty acid-induced enhancement of intestinal stem cell proliferation and self-renewal, despite evidence that PPAR-δ is dispensable for normal intestinal stem cell function [20•]. Another example is the nuclear receptor Tailless (TLX), which is directly required for the maintenance and proliferation of mammalian neural stem cells [70]. Gene knockout approaches in mammals for other nuclear hormone receptors, such as the retinoic acid receptor, have yielded mixed results, possibly due to considerable functional redundancy between receptors [71]. Indeed, the number of duplications in the nuclear hormone receptor superfamily makes defining functional roles in stem cells a challenge requiring sophisticated techniques for manipulating gene function. In this respect, Drosophila may be a prime organism in which to explore stem cell-specific roles for nuclear hormone receptors: while there are 48/49 (human/mouse) mammalian nuclear hormone receptors, there are only 18 encoded in the Drosophila genome, representing all of the mammalian subfamilies. Drosophila thus offer considerably less functional redundancy but evolutionarily conserved physiological functions [63].
Approximately half of the nuclear hormone receptors were identified based on primary sequence homology and have no known ligand. The existence of these so-called orphan nuclear hormone receptors suggests that many signaling molecules and pathways still remain to be identified. Indeed, although the first nuclear hormone receptors were isolated based on the binding properties of known steroid hormones, recent studies have suggested that some nuclear hormone receptors, particularly lipid-sensing receptors, are activated by a variety of different ligands, making it challenging to identify the primary endogenous ligand [68, 69]. Of note, in Drosophila, many orphan nuclear hormone receptors function interdependently with ecdysone signaling [36, 63]. Several EcR target genes encode nuclear hormone receptors, such as ftz transcription factor 1 (ftz-f1) and Hormone receptor 3 (Hr3, also known as Hr46), and are key components of the ecdysone-inducible gene network originally identified in the context of larval development [63]. One possibility is that other nuclear hormone receptors might serve as “partner” transcription factors to reinforce or refine the ecdysone signal in specific cell types, such as stem cells or their progeny, by co-regulating common gene targets. For example, during Drosophila embryonic and larval development, maximal induction of the EcR targets E74 and E75 requires the activity of Ftz-f1 [72–75]. Further, the ecdysone-responsive orphan nuclear hormone receptor ecdysone-induced protein 78C (E78) genetically interacts with EcR to control the growth and survival of germline cysts developing outside of the germarium, although the underlying molecular mechanisms remain unclear [51••]. Future studies testing the function of nuclear hormone receptors in stem cells and their progeny are thus likely to reveal an expansive gene regulatory network.
Identification of Steroid Hormone-Responsive Gene Networks
Recent work in Drosophila using genome-scale approaches to identify ecdysone-responsive genes has reinforced the hypothesis that steroid hormone signaling promotes distinct gene expression signatures in different cell types [76, 77•, 78]. Moving forward, it will be important to keep in mind that cells in different stages of differentiation, at distinct points in the cell cycle, or in varying microenvironments may have unique, although overlapping [12••], molecular networks. One example can be found in initial studies on ecdysone and cell proliferation in different cellular contexts. There is evidence to support direct regulation of cell cycle genes such as E2f1 and cyclin E by ecdysone [79]; however, other studies have shown that ecdysone signaling also controls cell cycle genes indirectly, for example by upregulating a transcription factor that suppresses Wnt signaling [80], or by promoting changes in glucose metabolism that accompany cell cycle exit and terminal differentiation [14•].
There is also emerging evidence to suggest that ecdysone signaling controls stem cell self-renewal by broadly enhancing the production of intrinsic factors necessary for gene regulation. Genome-wide surveys of ecdysone-responsive genes have found a large number of targets integral for RNA splicing, alternative start site usage, transport, and stability [77•, 78, 81, 82]. Indeed, our own recent work identified the heterogeneous nuclear ribonucleoprotein Hrb27C as a key regulator of GSC self-renewal downstream of ecdysone signaling [12••]. One possibility is that ecdysone signaling transcriptionally activates RNA-binding proteins whose function is to regulate transcripts that promote the stem cell fate and suppress differentiation. Ecdysone is also well-known to function with a variety of chromatin remodeling factors and epigenetic regulators, which may change the subset of genes available for expression by modulating chromatin accessibility [36]. These mechanisms are elegant ways to broadly impact gene expression by directly regulating only a limited number of gene targets in a given cell. Identifying the full complement of steroid hormone targets in a given stem cell will thus help us to answer important unresolved questions in stem cell biology, such as the molecular mechanisms controlling the cell cycle, cell metabolism, and cell fate.
With the advent of genomic approaches to study cellular function, we are on the verge of being able to identify networks of hormone-responsive genes that may promote cross-talk between paracrine and endocrine signals to fine tune cell function. Whole-genome analyses such as RNA-sequencing and chromatin immunoprecipitation followed by sequencing will be helpful for identifying key nodes in gene networks. More sophisticated approaches to identifying gene networks at a single-cell resolution in vivo will also help us to generate new hypotheses about molecular control. These approaches, however, must continue to be coupled with traditional genetic functional analyses to fully elucidate complex stem cell controls on a per-cell basis in the context of the physiology of the organism as a whole. Moreover, the complex nature of steroid hormone signaling demands that we carefully examine genetic and functional interactions between different targets or receptors as pieces of a larger gene regulatory network controlling stem cell fate and function.
Relevance of the Hormonal Milieu to Anticancer Therapy and Regenerative Medicine
The evidence demonstrating that undifferentiated cells, including stem cells and precursor cells, respond to complex combinations of hormonal signals has broad implications to our understanding of human disease. Epidemiological data show strong correlations between altered states of physiology, such as obesity, and tumorigenic transformation: indeed, it is estimated that the majority of human cancers arise due to diet or environmental factors rather than genetic factors [83–86]. Many cancers are thought to arise from a modified tissue-resident stem cell or by transformation of a differentiated cell into a proliferative, long-lived, self-renewing “cancer stem cell” [5, 87, 88]. The cancer stem cell hypothesis proposes that the cellular heterogeneity within tumors arises due to the presence and activity of cells that have qualities mimicking those of tissue-resident stem cells [89]. Regardless of the exact nature of the cancer stem cell, several recent studies suggest that, similar to tissue-resident stem cells, cancer stem cells also sense and respond to steroid hormone signals. In hepatocellular carcinoma, cancer stem cell self-renewal is promoted by thyroid hormone [90]. Breast cancer stem cells, like mammary stem cells, receive hormonal signals through paracrine mechanisms, as they typically do not express either estrogen or progesterone receptors [91]. PPAR-δ-mediated self-renewal of intestinal stem cells also enhances intestinal tumorigenesis [20•]. Since steroid hormone signaling in and around tissue-resident stem cells is clearly complex, additional studies aimed at understanding the molecular underpinnings of steroid hormone responses will likely inform clinical investigations toward more effective cancer therapeutics.
The discovery of induced pluripotent stem (iPS) cells has invigorated the prospects of cell/tissue transplantation and regenerative medicine as potential options for the treatment of many diseases [92, 93]. In principle, de-differentiation of patient-specific skin cells might obviate the need for prolonged immunosuppressant therapy and the risks of graft rejection. Transplantation of specific cell types (as is the case for replacing β cells in diabetics) generated in vitro from iPS cells, however, is still hindered by our limited knowledge of the full complement of factors and epigenetic signatures necessary to coax iPS cells to properly differentiate into a specific terminal fate [94]. Initial transplantation trials for diabetes ameliorated symptoms in rodents by using naive lineage-restricted cells and allowing differentiation to proceed in the graft post-transplantation; however, since it remains unclear what factors are influencing terminal differentiation [95, 96], it is possible that the hormonal physiology of the transplant recipient might play a role. Indeed, a recent study illustrates this important caveat: transplantation into diabetic mice with hypothyroidism can impede the success of the transplant [97]. This finding suggests that hormones can not only affect endogenous stem cell populations but also affect iPS-derived transplanted cells, potentially shaping their ability to self-renew or to effectively generate the terminally differentiated cell population of interest. As clinical approaches are steered progressively toward personalized medicine, the effect of steroid hormones, in particular the sex steroids [98], on regenerative and cancer therapies should not be overlooked.
Conclusions
Steroid hormones control virtually every aspect of human physiology, and the hormonal environment continually changes over the lifetime of the organism. Tissue-resident stem cells are sensitive to steroid hormones and other systemic factors via nuclear hormone receptor signaling, both directly and via hormone-responsive intermediate cells. Hormonal signaling broadly impacts gene expression transcriptionally and post-transcriptionally, through diverse subsets of target genes, including RNA binding proteins and chromatin modulators. While we may have identified a few key intrinsic self-renewal genes modulated by hormones, it is likely that broad networks of genes integrate cell fate and cell cycle progression with physiology. Going forward, elucidating the mechanisms of hormonal control of stem cell fate and function will require sophisticated genetic manipulations. Nuclear hormone receptors employ multiple cellular modes of action, integrating a cell-intrinsic gene regulatory network with other hormones and signals from intermediate cells or tissues, each with their own network of gene expression. The ability of these hormonal networks to rapidly sense and respond to changes in the external environment or in physiological processes allows them to finely modulate stem cell lineages, thereby serving as powerful mediators of organismal adaptation to perpetually changing conditions.
References
Papers of particular interest, published recently, have been highlighted as: •Of importance •• Of major importance
Lepage R, Albert C. Fifty years of development in the endocrinology laboratory. Clin Biochem. 2006;39(5):542–57. doi:10.1016/j.clinbiochem.2006.03.007.
Wilson JD. The evolution of endocrinology. Clin Endocrinol. 2005;62(4):389–96. doi:10.1111/j.1365-2265.2005.02209.x.
Ables ET, Laws KM, Drummond-Barbosa D. Control of adult stem cells in vivo by a dynamic physiological environment: diet-dependent systemic factors in Drosophila and beyond. Wiley interdisciplinary reviews Developmental biology. 2012;1(5):657–74. doi:10.1002/wdev.48.
Joshi PA, Di Grappa MA, Khokha R. Active allies: hormones, stem cells and the niche in adult mammopoiesis. Trends in endocrinology and metabolism: TEM. 2012;23(6):299–309. doi:10.1016/j.tem.2012.04.002.
Mihaylova MM, Sabatini DM, Yilmaz OH. Dietary and metabolic control of stem cell function in physiology and cancer. Cell Stem Cell. 2014;14(3):292–305. doi:10.1016/j.stem.2014.02.008.
Nakada D, Levi BP, Morrison SJ. Integrating physiological regulation with stem cell and tissue homeostasis. Neuron. 2011;70(4):703–18. doi:10.1016/j.neuron.2011.05.011.
Rafalski VA, Mancini E, Brunet A. Energy metabolism and energy-sensing pathways in mammalian embryonic and adult stem cell fate. J Cell Sci. 2012;125(Pt 23):5597–608. doi:10.1242/jcs.114827.
Ables ET, Drummond-Barbosa D. The steroid hormone ecdysone functions with intrinsic chromatin remodeling factors to control female germline stem cells in Drosophila. Cell Stem Cell. 2010;7(5):581–92. doi:10.1016/j.stem.2010.10.001.
•• Konig A, Shcherbata HR. Soma influences GSC progeny differentiation via the cell adhesion-mediated steroid-let-7-wingless signaling cascade that regulates chromatin dynamics. Biology open. 2015;4(3):285–300. doi:10.1242/bio.201410553. Demonstrates that non-autonomous regulation of germline differentiation by ecdysone signaling is modulated by the miRNA let-7 , which targets the ecdysone repressor Abrupt .
Konig A, Yatsenko AS, Weiss M, Shcherbata HR. Ecdysteroids affect Drosophila ovarian stem cell niche formation and early germline differentiation. EMBO J. 2011;30(8):1549–62. doi:10.1038/emboj.2011.73.
Morris LX, Spradling AC. Steroid signaling within Drosophila ovarian epithelial cells sex-specificall modulates early germ cell development and meiotic entry. PLoS One. 2012;7(10):e46109. doi:10.1371/journal.pone.0046109.
•• Ables ET, Hwang GH, Finger DS, Hinnant TD, Drummond-Barbosa D. A genetic mosaic screen reveals ecdysone-responsive genes regulating Drosophila oogenesis. G3 (Bethesda, Md). 2016;6(8):2629–42. doi:10.1534/g3.116.028951. Identifies over 30 putative ecdysone-responsive genes that control various points of oogenesis, including GSC and FSC maintenance and proliferation.
•• Li Y, Ma Q, Cherry CM, Matunis EL. Steroid signaling promotes stem cell maintenance in the Drosophila testis. Dev Biol. 2014;394(1):129–41. doi:10.1016/j.ydbio.2014.07.016. First study to demonstrate a role for ecdysone signaling in the maintenance and proliferation of cyst stem cells, which indirectly maintain adjacent GSCs in the Drosophila testes.
• Homem CC, Steinmann V, Burkard TR, Jais A, Esterbauer H, Knoblich JA. Ecdysone and mediator change energy metabolism to terminate proliferation in Drosophila neural stem cells. Cell. 2014;158(4):874–88. doi:10.1016/j.cell.2014.06.024. Demonstrates a functional interaction between EcR and the mediator transcriptional regulatory complex in controlling cell cycle exit in larval neuroblasts.
Asselin-Labat ML, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER, et al. Control of mammary stem cell function by steroid hormone signalling. Nature. 2010;465(7299):798–802. doi:10.1038/nature09027.
Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote PA, Clarke CL, et al. Progesterone induces adult mammary stem cell expansion. Nature. 2010;465(7299):803–7. doi:10.1038/nature09091.
• Nakada D, Oguro H, Levi BP, Ryan N, Kitano A, Saitoh Y, et al. Oestrogen increases haematopoietic stem-cell self-renewal in females and during pregnancy. Nature. 2014;505(7484):555–8. doi:10.1038/nature12932. First study to conclusively demonstrate direct regulation of mouse hematopoietic stem cell proliferation and self-renewal by steroid hormone signaling.
• Kim JH, Han GC, Seo JY, Park I, Park W, Jeong HW, et al. Sex hormones establish a reserve pool of adult muscle stem cells. Nat Cell Biol. 2016;18(9):930–40. doi:10.1038/ncb3401. Provides evidence that sex hormones promote mouse muscle satellite cells to enter quiescence following puberty.
• Contreras-Jurado C, Lorz C, Garcia-Serrano L, Paramio JM, Aranda A. Thyroid hormone signaling controls hair follicle stem cell function. Mol Biol Cell. 2015;26(7):1263–72. doi:10.1091/mbc.E14-07-1251. Provides evidence that mouse hair follicle stem cells are regulated by thyroid hormone signaling.
• Beyaz S, Mana MD, Roper J, Kedrin D, Saadatpour A, Hong SJ, et al. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature. 2016;531(7592):53–8. doi:10.1038/nature17173. Provides evidence that PPAR-δ directly regulates mouse intestinal stem cell proliferation in response to increased fatty acid levels, predisposing stem cells to tumorigenic transformation.
Sirin O, Lukov GL, Mao R, Conneely OM, Goodell MA. The orphan nuclear receptor Nurr1 restricts the proliferation of haematopoietic stem cells. Nat Cell Biol. 2010;12(12):1213–9. doi:10.1038/ncb2125.
Shi Y, Chichung Lie D, Taupin P, Nakashima K, Ray J, Yu RT, et al. Expression and function of orphan nuclear receptor TLX in adult neural stem cells. Nature. 2004;427(6969):78–83. doi:10.1038/nature02211.
• Shirazi HA, Rasouli J, Ciric B, Rostami A, Zhang GX. 1,25-Dihydroxyvitamin D3 enhances neural stem cell proliferation and oligodendrocyte differentiation. Experimental and molecular pathology. 2015;98(2):240–245. doi:10.1016/j.yexmp.2015.02.004. Demonstrates a novel role for vitamin D in the regulation of mouse neural stem cell proliferation via the nuclear hormone receptor VDR.
• Ghiaur G, Yegnasubramanian S, Perkins B, Gucwa JL, Gerber JM, Jones RJ. Regulation of human hematopoietic stem cell self-renewal by the microenvironment's control of retinoic acid signaling. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(40):16121–6. doi:10.1073/pnas.1305937110. Implicates retinoic acid signaling in the indirect control of human hematopoietic stem cell self-renewal.
Purton LE, Dworkin S, Olsen GH, Walkley CR, Fabb SA, Collins SJ, et al. RARgamma is critical for maintaining a balance between hematopoietic stem cell self-renewal and differentiation. J Exp Med. 2006;203(5):1283–93. doi:10.1084/jem.20052105.
Daley GQ. Stem cells and the evolving notion of cellular identity. Philosophical transactions of the Royal Society of London series B. Biological sciences. 2015;370(1680) doi:10.1098/rstb.2014.0376.
Warr MR, Pietras EM, Passegue E. Mechanisms controlling hematopoietic stem cell functions during normal hematopoiesis and hematological malignancies. Wiley Interdiscip Rev Syst Biol Med. 2011; doi:10.1002/wsbm.145.
Li H, Jasper H. Gastrointestinal stem cells in health and disease: from flies to humans. Disease models & mechanisms. 2016;9(5):487–99. doi:10.1242/dmm.024232.
Gage FH, Temple S. Neural stem cells: generating and regenerating the brain. Neuron. 2013;80(3):588–601. doi:10.1016/j.neuron.2013.10.037.
Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132(4):598–611.
Spradling A. Developmental genetics of oogenesis. In: Bate M, editor. The development of Drosophila melanogaster. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1993. p. 1–70.
Laws KM, Drummond-Barbosa D. Control of germline stem cell lineages by diet and physiology. In: Arur S, editor. Signaling-mediated control of cell division: from oogenesis to oocyte-to-embryo development. Results and problems in cell differentiation. USA: Springer International Publishing; 2016.
Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83(6):835–9. doi:0092-8674(95)90199-X
•• Sieber MH, Spradling AC. Steroid signaling establishes a female metabolic state and regulates SREBP to control oocyte lipid accumulation. Current biology : CB. 2015;25(8):993–1004. doi:10.1016/j.cub.2015.02.019. Demonstrates that neuronal ecdysone signaling controls female feeding rates, whole-body triglyceride and glycogen storage, and lipid uptake in the ovary, thus promoting a metabolic state that supports the energetic demands of oogenesis.
Riddiford LM, Cherbas P, Truman JW. Ecdysone receptors and their biological actions. Vitam Horm. 2000;60:1–73.
Yamanaka N, Rewitz KF, O'Connor MB. Ecdysone control of developmental transitions: lessons from Drosophila research. Annu Rev Entomol. 2013;58:497–516. doi:10.1146/annurev-ento-120811-153608.
Niwa R, Niwa YS. Enzymes for ecdysteroid biosynthesis: their biological functions in insects and beyond. Biosci Biotechnol Biochem. 2014;78(8):1283–92. doi:10.1080/09168451.2014.942250.
Gancz D, Lengil T, Gilboa L. Coordinated regulation of niche and stem cell precursors by hormonal signaling. PLoS Biol. 2011;9(11):e1001202. doi:10.1371/journal.pbio.1001202.
Hodin J, Riddiford LM. The ecdysone receptor and ultraspiracle regulate the timing and progression of ovarian morphogenesis during Drosophila metamorphosis. Dev Genes Evol. 1998;208(6):304–17.
•• Mendes CC, Mirth CK. Stage-specific plasticity in ovary size is regulated by insulin/insulin-like growth factor and ecdysone signaling in Drosophila. Genetics. 2016;202(2):703–19. doi:10.1534/genetics.115.179960. Defines the window of sensitivity to nutritional input during ovary development and implicates cross-talk between ecdysone and insulin signaling in the control of ovariole number in response to diet.
Huang X, Warren JT, Gilbert LI. New players in the regulation of ecdysone biosynthesis. Journal of genetics and genomics. Yi chuan xue bao. 2008;35(1):1–10. doi:10.1016/S1673-8527(08)60001-6.
Uryu O, Ameku T, Niwa R. Recent progress in understanding the role of ecdysteroids in adult insects: germline development and circadian clock in the fruit fly Drosophila melanogaster. Zoological letters. 2015;1:32. doi:10.1186/s40851-015-0031-2.
•• Ameku T, Niwa R. Mating-induced increase in germline stem cells via the neuroendocrine system in female Drosophila. PLoS Genet. 2016;12(6):e1006123. doi:10.1371/journal.pgen.1006123. Demonstrates that neuronal inputs regulate ecdysone biosynthesis in the adult ovary and provides evidence that ecdysone signaling in escort cells indirectly regulates GSC proliferation.
•• Belles X, Piulachs MD. Ecdysone signalling and ovarian development in insects: from stem cells to ovarian follicle formation. Biochim Biophys Acta. 2015;1849(2):181–6. doi:10.1016/j.bbagrm.2014.05.025. Comprehensive review of the roles of ecdysone signaling in the Drosophila ovary.
Avila FW, Sirot LK, LaFlamme BA, Rubinstein CD, Wolfner MF. Insect seminal fluid proteins: identification and function. Annu Rev Entomol. 2011;56:21–40. doi:10.1146/annurev-ento-120709-144823.
Feng K, Palfreyman MT, Hasemeyer M, Talsma A, Dickson BJ. Ascending SAG neurons control sexual receptivity of Drosophila females. Neuron. 2014;83(1):135–48. doi:10.1016/j.neuron.2014.05.017.
Hasemeyer M, Yapici N, Heberlein U, Dickson BJ. Sensory neurons in the Drosophila genital tract regulate female reproductive behavior. Neuron. 2009;61(4):511–8. doi:10.1016/j.neuron.2009.01.009.
Buszczak M, Freeman MR, Carlson JR, Bender M, Cooley L, Segraves WA. Ecdysone response genes govern egg chamber development during mid-oogenesis in Drosophila. Development (Cambridge, England). 1999;126(20):4581–9.
Carney GE, Bender M. The Drosophila ecdysone receptor (EcR) gene is required maternally for normal oogenesis. Genetics. 2000;154(3):1203–11.
Garen A, Kauvar L, Lepesant JA. Roles of ecdysone in Drosophila development. Proc Natl Acad Sci U S A. 1977;74(11):5099–103.
•• Ables ET, Bois KE, Garcia CA, Drummond-Barbosa D. Ecdysone response gene E78 controls ovarian germline stem cell niche formation and follicle survival in Drosophila. Dev Biol. 2015;400(1):33–42. doi:10.1016/j.ydbio.2015.01.013. First demonstration that nuclear hormone receptor E78 promotes niche cell development and survival of germline cysts during early oogenesis.
Jang AC, Chang YC, Bai J, Montell D. Border-cell migration requires integration of spatial and temporal signals by the BTB protein abrupt. Nat Cell Biol. 2009;11(5):569–79. doi:10.1038/ncb1863.
Romani P, Bernardi F, Hackney J, Dobens L, Gargiulo G, Cavaliere V. Cell survival and polarity of Drosophila follicle cells require the activity of ecdysone receptor B1 isoform. Genetics. 2009;181(1):165–75. doi:10.1534/genetics.108.096008.
Tzolovsky G, Deng WM, Schlitt T, Bownes M. The function of the broad-complex during Drosophila melanogaster oogenesis. Genetics. 1999;153(3):1371–83.
Terashima J, Bownes M. E75A and E75B have opposite effects on the apoptosis/development choice of the Drosophila egg chamber. Cell Death Differ. 2006;13(3):454–64. doi:10.1038/sj.cdd.4401745.
Bownes M, Ronaldson E, Mauchline D. 20-hydroxyecdysone, but not juvenile hormone, regulation of yolk protein gene expression can be mapped to cis-acting DNA sequences. Dev Biol. 1996;173(2):475–89. doi:10.1006/dbio.1996.0041.
LaFever L, Drummond-Barbosa D. Direct control of germline stem cell division and cyst growth by neural insulin in Drosophila. Science (New York, NY). 2005;309(5737):1071–3. doi:10.1126/science.1111410.
Bai J, Uehara Y, Montell DJ. Regulation of invasive cell behavior by taiman, a Drosophila protein related to AIB1, a steroid receptor coactivator amplified in breast cancer. Cell. 2000;103(7):1047–58.
•• Domanitskaya E, Anllo L, Schupbach T. Phantom, a cytochrome P450 enzyme essential for ecdysone biosynthesis, plays a critical role in the control of border cell migration in Drosophila. Dev Biol. 2014;386(2):408–18. doi:10.1016/j.ydbio.2013.12.013. Provides evidence that ecdysone biosynthesis specifically in ovarian follicle cells is necessary for the ecdysone-induced migration of somatic border cells.
Hackney JF, Pucci C, Naes E, Dobens L. Ras signaling modulates activity of the ecdysone receptor EcR during cell migration in the Drosophila ovary. Developmental dynamics : an official publication of the American Association of Anatomists. 2007;236(5):1213–26. doi:10.1002/dvdy.21140.
•• Romani P, Gargiulo G, Cavaliere V. The ecdysone receptor signalling regulates microvilli formation in follicular epithelial cells. Cellular and molecular life sciences : CMLS. 2016;73(2):409–25. doi:10.1007/s00018-015-1999-7. Demonstrates that ecdysone signaling is required to properly position ovarian follicle cells within an epithelium via stabilizing the actin cytoskeleton.
Sun J, Smith L, Armento A, Deng WM. Regulation of the endocycle/gene amplification switch by Notch and ecdysone signaling. J Cell Biol. 2008;182(5):885–96. doi:10.1083/jcb.200802084.
King-Jones K, Thummel CS. Nuclear receptors--a perspective from Drosophila. Nat Rev Genet. 2005;6(4):311–23. doi:10.1038/nrg1581.
Brisken C, Hess K, Jeitziner R. Progesterone and overlooked endocrine pathways in breast cancer pathogenesis. Endocrinology. 2015;156(10):3442–50. doi:10.1210/en.2015-1392.
Visvader JE, Stingl J. Mammary stem cells and the differentiation hierarchy: current status and perspectives. Genes Dev. 2014;28(11):1143–58. doi:10.1101/gad.242511.114.
Evans RM, Mangelsdorf DJ. Nuclear receptors, RXR, and the big bang. Cell. 2014;157(1):255–66. doi:10.1016/j.cell.2014.03.012.
Pardee K, Necakov AS, Krause H. Nuclear receptors: small molecule sensors that coordinate growth. Metabolism and Reproduction Sub-cellular biochemistry. 2011;52:123–53. doi:10.1007/978-90-481-9069-0_6.
Vacca M, Degirolamo C, Mariani-Costantini R, Palasciano G, Moschetta A. Lipid-sensing nuclear receptors in the pathophysiology and treatment of the metabolic syndrome. Wiley Interdiscip Rev Syst Biol Med. 2011; doi:10.1002/wsbm.137.
Wollam J, Antebi A. Sterol regulation of metabolism, homeostasis, and development. Annu Rev Biochem. 2011;80:885–916. doi:10.1146/annurev-biochem-081308-165917.
Islam MM, Zhang CL. TLX: a master regulator for neural stem cell maintenance and neurogenesis. Biochim Biophys Acta. 2015;1849(2):210–6. doi:10.1016/j.bbagrm.2014.06.001.
Leid M, Kastner P, Chambon P. Multiplicity generates diversity in the retinoic acid signalling pathways. Trends Biochem Sci. 1992;17(10):427–33.
Broadus J, McCabe JR, Endrizzi B, Thummel CS, Woodard CT. The Drosophila beta FTZ-F1 orphan nuclear receptor provides competence for stage-specific responses to the steroid hormone ecdysone. Mol Cell. 1999;3(2):143–9.
Ruaud AF, Lam G, Thummel CS. The Drosophila nuclear receptors DHR3 and betaFTZ-F1 control overlapping developmental responses in late embryos. Development (Cambridge, England). 2010;137(1):123–31. doi:10.1242/dev.042036.
Sullivan AA, Thummel CS. Temporal profiles of nuclear receptor gene expression reveal coordinate transcriptional responses during Drosophila development. Molecular endocrinology (Baltimore, Md). 2003;17(11):2125–37. doi:10.1210/me.2002-0430.
Woodard CT, Baehrecke EH, Thummel CS. A molecular mechanism for the stage specificity of the Drosophila prepupal genetic response to ecdysone. Cell. 1994;79(4):607–15.
Li TR, White KP. Tissue-specific gene expression and ecdysone-regulated genomic networks in Drosophila. Dev Cell. 2003;5(1):59–72.
• Stoiber M, Celniker S, Cherbas L, Brown B, Cherbas P. Diverse hormone response networks in 41 independent Drosophila cell lines. G3 (Bethesda, Md). 2016;6(3):683–94. doi:10.1534/g3.115.023366. Comprehensive genome-wide characterization of ecdysone-responsive genes in 41 different ecdysone-responsive Drosophila cell lines.
Shlyueva D, Stelzer C, Gerlach D, Yanez-Cuna JO, Rath M, Boryn LM, et al. Hormone-responsive enhancer-activity maps reveal predictive motifs, indirect repression, and targeting of closed chromatin. Mol Cell. 2014;54(1):180–92. doi:10.1016/j.molcel.2014.02.026.
Qian W, Kang L, Zhang T, Meng M, Wang Y, Li Z, et al. Ecdysone receptor (EcR) is involved in the transcription of cell cycle genes in the silkworm. Int J Mol Sci. 2015;16(2):3335–49. doi:10.3390/ijms16023335.
Mitchell NC, Lin JI, Zaytseva O, Cranna N, Lee A, Quinn LM. The ecdysone receptor constrains wingless expression to pattern cell cycle across the Drosophila wing margin in a Cyclin B-dependent manner. BMC Dev Biol. 2013;13:28. doi:10.1186/1471-213x-13-28.
Beckstead RB, Lam G, Thummel CS. The genomic response to 20-hydroxyecdysone at the onset of Drosophila metamorphosis. Genome Biol. 2005;6(12):R99. doi:10.1186/gb-2005-6-12-r99.
Gauhar Z, Sun LV, Hua S, Mason CE, Fuchs F, Li TR, et al. Genomic mapping of binding regions for the ecdysone receptor protein complex. Genome Res. 2009;19(6):1006–13. doi:10.1101/gr.081349.108.
Baena Ruiz R, Salinas HP. Diet and cancer: risk factors and epidemiological evidence. Maturitas. 2014;77(3):202–8. doi:10.1016/j.maturitas.2013.11.010.
Faulds MH, Dahlman-Wright K. Metabolic diseases and cancer risk. Curr Opin Oncol. 2012;24(1):58–61. doi:10.1097/CCO.0b013e32834e0582.
Mayne ST, Playdon MC, Rock CL. Diet, nutrition, and cancer: past, present and future. Nat Rev Clin Oncol. 2016;13(8):504–15. doi:10.1038/nrclinonc.2016.24.
Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and cancer—viewpoint of the IARC working group. N Engl J Med. 2016;375(8):794–8. doi:10.1056/NEJMsr1606602.
Adams PD, Jasper H, Rudolph KL. Aging-induced stem cell mutations as drivers for disease and cancer. Cell Stem Cell. 2015;16(6):601–12. doi:10.1016/j.stem.2015.05.002.
Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med. 2011;17(3):313–9. doi:10.1038/nm.2304.
Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–11. doi:10.1038/35102167.
Wang T, Xia L, Ma S, Qi X, Li Q, Xia Y, et al. Hepatocellular carcinoma: thyroid hormone promotes tumorigenicity through inducing cancer stem-like cell self-renewal. Scientific reports. 2016;6:25183. doi:10.1038/srep25183.
Simoes BM, Alferez DG, Howell SJ, Clarke RB. The role of steroid hormones in breast cancer stem cells. Endocrine-related cancer. 2015;22(6):T177–86. doi:10.1530/erc-15-0350.
Liu K, Yu C, Xie M, Li K, Ding S. Chemical modulation of cell fate in stem cell therapeutics and regenerative medicine. Cell Chem Biol. 2016;23(8):893–916. doi:10.1016/j.chembiol.2016.07.007.
Mao AS, Mooney DJ. Regenerative medicine: current therapies and future directions. Proc Natl Acad Sci U S A. 2015;112(47):14452–9. doi:10.1073/pnas.1508520112.
Kahraman S, Okawa ER, Kulkarni RN. Is transforming stem cells to pancreatic Beta cells still the holy grail for type 2 diabetes? Current diabetes reports. 2016;16(8):70. doi:10.1007/s11892-016-0764-0.
Pagliuca FW, Millman JR, Gurtler M, Segel M, Van Dervort A, Ryu JH, et al. Generation of functional human pancreatic beta cells in vitro. Cell. 2014;159(2):428–39. doi:10.1016/j.cell.2014.09.040.
Rezania A, Bruin JE, Arora P, Rubin A, Batushansky I, Asadi A, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol. 2014;32(11):1121–33. doi:10.1038/nbt.3033.
Bruin JE, Saber N, O'Dwyer S, Fox JK, Mojibian M, Arora P, et al. Hypothyroidism impairs human stem cell-derived pancreatic progenitor cell maturation in mice. Diabetes. 2016;65(5):1297–309. doi:10.2337/db15-1439.
Miller VM. Why are sex and gender important to basic physiology and translational and individualized medicine? Am J Physiol Heart Circ Physiol. 2014;306(6):H781–8. doi:10.1152/ajpheart.00994.2013.
Acknowledgements
We apologize to our colleagues whose original work could not be cited due to space limitations. Many thanks to Danielle Finger for the assistance with the figure preparation and to Lesley Weaver and members of the Ables laboratory for critical reading of this review. Work from our laboratories is supported by National Institutes of Health (NIH) R01 GM069875 (D.D.-B.), NIH R15 GM117502 (E.T.A.), and March of Dimes Basil O’Connor Research Starter Award 5-FY14-62 (E.T.A.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
Elizabeth T. Ables and Daniela Drummond-Barbosa declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Metabolism and Stem Cells
Rights and permissions
About this article
Cite this article
Ables, E.T., Drummond-Barbosa, D. Steroid Hormones and the Physiological Regulation of Tissue-Resident Stem Cells: Lessons from the Drosophila Ovary. Curr Stem Cell Rep 3, 9–18 (2017). https://doi.org/10.1007/s40778-017-0070-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40778-017-0070-z