Abstract
Purposeof review
This review examines the role of prophylactic peritoneal dialysis in pediatric patients after cardiac surgery. We identify benefits and risks of the intervention while highlighting gaps in current knowledge.
Recent findings
The usage of peritoneal dialysis to manage and acute kidney injury (AKI) and fluid overload (FO) after pediatric cardiac surgery may demonstrate advantages over conventional diuresis and more invasive forms of renal replacement therapies (RRT) in some patients. Outcomes vary by population in limited single and multicenter studies but suggest decreased mechanical ventilation time, earlier time to net negative fluid balance, and shorter ICU stays when compared to conventional therapies.
Summary
Prophylactic peritoneal dialysis use in the pediatric cardiac population varies widely across pediatric cardiac centers but is increasingly being demonstrated as a safe, effective treatment of AKI and FO, both of which are common and associated with deleterious outcomes in this population. Clinical decision support tools have been developed to assist in identifying patients most likely to benefit from RRT outside of the cardiac population, but further work is needed to determine if they can be applied to postoperative cardiac patients. Complications are relatively infrequent but may indicate benefits for center-specific peritoneal dialysis management protocols.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute kidney injury (AKI) and fluid overload (FO) are common and associated with poor outcomes in heterogenous populations of critically ill children [1,2,3]. A detailed discussion of the epidemiology of cardiac surgery-associated AKI and FO are beyond the scope of this review. Briefly, recent epidemiologic data from the Neonatal and Pediatric Heart and Renal Outcomes Network (NEPHRON) demonstrated highly variable rates of AKI across centers when using the modified neonatal Kidney Disease Improving Global Outcomes serum creatinine and urine output criteria [1]. Unlike prior studies, where AKI was associated with increased resources utilization and mortality [4,5,6], in the NEPHRON study by Alten and colleagues, only stage 3 AKI was associated with mortality, but not other clinically relevant outcomes. Bailly and colleagues also used the NEPHRON data for evaluation of the impact of FO on outcomes [7]. In this study, the degree of FO was much lower than has previously reported in children undergoing cardiac surgery [5, 8] measuring approximately 4.9% [7]. Peak FO and postoperative day 1 FO were not associated with clinically relevant outcomes; however, hospital resource utilization, characterized by longer duration of ventilation and ICU length of stay, was increased on each successive day of not achieving a negative fluid balance [7]. This lower peak FO suggests that clinicians may be more aware and mindful of the deleterious effects of FO limiting excessive fluid accumulation in their patients. Thus, it is imperative that focus now be shifted to actionable targets such as earlier achievement of negative fluid balance to minimize hospital resource utilization.
The etiology of AKI after pediatric cardiac surgery is multifactorial. Intra- and post-operative factors that are associated with AKI include hemolysis, blood product transfusion, systemic inflammatory response from deep hypothermic circulatory arrest, long cardiopulmonary bypass (CPB) duration, and nephrotoxic medication exposures [9]. These mechanistic factors may lead to exacerbation of either AKI and FO, or both. Indeed, there is a bidirectional risk associated with the presence of AKI and FO and in critically ill children, and they appear to have a synergistic effect in negatively impacting outcomes [10]. In the most severe form of both AKI and FO, renal replacement therapy (RRT) is used as supportive therapy when there is oliguria, electrolyte disturbances, and uremia present. Peritoneal dialysis has been shown to be an effective alternative method of RRT for AKI and fluid management in infants undergoing cardiac surgery and is the modality of choice in some centers in the immediate postoperative period to prevent AKI and FO. Peritoneal dialysis has demonstrated comparable morbidity and mortality outcomes to extracorporeal therapies in both cardiac and non-cardiac pediatric populations. The purpose of this review is to discuss utilization of peritoneal drains and peritoneal dialysis in children undergoing cardiac surgery.
Peritoneal drains and dialysis
Peritoneal dialysis is a commonly employed technique in children in the acute and chronic setting, particularly in neonates and infants, and is the primary therapy in low resource settings [11, 12]. The utility of peritoneal dialysis has been extended to children undergoing cardiac surgery in the absence of standard indications. In this setting, peritoneal drains are used either for passive peritoneal drainage or prophylactic peritoneal dialysis [13, 14]. Cardiac surgery, particularly with CPB and circulatory arrest, can result in activation of pro- and anti-inflammatory cytokines with a resultant significant inflammatory response, capillary leak, and low cardiac output syndrome [15]. These factors are often associated with the development of AKI and FO. For this reason, preoperative decision making has been employed in some centers for placement of peritoneal catheters for either postoperative passive drainage or prophylactic peritoneal dialysis to mitigate the deleterious inflammatory response by augmenting cytokine and fluid removal and perhaps reduce the burden of AKI [16,17,18,19,20]. Table 1 summarizes considerations for identifying high- and medium risk patients in whom peritoneal dialysis catheter (PDC) placement would most likely be beneficial following cardiac surgery.
Peritoneal catheter placement and dialysis prescription.
Peritoneal drain catheters (PDC) can either be placed in the operating room or at bedside in the intensive care unit (ICU) as the need arises. The single or double cuffed pediatric sized Tenckhoff pediatric PD catheter is the most used catheter in children after cardiac surgery. The standard approach in the operating room is performed after cardiac surgery and prior to chest closure, where a small incision is made into the peritoneum at the inferior portion of the sternotomy incision and the catheter tunneled into the peritoneal cavity under direct visualization. This is followed by closing the peritoneal incision and fixing the catheter to the skin with a stay suture. In the ICU, drain placement can be performed with or without ultrasound guidance. The most common approach involves making an incision either inferior to the umbilicus or at the midpoint between the umbilicus and anterior inferior iliac spine to avoid the inferior epigastric artery that traverses along the lateral aspect of the rectus abdominis sheath [11, 21]. The PDC is then placed into the peritoneum and secured in a similar fashion. For both operating room and ICU placement, the catheter is then connected to a standard collection bag, which can be exchanged for a closed system to allow flushes for patency and passive drainage, or regular cycles of peritoneal dialysis. The only catheter-related interventions that have been shown by meta-analysis to be successful at preventing catheter-associated peritonitis is the usage of disconnect (twin-bag and Y-set) systems as opposed to conventional spike systems [22]. Prophylactic perioperative antibiotics should be administered 60 min prior to PDC insertion to prevent early-onset peritonitis [23, 24].
Once placed, the peritoneal catheter may be used either for passive drainage or dialysis depending on the clinical status of the patient and clinician discretion. Passive drainage is often used but when augmented fluid removal is necessary, prophylactic peritoneal dialysis can instead be implemented at any time during the patient’s post-operative recovery. The most common starting prescription for prophylactic peritoneal dialysis is Dianeal with 2.5% dextrose with a fill volume of 10 mL/kg to prevent peritoneal fluid leakage. Per the International Society for Peritoneal Dialysis, fill volumes may be gradually increased to 30–40 mL/kg if higher prescriptions are needed to achieve optimal fluid status [11]. The near-physiologic dialysate infusion then facilitates diffusion of fluid, metabolites, and molecules between blood and peritoneal fluid through the semi-permeable peritoneal membrane. Higher dialysate dextrose concentrations can be used to enhance fluid removal by increasing the osmotic gradient for ultrafiltration. Diffusion of molecules such as potassium, creatinine, and urea across the peritoneal membrane are dependent on concentrations in the dialysate, which allows for targeted ultrafiltration and removal of toxic metabolites in the setting of AKI. The resultant ultrafiltrate is drained from the peritoneal cavity to achieve improved metabolic and hemodynamic status [11, 25]. The prescription usually allows for a 5-–10-min fill time, a 30-–45-min dwell time and drainage of 10 min. Both the fill volume and dwell time can be increased to optimize dialysis.
Peritoneal dialysis complications
Despite the known benefits of peritoneal dialysis in this cohort, risk–benefit analysis should be individualized for each potential candidate as there is always risk for complications. A potentially life-threatening complication of peritoneal dialysis is peritonitis. Leakage of peritoneal fluid through the dialysis catheter can increase the risk of infection. Advancements in PDC insertion techniques and antibiotic usage have reduced rates of peritonitis in patients. Numerous studies have reported on PD catheter related complications after pediatric cardiac surgery. Flores et al. demonstrated no increase in peritonitis among those with PDC [26]. Another study identified peritonitis in 2 out of 23 patients treated with peritoneal dialysis [13]. In an older study at a single institution from 1993 to 2002, only 2 patients transitioned to hemodialysis as a result of peritonitis. Risk factors associated with peritoneal dialysis complications included longer duration of peritoneal dialysis, higher Risk Adjusted Classification for Congenital heart Surgery (RACHS-1) score and hyperkalemia at time of peritoneal dialysis initiation [20].
Metabolic derangements are an additional potential complication that necessitates routine lab monitoring. The semi-permeable quality of the peritoneal membrane is bi-directional, permitting diffusion of dextrose from the high concentration dialysate into the blood stream which can cause hyperglycemia. Potassium may diffuse across the peritoneal membrane from the blood into the low concentration dialysate, resulting in hypokalemia. Up to one-third of patients have been reported to have hypokalemia, and increased morbidity and mortality have been associated with persistent hypokalemia [27,28,29]. To mitigate the risk of peritoneal dialysis-associated hypokalemia, potassium should be added to dialysate solutions once serum potassium drops below 4 mmol/L. In addition, a bicarbonate buffer is recommended for patients exhibiting significant metabolic acidosis, hemodynamic instability, and hepatic dysfunction during peritoneal dialysis. Small solute clearance can be assessed as a measured of PD efficacy. Residual kidney function, if present, would contribute to total clearance. Routine monitoring of electrolytes, albumin, and bicarbonate is recommended to prevent electrolyte derangement and identify nutritional deficiencies associated with peritoneal dialysis [11, 21].
Another potential complication of peritoneal dialysis includes intra-abdominal organ damage from the indwelling catheter [30]. In addition, long-term use of high concentration dextrose solutions may lead to sclerosis of the peritoneal membrane rendering dialysis ineffective. Fortunately, this is uncommon with prophylactic peritoneal dialysis after cardiac surgery as it is most often used only for the first few postoperative days. The PDC can also communicate with the pleural space, which can lead to numerous complications and negates the ability to use the PDC for prophylactic dialysis. Removal of catheters in awake children with an acute increase in intraabdominal pressure, such as during cough, can increase the risk of omental herniation [31]. Finally, the development of an abdominal hernia during catheter removal is a relatively common complication that has been identified in certain patient populations [32].
Peritoneal dialysis utilization in the pediatric cardiac population
There is significant heterogeneity in utilization of peritoneal drains for passive drainage or prophylactic dialysis across pediatric cardiac surgery centers. In the large epidemiology study, prophylactic dialysis was used in 13.8% of patients within the first 24 h [1]. A more detailed report on the impact and outcomes of patients receiving prophylactic dialysis from NEPHRON is forthcoming. There are several perceived benefits to using peritoneal drains in children after cardiac surgery: [1] relative ease in catheter placement, [2] lack of requirement for a large intravenous dialysis catheter and anticoagulation (regional or systemic), [3] cytokine removal that occurs with passive drainage and is enhanced by prophylactic dialysis, [4] less need for electrolyte replacements with prophylactic dialysis, and [5] small amount of supplemental nutrition from the dextrose-containing dialysis solution [14, 18,19,20, 33,34,35,36].
Peritoneal dialysis has been shown to effectively mitigate FO and promote diuresis with improved outcomes following cardiac surgery. Among patients who do undergo PDC placement, Sasser et al. evaluated the use of prophylactic peritoneal dialysis in comparison with passive drainage plus diuretic therapy in 52 high-risk neonates and infants undergoing cardiac surgery with similar intraoperative variables [37]. The dialysis group had a more negative median net fluid balance 24 h after surgery. The dialysis group was also found to have earlier time to sternal closure, lower mean inotrope scores, and lower serum concentrations of inflammatory markers (interleukin-6 and interleukin-8) 24-h after surgery [37]. Kwiatkowski et al. published a retrospective study which found that in 42 high-risk infants with planned PDC placement at time of cardiac surgery, there was a faster time to negative fluid balance when compared to age and procedure-matched infants. Notably, time to extubation, inotropy scores, and frequency of medications administered for electrolyte correction were also reduced in the cohort with planned PDC placement [19]. In a subsequent single center randomized controlled trial of infants undergoing cardiac surgery who were either randomized to a standard regimen of furosemide or prophylactic peritoneal dialysis withing 6 h of arrival to the ICU, those who received prophylactic dialysis had lower % FO, shorter ventilation time, shorter inotropic medication use, and fewer electrolyte replacements [18]. In a single-center retrospective study of neonates undergoing arterial switch operation, fluid accumulation was reduced among those receiving prophylactic peritoneal dialysis [38]. In addition, there was a 42% reduction in mechanical ventilation and 34% reduction in ICU length of stay among patients who received peritoneal dialysis [38]. Whether the benefits of prophylactic peritoneal dialysis translate across multiple centers is unknown, especially given the recent reports of heterogenous rates of AKI and FO across centers included in the NEPHRON study [1, 7]. A recent meta-analysis evaluated the efficacy, safety and outcomes (duration of ventilation, length of stay, and mortality) of using diuretics compared to prophylactic peritoneal dialysis across 8 studies including 507 pediatric patients after cardiac surgery. Patients who received prophylactic peritoneal dialysis had longer cardiopulmonary bypass. There was also a notable reduction in duration of mechanical ventilation among those who received prophylactic dialysis. This study found no significant difference in length of ICU or hospital stay between those who did and did not receive prophylactic peritoneal dialysis. However, there was insufficient power to determine differences between negative fluid balance on postoperative day 1 and time to negative fluid balance between the groups [26].Two of the eight studies were from the same center and randomized in their approach, two were prospective and observational, and the remaining studies were retrospective. This highlights the potential bias in perceived benefits of PD in the small number of studies which included centers that were already routinely performing prophylactic peritoneal dialysis in their patients. A more recent meta-analysis demonstrated some benefits in select patients but the authors recommended prospective studies [39]. Additionally, further investigation is needed to assess if a standardized approach can be feasibly applied to all patients or if it should be implemented using a clinical decision support tool after appropriate risk stratification. Indeed, it is likely not a one size fits all approach, and therapy should be individually considered and tailored based on pre-, peri-, and postoperative factors.
Considerations for risk stratification for passive peritoneal drains or prophylactic PD
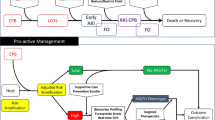
Recent work by the 26th acute disease quality initiative (ADQI) working group suggested that risk stratification tools be developed and implemented to identify those most likely to develop AKI and FO [40]. This working group also suggested that there are multiple different AKI phenotypes exists. Perhaps the reason we have identified variable benefit of prophylactic peritoneal dialysis across pediatric studies is because it is currently applied to patients who clinicians perceived to be at high risk for AKI and FO based on experience rather than real-time clinical decision support [40]. The renal angina index (RAI), a calculable bedside clinical risk score has been used in the pediatric intensive care unit within 12 h of admission to identify those at highest risk for day 2–4 severe AKI [41]. The performance of the RAI was enhanced by integration of a urinary biomarker (neutrophil gelatinase associated lipocalin – NGAL) [42]. At Cincinnati Children’s Hospital, the RAI is now automatically calculated for patients within 12 h of admission to the pediatric ICU and if the score is greater than or equal to 8, a urine NGAL is sent. Clinical decision support is then provided to clinicians to either fluid restrict or consider early implementation of renal replacement therapy among those with very elevated urine NGAL or who fail to respond to diuretics [43]. Results of the implementation of this clinical decision support tool for mitigation of FO in those at risk for severe day 2–4 AKI are forthcoming. Unfortunately, the RAI, in its current form is not applicable for use in children following cardiac surgery. However, a cardiac modification of the RAI was recently published (44) and could certainly be optimized by studying the integration of biomarkers as well as developing a clinical decision support tool for which prophylactic peritoneal dialysis could be used as the fluid removal modality of choice. We have proposed a clinical decision support tool for use of peritoneal dialysis in this population (Fig. 1). This would need to be tested prospectively, preferably in a multi-center fashion to determine whether it improves AKI and FO related outcomes in children undergoing cardiac surgery.
In summary, peritoneal drains are used with variable frequency after cardiac surgery, but most commonly in neonates and infants. The benefits of prophylactic peritoneal dialysis also vary across institutions and patient populations, and to date, no multicenter trials exist. Risk stratification and clinical decision support can aid in identification of who should receive prophylactic dialysis to improve outcomes.
References
Alten JA, Cooper DS, Blinder JJ, Selewski DT, Tabbutt S, Sasaki J, et al. Epidemiology of Acute Kidney Injury After Neonatal Cardiac Surgery: A Report From the Multicenter Neonatal and Pediatric Heart and Renal Outcomes Network. Crit Care Med. 2021;49(10):e941–51.
Jetton JG, Boohaker LJ, Sethi SK, Wazir S, Rohatgi S, Soranno DE, et al. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc Health. 2017;1(3):184–94.
Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL, Investigators A. Epidemiology of Acute Kidney Injury in Critically Ill Children and Young Adults. N Engl J Med. 2017;376(1):11–20.
Blinder JJ, Goldstein SL, Lee VV, Baycroft A, Fraser CD, Nelson D, et al. Congenital heart surgery in infants: effects of acute kidney injury on outcomes. J Thorac Cardiovasc Surg. 2012;143(2):368–74.
Mah KE, Hao S, Sutherland SM, Kwiatkowski DM, Axelrod DM, Almond CS, et al. Fluid overload independent of acute kidney injury predicts poor outcomes in neonates following congenital heart surgery. Pediatr Nephrol. 2018;33(3):511–20.
Li S, Krawczeski CD, Zappitelli M, Devarajan P, Thiessen-Philbrook H, Coca SG, et al. Incidence, risk factors, and outcomes of acute kidney injury after pediatric cardiac surgery: a prospective multicenter study. Crit Care Med. 2011;39(6):1493–9.
Bailly DK, Alten JA, Gist KM, Mah KE, Kwiatkowski DM, Valentine KM, et al. Fluid accumulation after neonatal congenital cardiac operation: clinical implications and outcomes. Ann Thorac Surg. 2022;114(6):2288–94.
Wilder NS, Yu S, Donohue JE, Goldberg CS, Blatt NB. Fluid Overload is associated with late poor outcomes in neonates following cardiac surgery. Pediatr Crit Care Med. 2016;17(5):420–7.
Bellomo R, Auriemma S, Fabbri A, D’Onofrio A, Katz N, McCullough PA, et al. The pathophysiology of cardiac surgery-associated acute kidney injury (CSA-AKI). Int J Artif Organs. 2008;31(2):166–78.
Gist KM, Selewski DT, Brinton J, Menon S, Goldstein SL, Basu RK. Assessment of the independent and synergistic effects of fluid overload and acute kidney injury on outcomes of critically ill children. Pediatr Crit Care Med. 2020;21(2):170–7.
Nourse P, Cullis B, Finkelstein F, Numanoglu A, Warady B, Antwi S, et al. ISPD guidelines for peritoneal dialysis in acute kidney injury: 2020 Update (paediatrics). Perit Dial Int. 2021;41(2):139–57.
McCulloch M, Luyckx VA, Cullis B, Davies SJ, Finkelstein FO, Yap HK, et al. Challenges of access to kidney care for children in low-resource settings. Nat Rev Nephrol. 2021;17(1):33–45.
Santos CR, Branco PQ, Gaspar A, Bruges M, Anjos R, Goncalves MS, et al. Use of peritoneal dialysis after surgery for congenital heart disease in children. Perit Dial Int. 2012;32(3):273–9.
Sahu MK, Bipin C, Arora Y, Singh SP, Devagouru V, Rajshekar P, et al. Peritoneal dialysis in pediatric postoperative cardiac surgical patients. Indian J Crit Care Med. 2019;23(8):371–5.
Frering B, Philip I, Dehoux M, Rolland C, Langlois JM, Desmonts JM. Circulating cytokines in patients undergoing normothermic cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1994;108(4):636–41.
Bokesch PM, Kapural MB, Mossad EB, Cavaglia M, Appachi E, Drummond-Webb JJ, et al. Do peritoneal catheters remove pro-inflammatory cytokines after cardiopulmonary bypass in neonates? Ann Thorac Surg. 2000;70(2):639–43.
Dittrich S, Aktuerk D, Seitz S, Mehwald P, Schulte-Monting J, Schlensak C, et al. Effects of ultrafiltration and peritoneal dialysis on proinflammatory cytokines during cardiopulmonary bypass surgery in newborns and infants. Eur J Cardiothorac Surg. 2004;25(6):935–40.
Kwiatkowski DM, Goldstein SL, Cooper DS, Nelson DP, Morales DL, Krawczeski CD. Peritoneal Dialysis vs furosemide for prevention of fluid overload in infants after cardiac surgery: a randomized clinical trial. JAMA Pediatr. 2017;171(4):357–64.
Kwiatkowski DM, Menon S, Krawczeski CD, Goldstein SL, Morales DL, Phillips A, et al. Improved outcomes with peritoneal dialysis catheter placement after cardiopulmonary bypass in infants. J Thorac Cardiovasc Surg. 2015;149(1):230–6.
Pedersen KR, Hjortdal VE, Christensen S, Pedersen J, Hjortholm K, Larsen SH, et al. Clinical outcome in children with acute renal failure treated with peritoneal dialysis after surgery for congenital heart disease. Kidney Int Suppl. 2008;108:S81–6.
Brown EA, Blake PG, Boudville N, Davies S, de Arteaga J, Dong J, et al. International Society for Peritoneal Dialysis practice recommendations: Prescribing high-quality goal-directed peritoneal dialysis. Perit Dial Int. 2020;40(3):244–53.
Strippoli GF, Tong A, Johnson D, Schena FP, Craig JC. Catheter-related interventions to prevent peritonitis in peritoneal dialysis: a systematic review of randomized, controlled trials. J Am Soc Nephrol. 2004;15(10):2735–46.
Eklund B, Honkanen E, Kyllonen L, Salmela K, Kala AR. Peritoneal dialysis access: prospective randomized comparison of single-cuff and double-cuff straight Tenckhoff catheters. Nephrol Dial Transplant. 1997;12(12):2664–6.
Warady BA, Bakkaloglu S, Newland J, Cantwell M, Verrina E, Neu A, et al. Consensus guidelines for the prevention and treatment of catheter-related infections and peritonitis in pediatric patients receiving peritoneal dialysis: 2012 update. Perit Dial Int. 2012;32 Suppl 2(Suppl 2):S32–86.
Misra M, Phadke GM. Historical Milestones in Peritoneal Dialysis. Contrib Nephrol. 2019;197:1–8.
Flores S, Loomba RS, Elhoff JJ, Bronicki RA, Mery CM, Alsaied T, et al. Peritoneal Dialysis vs diuretics in children after congenital heart surgery. Ann Thorac Surg. 2019;108(3):806–12.
Davies SJ, Zhao J, Morgenstern H, Zee J, Bieber B, Fuller DS, et al. Low serum potassium levels and clinical outcomes in peritoneal dialysis-international results from PDOPPS. Kidney Int Rep. 2021;6(2):313–24.
Hamad A, Hussain ME, Elsanousi S, Ahmed H, Navalta L, Lonappan V, et al. Prevalence and management of hypokalemia in peritoneal dialysis patients in Qatar. Int J Nephrol. 2019;2019:1875358.
Szeto CC, Chow KM, Kwan BC, Leung CB, Chung KY, Law MC, et al. Hypokalemia in Chinese peritoneal dialysis patients: prevalence and prognostic implication. Am J Kidney Dis. 2005;46(1):128–35.
Werner HA, Wensley DF, Lirenman DS, LeBlanc JG. Peritoneal dialysis in children after cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1997;113(1):64–8; discussion 8–70.
Morris KP, Butt WW, Karl TR. Effect of peritoneal dialysis on intra-abdominal pressure and cardio-respiratory function in infants following cardiac surgery. Cardiol Young. 2004;14(3):293–8.
Yang SF, Liu CJ, Yang WC, Chang CF, Yang CY, Li SY, et al. The risk factors and the impact of hernia development on technique survival in peritoneal dialysis patients: a population-based cohort study. Perit Dial Int. 2015;35(3):351–9.
Auron A, Warady BA, Simon S, Blowey DL, Srivastava T, Musharaf G, et al. Use of the multipurpose drainage catheter for the provision of acute peritoneal dialysis in infants and children. Am J Kidney Dis. 2007;49(5):650–5.
Bonilla-Felix M. Peritoneal dialysis in the pediatric intensive care unit setting: techniques, quantitations and outcomes. Blood Purif. 2013;35(1–3):77–80.
Goes CR, Berbel MN, Balbi AL, Ponce D. Metabolic implications of peritoneal dialysis in patients with acute kidney injury. Perit Dial Int. 2013;33(6):635–45.
Golej J, Kitzmueller E, Hermon M, Boigner H, Burda G, Trittenwein G. Low-volume peritoneal dialysis in 116 neonatal and paediatric critical care patients. Eur J Pediatr. 2002;161(7):385–9.
Sasser WC, Dabal RJ, Askenazi DJ, Borasino S, Moellinger AB, Kirklin JK, et al. Prophylactic peritoneal dialysis following cardiopulmonary bypass in children is associated with decreased inflammation and improved clinical outcomes. Congenit Heart Dis. 2014;9(2):106–15.
Gist KM, Henry BM, Borasino S, Rahman A, Webb T, Hock KM, et al. Prophylactic Peritoneal dialysis after the arterial switch operation: a retrospective cohort study. Ann Thorac Surg. 2021;111(2):655–61.
Namachivayam SP, Law S, Millar J, d’Udekem Y. Early Peritoneal dialysis and postoperative outcomes in infants after pediatric cardiac surgery: a systematic review and meta-analysis. Pediatr Crit Care Med. 2022;23(10):793–800.
Goldstein SL, Akcan-Arikan A, Alobaidi R, Askenazi DJ, Bagshaw SM, Barhight M, et al. Consensus-based recommendations on priority activities to address acute kidney injury in children: a modified delphi consensus statement. JAMA Network Open. 2022;5(9):e2229442-e.
Basu RK, Zappitelli M, Brunner L, Wang Y, Wong HR, Chawla LS, et al. Derivation and validation of the renal angina index to improve the prediction of acute kidney injury in critically ill children. Kidney Int. 2014;85(3):659–67.
Basu RK, Wang Y, Wong HR, Chawla LS, Wheeler DS, Goldstein SL. Incorporation of biomarkers with the renal angina index for prediction of severe AKI in critically ill children. Clin J Am Soc Nephrol. 2014;9(4):654–62.
Goldstein SL, Krallman KA, Kirby C, Roy JP, Collins M, Fox K, et al. Integration of the renal angina index and urine neutrophil gelatinase-associated lipocalin improves severe acute kidney injury prediction in critically ill children and young adults. Kidney Int Rep. 2022;7(8):1842–9.
Gist KM, SooHoo M, Mack E, Ricci Z, Kwiatkowski DM, Cooper DS, et al. Modifying the renal angina index for predicting AKI and related adverse outcomes in pediatric heart surgery. World J Pediatr Congenit Heart Surg. 2022;13(2):196–202.
Acknowledgements
The authors wish to thank Dr. Shina Menon for reviewing their manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Katie Brandewie, Katherine Melink and Katja M Gist DO each declare no potential conflicts of interest.
Human and Animal Rights and Informed Concent
The article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cardiology/CT Surgery
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brandewie, K., Melink, K. & Gist, K. Prophylactic Peritoneal Dialysis in Pediatric Cardiac Surgery. Curr Treat Options Peds 9, 136–145 (2023). https://doi.org/10.1007/s40746-023-00268-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40746-023-00268-z