Abstract
Purpose of Review
Acute pancreatitis (AP) is an increasingly recognized disorder in the pediatric population. Despite the increasing incidence of AP in children, most management recommendations are based on extrapolated data from adult studies (despite having distinctly different etiologies and clinical courses). The purpose of this paper will be to review the etiology, diagnosis, and complications of AP in children and to discuss current recommendations for the management of pediatric AP, including areas of future research.
Recent Findings
Treatment of pediatric AP is mainly supportive, centered around hydration, pain control, and nutritional support while monitoring for potential complications. Aggressive fluid resuscitation with crystalloid is critical in the treatment of AP, with recent studies supporting the use of lactated ringers over normal saline. Adequate pain control should be achieved with the use of opioid sparing agents, reserving opioids for those not responding to non-opioid analgesia. Early enteral nutrition should be adopted and is associated with improved outcomes.
Summary
Increasing awareness of pediatric acute pancreatitis had led to recent advancements in understanding the epidemiology, risk factors, genetics, and natural history of AP in children. However, future multicenter prospective and randomized controlled studies are needed to better understand and optimize the treatment of pediatric AP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, there has been a growing interest in pediatric acute pancreatitis (AP), as the incidence of AP in children has been on the rise over the last two decades. It is estimated that AP affects 3–13 per 100,000 children each year, approaching the incidence seen in adults (5–40 per 100,000 persons per year) [1, 2]. It is unclear why this trend has been observed but is likely multifactorial and not solely due to improved diagnostic methods [3]. While most cases of pediatric pancreatitis are mild, a subset of patients develop severe pancreatitis, which can be associated with significant morbidity and mortality [4]. Additionally, there are no validated tools to predict which patients will have a mild or severe course. Until recently, most of the guidelines for the management of pediatric AP had been based on extrapolated data from adult studies. Recognizing the need for pediatric specific treatment recommendations, new guidelines have been developed for the diagnosis and management of pediatric AP [5••, 6]. This paper will review the etiology, diagnostic approach, and complications of AP in children and discuss the current recommendations for the management of pediatric AP including areas of future research.
Definition
Acute pancreatitis is the reversible process of inflammation within the parenchyma of the pancreas with variable degrees of edema, necrosis, and/or hemorrhage [7]. In 2012, the INSPPIRE (International Study group of Pediatric Pancreatitis: In Search of a CuRE) group proposed diagnostic criteria for AP in children, modeled after the Atlanta criteria used in adults [8••], which has since been widely adopted [9]. It defines pediatric AP as a clinical diagnosis having at least 2 of the following: (1) abdominal pain compatible with AP (2) serum amylase and/or lipase value at least 3 times greater than the upper limit of normal or (3) imaging findings compatible with AP. Acute recurrent pancreatitis (ARP) is defined by 2 distinct episodes of AP separated by either (1) complete resolution of pain or (2) complete normalization of amylase and/or lipase between AP episodes. Additionally, chronic pancreatitis (CP) is defined by having at least one of the following: (1) pancreatic abdominal pain and radiographic findings of chronic pancreatitis OR (2) exocrine pancreatic insufficiency and radiographic chronic pancreatitis OR (3) endocrine pancreatic insufficiency and radiographic chronic pancreatitis.
The severity of AP can be classified as mild, moderate, or severe. While rare, severe acute pancreatitis (SAP) has been defined as AP with multisystem organ failure or local/systemic complications [8••]. Unlike adults in which there are validated clinical scoring systems (Ranson criteria, APACHE-II, and Glasgow, BISAP) [10, 11] that predict disease severity, these scoring systems do not accurately predict severity in pediatric AP [10, 12]. Pediatric specific scoring systems have been created, including the Pediatric Acute Pancreatitis Severity (PAPS) score, but these have low sensitivity in predicting SAP in children [13]. Radiographic scoring systems, such as the computed tomography severity index (CTSI or Balthazar score), have shown to be superior to clinical scoring systems in adults [14] and have also demonstrated superiority to clinical scoring systems in predicting disease severity in children, with sensitivity of 81% and specificity of 76% [15]. However, CTSI is an undesirable scoring system in children given the associated radiation exposure. There are known independent risk factors for the development of SAP in children, which include elevated WBC, elevated BUN, and low albumin [5••]. The systematic inflammatory response syndrome (SIRS) score is a simple widespread scoring tool that has also been shown to successfully predict disease severity in children. One study demonstrated that having a score of 2 or greater on admission was associated with increased risk of ICU admission and longer length of stay [16], findings which have been supported by other studies [17]. However, there is a clear need for a validated scoring system to risk stratify children with AP.
Etiologies
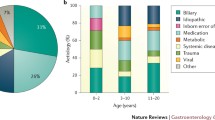
Unlike adults where the majority of AP is due to alcohol or gallstones [18], pediatric AP is associated with numerous distinct etiologies. These include systemic illness, biliary disease, congenital anatomic variants, medications, trauma, metabolic conditions, and genetic predispositions (Table 1).
Among the known causes of AP in children, medications and biliary disease (such as cholelithiasis) are among the most common etiologies [1, 19]. A list of the most common medications which can cause pancreatitis are seen in Table 1 [19, 20]. Systemic illnesses, traumatic injury, metabolic conditions, and various infections can also predispose to the development of AP. Rare etiologies include congenital malformations, or specific gene mutations.
Most pediatric pancreatologists would recommend obtaining liver transaminases, serum triglyceride and calcium levels, and abdominal ultrasound (US) as part of their routine evaluation for a child presenting with AP with unclear etiology and negative family history. The use of advanced imaging such as magnetic resonance cholangiopancreatography (MRCP) and genetic testing should be reserved for patients presenting with ARP or chronic pancreatitis (CP) [8••]. Despite increased knowledge on the various etiologies of AP, a sizeable percentage (10–25%) of pediatric AP is defined as idiopathic where patients will have no identifiable cause [21,22,23].
Evaluation
Acute pancreatitis is a clinical diagnosis based on clinical presentation, serum biomarkers, and/or radiographic imaging. Pediatric AP should be suspected in patients who present with compatible symptoms, including abdominal pain, epigastric tenderness, nausea, and vomiting. The most common findings in older children are pain and epigastric tenderness [24], whereas younger children more commonly present with vomiting and irritability [4]. Infants can present solely with fever [4]. Given that symptoms are often nonspecific and can be subtle, the diagnosis requires a high index of suspicion. This is most relevant in pediatric patients who are non-verbal, noncommunicative, or have some degree of developmental delay. Subtle changes in feeding or feeding intolerance, along with vital sign abnormalities, should alert the evaluating clinician. The most common biomarkers used for the diagnosis of AP include serum lipase and amylase. Both amylase and lipase are elevated early on in the disease course; however, lipase alone may be enough to diagnose AP as some studies have shown increased sensitivity and specificity as well as longer time to return to baseline as compared to amylase. Multiple studies have assessed the accuracy of serum lipase and amylase levels for AP and found conflicting results, but overall, the sensitivity of lipase and amylase in diagnosing AP in children appears to range from 77 to 95% and 39 to 85%, respectively [1, 25]. It is also important to note that elevations in amylase and lipase can also be observed in other non-pancreatic conditions, including liver disease, renal failure, intestinal inflammation, diabetic ketoacidosis (DKA), and trauma [1]. Other common biomarkers obtained as part of the initial evaluation of AP include hepatic panel, serum triglycerides, and calcium level [8••]. A complete blood count with differential can identify a leukocytosis, which may suggest an infectious etiology. This is relevant when patients may present with a fever or viral prodrome. An extensive electrolyte panel or serum chemistries can be useful in identifying any electrolyte abnormalities, such as hyponatremia, which can be seen in early AP. Glycemic control can be impaired, especially in cases with co-existing pancreatic diseases such as type I diabetes or patients in DKA. A serum blood urea nitrogen (BUN), hematocrit (HCT), and creatinine (Cr) can also give insight to the degree of hemoconcentration a patient with AP is experiencing. Patients who are hemoconcentrated have been demonstrated to have more severe disease, and the BUN and HCT have been reported as single predictors of severe disease [26].
Imaging in AP
The use of imaging is not necessary for the diagnosis of AP in patients who present with abdominal pain and elevated lipase or amylase. It becomes useful in cases where there are signs of severe disease or if there is suspicion of an obstructive etiology, such as biliary disease. Transabdominal ultrasound (US) is often recommended as a first line imaging modality given its widespread availability, safety profile, and ability to evaluate the biliary system well [27]. However, the sensitivity of ultrasound in diagnosing AP is lower compared to contrast enhanced computed tomography (CECT) and often limited by overlying bowel gas [27]. CECT is considered the mainstay for imaging in AP in adults; however, its use is limited in pediatrics due to added risk of exposing the patient to ionizing radiation. The ability to detect pancreatic inflammation, perfusion, and ductal anatomy, as well as the presence of peripancreatic complications, such as evolving necrosis or fluid collections in a very quick manner, makes it a useful tool in cases where US is inconclusive and MRCP unavailable [27]. Based on adult guidelines, optimal timing for when to obtain CECT is 72–96 h after symptom onset in assessment of disease severity and in evaluating for complications. Early CECT (< 72 h from presentation) is not recommended in adults as early CT has not been shown to improve clinical outcomes and findings may be unreliable as the full extent of pancreatic necrosis may not be seen on CT until 72 h after attack onset [28]. Additionally, the accuracy of early CT in predicting severity of AP in adults has been shown to be similar to clinical scoring systems; therefore, obtaining CT at admission is not recommended for the purposes of assessing clinical severity [29]. MRCP can provide excellent evaluation of pancreatic tissue and fluid collections, but with the drawback of requiring sedation in younger patients or those who cannot remain still. MRCP with intravenous secretin administration is the best imaging tool for evaluating the ductal system and is therefore recommended in cases where there is ductal leak or injury or if there is suspected anatomic or obstructive ductal abnormalities [28]. Endoscopic ultrasound (EUS) allows for detailed evaluation of the pancreas as well as the hepato-biliary system. The use of EUS has been widespread and well established in adults with pancreatic and biliary diseases for over 40 years; however, its use in pediatric patients has only recently come to light, most likely due to size limitations of the endoscope, as well as lack of pediatric endoscopists with expertise in performing EUS [27]. EUS has been shown to be safe and very effective in children in evaluating for the etiology of AP, especially gallstones pancreatitis and autoimmune pancreatitis, as well as evaluating for parenchymal changes suggestive of chronic disease [27, 30]. EUS also provides a modality for tissue sampling of pancreatic lesions, as well as parenchyma. In addition to its utility in diagnosis, EUS has evolved as a therapeutic modality in which skilled endoscopists can obtain guided tissue samples as well as endoscopically drain fluid collections. In the case of a pancreatic mass or suspected autoimmune pancreatitis, EUS-guided biopsy is the preferred modality for tissue sampling [31]. EUS can also be used to endoscopically drain pancreatic fluid collections or necrotic collections, a favorable alternative to surgical or percutaneous drainage [27].
Management
Treatment of AP is mainly supportive, with the goal to provide aggressive hydration, adequate pain control, and early nutritional support while also monitoring for and managing potential complications. Until recently, management of pediatric AP has been largely influenced by data extrapolated from adult literature. Due to the sparse guidelines for the management of AP in children, there are wide variations in how these patients are managed [32].
Hydration
The use of aggressive fluid resuscitation is a critical component of the treatment of AP, as it not only allows for adequate fluid balance but also prevents potential complications. Due to the pathogenesis of AP, including arteriolar vasoconstriction, increased capillary permeability, and hypercoagulable state with microvascular thrombosis, fluid resuscitation helps to prevent further tissue ischemia and counterbalances the interstitial fluid losses that occur. In the adult literature, early aggressive intravenous hydration is associated with improved outcomes. Currently, recommendations for initial fluid resuscitation include providing 5–10 mL/kg/hour until resuscitation goals are met, usually needing 2.5–4 L within the first 24 h [28]. While there have been no studies to date comparing ideal initial resuscitation volumes in pediatrics, current guidelines recommend 10–20 mL/kg bolus of an isotonic solution. Additionally, there is limited data regarding ideal rate of fluid resuscitation in the first 24–48 h, but consensus guidelines agree that children with AP should be provided 1.5–2× maintenance IV fluids with a dextrose containing isotonic solution after initial bolus [5••].
The type of fluids has also been debated, and the data in the pediatric population are sparse. Crystalloid is regarded as the preferred fluid for initial volume replacement; however, the data regarding whether to use normal saline (NS) or lactated ringers (LR) in pediatric AP has only recently been investigated [33•]. In adult AP, fluid resuscitation with LR has shown to reduce the incidence of systemic inflammation compared to NS, likely by providing a more favorable pH buffer [34]. A recent study has also shown that the use of LR may be preferred over NS in pediatric AP as it is associated with shortened hospital stay [33•]. Therefore, it should be recommended that children with AP receive LR with dextrose 5% as the preferred crystalloid for fluid resuscitation in the first 24–48 h.
The use of colloids is not routinely recommended in the initial fluid resuscitation of AP. Based on adult data, the use of colloids (albumin, packed red blood cells, or fresh frozen plasma) should be reserved for specific situations. Current adult guidelines recommend its use for cases where the hematocrit is < 25% or albumin level is < 2 g/dL [28].
Monitoring for adequate fluid resuscitation is imperative during in the initial disease course as vigorous fluid replacement is necessary to prevent multiorgan dysfunction but can result in fluid overload and electrolyte abnormalities. Recommendations for monitoring include frequent vital signs check during the first 48 h to assess for developing complications (such as SIRS and organ dysfunction) and monitoring both urine output and BUN/Cr levels to ensure adequate fluid resuscitation.
Pain control
Pain control is imperative in the management of AP, especially considering that pain is the most frequent presenting symptom in children with AP [1]. Despite its critical role in the treatment of AP, there is limited data in the pediatric literature that shows the optimal analgesia for AP in children. Opioid sparing medications, such as parental acetaminophen or nonsteroidal anti-inflammatory medications, should be considered for the pain management of all pediatric patients with AP. When pancreatitis pain does not respond to opioid sparing agents, then opioids should be used for additional pain control. No single opiate has been found to be superior to another for the treatment of pain in AP in adults, and there is no data to support that opioids cause sphincter of Oddi dysfunction [35]. Thus, opioids can be safely used for pediatric pancreatitis pain that does not respond to opioid sparing medications, although caution should be used given the risk associated with opioid use. Current consensus guidelines recommend that non-opioid medications should be used in all children with AP, reserving opioids for those not responding to non-opioid analgesia [5••]. Despite recommendations, a recent study demonstrated that pediatric pancreatitis pain is treated far more frequently with opioids compared to non-opioids, and over 50% of patients receive opioids alone for analgesia [36•]. This further highlights the need for future prospective studies to determine the optimal pain management in pediatric AP.
Nutrition
Historically, management of AP included keeping patients nil per os in an effort to “rest the pancreas” and reduce ongoing pancreatic injury and subsequent systemic inflammatory response. However, this practice is no longer supported after systematic review of multiple randomized controlled studies demonstrated that early enteral nutrition is associated with improved outcomes in AP [37]. Early enteral nutrition is thought to preserve the integrity of the intestinal mucosa and prevent bacterial translocation, thus decreasing the risk of infection and mortality [38]. It was also previously thought that enteral feeds should be given straight into the jejunum (through nasojejunal feeding tube) rather than into the stomach, similarly with the thought of bypassing the pancreatic duct to decrease pancreatic enzyme release and therefore “rest the pancreas.” However, systematic review of the literature has shown no difference in outcomes between receiving nasogastric versus nasojejunal feeds [39].
Children with mild AP should be started on a regular diet as early as possible, ideally within the first 24–48 h of admission. Early enteral nutrition in children with mild AP is associated with shorter hospital stay, less admissions to an intensive care unit, and lower rates of progression to SAP [40•]. Additionally, early enteral nutrition is well tolerated and has not been shown to be associated with increased pain [41].
Children with SAP should also receive early enteral nutrition, preferably within 72 h of admission. While there is no pediatric data examining early enteral nutrition in SAP, adult data has shown that early enteral nutrition (within 48 h of admission) in patients with SAP is associated with reductions in both infections and mortality compared to those receiving delayed enteral nutrition. It is therefore recommended that children with SAP should receive early enteral nutrition once deemed hemodynamically stable [42]. Additionally, children with SAP can be safely fed orally or by nasogastric tube and should reserve the use of nasojejunal tube for children who cannot tolerate nasogastric feeds. In children who are unable to tolerate enteral nutrition for a prolonged period, then parental nutrition should be considered. However, enteral nutrition should be started as soon as possible, as a combination of enteral and parental nutrition is preferable to parental nutritional alone [42].
Antibiotics
The role of antibiotics in adults have been studied extensively as approximately 30% of patients with necrotizing pancreatitis will develop infected necrosis, which is associated with significant morbidity and mortality [43]. Identifying patients at risk for infected necrosis is difficult as the adult literature has shown no correlation between extent of necrosis and the risk of infection [44] and the risk of infected necrosis is relatively rare early in the disease course (first 7 days) [45]. Despite the high mortality rate of infected pancreatic necrosis, systematic analysis of the adult literature has shown that there is no benefit of prophylactic antibiotics [46]. While there have not been any studies in children examining antibiotic use in AP, prophylactic antibiotic use is not recommended in the management of pediatric AP. In cases in which either infected pancreatic necrosis or extra-pancreatic infection is suspected, then antibiotics should be used [5••]. Infected pancreatic necrosis should be suspected in patients with fevers, leukocytosis, bacteremia, worsening clinical status, or imaging demonstrating gas within a pancreatic/peripancreatic collection.
Therapeutic interventions
Due to the advances of improved imaging modalities as well as increased use of endoscopic ultrasound (EUS), ERCP is now used primarily for therapeutic interventions and rarely used for diagnostic purposes. The indications for ERCP in pediatric AP include biliary pancreatitis, pancreatic ductal stones, or pancreatic duct leaks/fistulas or strictures [47].
The role of surgery in the management of pediatric AP is limited and not routinely necessary. In cases of pancreatitis due to gallstones or biliary sludge, cholecystectomy should be done to prevent recurrence. Cholecystectomy should be done early, ideally during the same hospitalization for mild gallstone pancreatitis, due to the risk of recurrence with interval cholecystectomy [48•].
In cases of mature fluid collections (such as a pseudocyst or walled off necrosis), therapeutic drainage may be necessary. Asymptomatic pancreatic fluid collections (PFCs) should be managed conservatively; however, symptomatic PFCs require therapeutic drainage [49]. Drainage of PFCs can be done endoscopically, percutaneously, or surgically. Historically, surgery was the preferred management of PFCs, which included open or laparoscopic cyst gastrostomy for drainage of pseudocysts and necrosectomy for walled off necrosis [50]. However, with advancement in endoscopic therapies and techniques, studies have demonstrated improved outcomes with endoscopic drainage as compared to surgery and have become the preferred initial treatment approach [50,51,52]. Additionally, surgical debridement (necrosectomy) for pancreatic necrosis has been shown to carry a high mortality risk, especially when done early on in the disease course [53]. Randomized control studies have shown that in adults, the step-up approach reduces mortality rates, in which endoscopic or percutaneous drainage is attempted first followed by surgery if necessary for the management of infected pancreatic necrosis [54••]. Endoscopic drainage of PFCs is usually done under EUS guidance, allowing the endoscopist the ability to confirm the location and adequate wall maturity of the collection as well as identify and avoid vascular structures between the cyst and gastric lumen [50]. EUS-guided cystogastrostomy involves creating a fistula between the gastric lumen and cyst cavity and deployment of plastic or metal stent to facilitate continuous drainage [50]. The use of metal stents offers several advantages, which includes larger luminal diameter and shorter procedural time as they only require a single access point into the cyst [50]. More recent advancements include the development of lumen-apposing metal stents (LAMS), which is characterized by a “dumbbell” configuration with two flanges on either side of the stent to avoid stent migration and a large luminal diameter for improved endoscopic drainage [55, 56]. The use of LAMS for EUS-guided drainage in pediatric patients has been shown to be safe and effective, with high rates of technical and clinical success [57].
Outcomes
The majority of AP in children are mild with return of normal pancreatic parenchyma within 6 months. Those with SAP account for a small fraction of all AP cases in children, but those patients have a much more prolonged course with associated complications [1]. Complications of AP in children are similar to those complications seen in adults. These include both local and systemic complications. Systemic complications include pulmonary edema, coagulopathy, acute renal failure, sepsis, and multiorgan failure. Local complications include peripancreatic fluid collection, pancreatic pseudocyst, pancreatic necrosis (sterile or infected), and walled off necrosis.
Pancreatic fluid collections are common and can be seen in approximately 60% of patients [49, 58]. Classifications of fluid collections are based upon the nature of the fluid collection as well as its duration [59]. Acute peripancreatic fluid collections occur in the setting of interstitial edematous pancreatitis and can mature into pseudocyst (usually after 4 weeks), and similarly acute necrotic collection can mature into a walled off necrosis (usually after 4 weeks) [59]. Pancreatic fluid collections often resolve spontaneously but may require intervention if they cause persistent symptoms or if there is evidence of a complicated pseudocyst. Pancreatic necrosis is a relatively rare complication in children, but when it occurs, it can mature into a walled off necrotic collection (WON). The management of pseudocyst and WON includes drainage of the collection. This can be done either through endoscopic ultrasound-guided drainage, percutaneously or surgically.
While most cases of AP in children resolve without additional attacks or long-term sequelae, approximately 10–35% of children will have another attack. Recent data has demonstrated that the progression from acute to acute recurrent pancreatitis (ARP) can occur in as little as 5 months and that 20–40% of children with ARP ultimately progress to chronic pancreatitis within 2–5 years [60, 61]. Risk factors for the development of ARP include underlying genetic predisposition, anatomic abnormalities, and the occurrence of pancreatic necrosis during the initial attack [21, 62, 63]. Those children who develop recurrent episodes of pancreatitis should undergo further evaluation, which may include more detailed imaging and genetic testing, to try to identify an underlying etiology.
Conclusions
Recently, there has been increased awareness of pancreatitis in children given the increasing incidence of pediatric AP, resulting in significant disease burden in the pediatric population [64]. The economic burden of pediatric AP has increased in the last decade, estimating that the inpatient cost alone is approximately $200 million per year [1, 65]. While there have been significant advancements in understanding the epidemiology, risk factors, and genetics of AP in children, there is limited data regarding the optimal management of pediatric AP. Future multicenter prospective and randomized controlled studies are needed to better understand and optimize the management of AP in children.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Bai HX, Lowe ME, Husain SZ. What have we learned about acute pancreatitis in children? J Pediatr Gastroenterol Nutr. 2011;52(3):262–70. https://doi.org/10.1097/MPG.0b013e3182061d75.
Epidemiology of Pancreatitis. GI Epidemiology. 2014. p. 306-12.
Lopez MJ. The changing incidence of acute pancreatitis in children: a single-institution perspective. J Pediatr. 2002;140(5):622–4. https://doi.org/10.1067/mpd.2002.123880.
Kandula L, Lowe ME. Etiology and outcome of acute pancreatitis in infants and toddlers. J Pediatr. 2008;152(1):106–10, 10.e1. https://doi.org/10.1016/j.jpeds.2007.05.050.
•• Abu-El-Haija M, Kumar S, Quiros JA, Balakrishnan K, Barth B, Bitton S et al. Management of acute pancreatitis in the pediatric population: a clinical report from the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition Pancreas Committee. J Pediatr Gastroenterol Nutr. 2018;66(1):159-176. doi:10.1097/mpg.0000000000001715.This clinical report is the first paper providing recommendations for the treatment of pediatric acute pancreatitis. This report reviews existing studies of pediatric acute pancreatitis and draws from both expert opinion as well as relevant adult literature. It also highlights areas in which pediatric data is lacking and direction for future research.
Párniczky A, Abu-El-Haija M, Husain S, Lowe M, Oracz G, Sahin-Tóth M, et al. EPC/HPSG evidence-based guidelines for the management of pediatric pancreatitis. Pancreatology. 2018;18(2):146–60. https://doi.org/10.1016/j.pan.2018.01.001.
Bradley EL 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993;128(5):586–90. https://doi.org/10.1001/archsurg.1993.01420170122019.
•• Morinville VD, Husain SZ, Bai H, Barth B, Alhosh R, Durie PR, et al. Definitions of pediatric pancreatitis and survey of present clinical practices. J Pediatr Gastroenterol Nutr. 2012;55(3):261–5. https://doi.org/10.1097/MPG.0b013e31824f1516 This study represents the creation of the INSPPIRE consortium, the first multicenter initiative to improve our understanding of the epidemiology, etiology, and outcomes of acute pancreatitis in children. This study resulted in established criteria for the diagnosis of pediatric acute pancreatitis as well as definitions of acute, recurrent, and chronic pancreatitis.
Abu-El-Haija M, Kumar S, Szabo F, Werlin S, Conwell D, Banks P, et al. Classification of acute pancreatitis in the pediatric population: clinical report from the NASPGHAN Pancreas Committee. J Pediatr Gastroenterol Nutr. 2017;64(6):984–90. https://doi.org/10.1097/mpg.0000000000001583.
Valverde-Lopez F, Matas-Cobos AM, Alegria-Motte C, Jimenez-Rosales R, Ubeda-Munoz M, Redondo-Cerezo E. BISAP, RANSON, lactate and others biomarkers in prediction of severe acute pancreatitis in a European cohort. J Gastroenterol Hepatol. 2017;32(9):1649–56. https://doi.org/10.1111/jgh.13763.
Papachristou GI, Muddana V, Yadav D, O'Connell M, Sanders MK, Slivka A, et al. Comparison of BISAP, Ranson's, APACHE-II, and CTSI scores in predicting organ failure, complications, and mortality in acute pancreatitis. Am J Gastroenterol. 2010;105(2):435–41;quiz 42. https://doi.org/10.1038/ajg.2009.622.
Ikeura T, Horibe M, Sanui M, Sasaki M, Kuwagata Y, Nishi K, et al. Validation of the efficacy of the prognostic factor score in the Japanese severity criteria for severe acute pancreatitis: A large multicenter study. United European Gastroenterol J. 2017;5(3):389–97. https://doi.org/10.1177/2050640616670566.
Lautz TB, Chin AC, Radhakrishnan J. Acute pancreatitis in children: spectrum of disease and predictors of severity. J Pediatr Surg. 2011;46(6):1144–9. https://doi.org/10.1016/j.jpedsurg.2011.03.044.
Balthazar EJ, Ranson JH, Naidich DP, Megibow AJ, Caccavale R, Cooper MM. Acute pancreatitis: prognostic value of CT. Radiology. 1985;156(3):767–72. https://doi.org/10.1148/radiology.156.3.4023241.
Lautz TB, Turkel G, Radhakrishnan J, Wyers M, Chin AC. Utility of the computed tomography severity index (Balthazar score) in children with acute pancreatitis. J Pediatr Surg. 2012;47(6):1185–91. https://doi.org/10.1016/j.jpedsurg.2012.03.023.
Grover AS, Kadiyala V, Banks PA, Grand RJ, Conwell DL, Lightdale JR. The utility of the systemic inflammatory respsonse syndrome score on admission in children with acute pancreatitis. Pancreas. 2017;46(1):106–9. https://doi.org/10.1097/MPA.0000000000000681.
Nauka PC, Weinstein TA, Dolinger MT, Miller JM, Kohn N, Bitton S, et al. Validation of lipase and systemic inflammatory response syndrome as prognostic indicators in pediatric acute pancreatitis: a retrospective analysis. J Pediatr Gastroenterol Nutr. 2019;68(3):389–93. https://doi.org/10.1097/MPG.0000000000002217.
Lankisch PG, Apte M, Banks PA. Acute pancreatitis. Lancet. 2015;386(9988):85–96. https://doi.org/10.1016/S0140-6736(14)60649-8.
Abu-El-Haija M, Hornung L, Lin TK, Nathan JD, Thompson T, Vitale DS, et al. Drug induced pancreatitis is the leading known cause of first attack acute pancreatitis in children. Pancreatology. 2020;20(6):1103–8. https://doi.org/10.1016/j.pan.2020.07.008.
Trivedi CD, Pitchumoni CS. Drug-induced pancreatitis: an update. J Clin Gastroenterol. 2005;39(8):709–16. https://doi.org/10.1097/01.mcg.0000173929.60115.b4.
Weizman Z, Durie PR. Acute pancreatitis in childhood. J Pediatr. 1988;113(1 Pt 1):24–9. https://doi.org/10.1016/s0022-3476(88)80523-7.
Werlin SL, Kugathasan S, Frautschy BC. Pancreatitis in children. J Pediatr Gastroenterol Nutr. 2003;37(5):591–5. https://doi.org/10.1097/00005176-200311000-00017.
Hyams JS, Kay M. Wyllie R. Pediatric gastrointestinal and liver disease: Elsevier; 2015.
Park AJ, Latif SU, Ahmad MU, Bultron G, Orabi AI, Bhandari V, et al. A comparison of presentation and management trends in acute pancreatitis between infants/toddlers and older children. J Pediatr Gastroenterol Nutr. 2010;51(2):167–70. https://doi.org/10.1097/MPG.0b013e3181cea545.
Orkin SH, Trout AT, Fei L, Lin TK, Nathan JD, Thompson T, et al. Sensitivity of biochemical and imaging findings for the diagnosis of acute pancreatitis in children. J Pediatr. 2019;213:143–8.e2. https://doi.org/10.1016/j.jpeds.2019.06.028.
Koutroumpakis E, Wu BU, Bakker OJ, Dudekula A, Singh VK, Besselink MG, et al. Admission hematocrit and rise in blood urea nitrogen at 24 h outperform other laboratory markers in predicting persistent organ failure and pancreatic necrosis in acute pancreatitis: a post hoc analysis of three large prospective databases. Am J Gastroenterol. 2015;110(12):1707–16. https://doi.org/10.1038/ajg.2015.370.
Lin TK, Troendle DM, Wallihan DB, Barth B, Fox VL, Fishman DS, et al. Specialized imaging and procedures in pediatric pancreatology: a North American Society for pediatric gastroenterology, hepatology, and nutrition clinical report. J Pediatr Gastroenterol Nutr. 2017;64(3):472–84. https://doi.org/10.1097/mpg.0000000000001371.
IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13(4 Suppl 2):e1-15. doi:10.1016/j.pan.2013.07.063.
Bollen TL, Singh VK, Maurer R, Repas K, van Es HW, Banks PA et al. A comparative evaluation of radiologic and clinical scoring systems in the early prediction of severity in acute pancreatitis. Official journal of the American College of Gastroenterology | ACG. 2012;107(4):612-9. https://doi.org/10.1038/ajg.2011.438.
Fugazza A, Bizzarri B, Gaiani F, Manfredi M, Ghiselli A, Crafa P, et al. The role of endoscopic ultrasound in children with pancreatobiliary and gastrointestinal disorders: a single center series and review of the literature. BMC Pediatr. 2017;17(1):203. https://doi.org/10.1186/s12887-017-0956-z.
Committee ASoP, Eloubeidi MA, Decker GA, Chandrasekhara V, Chathadi KV, Early DS, et al. The role of endoscopy in the evaluation and management of patients with solid pancreatic neoplasia. Gastrointest Endosc. 2016;83(1):17–28. https://doi.org/10.1016/j.gie.2015.09.009.
Abu-El-Haija M, Palermo JJ, Fei L, Lin TK. Variability in pancreatitis care in pediatrics: a single institution's survey report. Pancreas. 2016;45(1):40–5. https://doi.org/10.1097/mpa.0000000000000436.
• Farrell PR, Farrell LM, Hornung L, Abu-El-Haija M. Use of lactated ringers solution compared with normal saline is associated with shorter length of stay in pediatric acute pancreatitis. Pancreas. 2020;49(3):375–80. https://doi.org/10.1097/mpa.0000000000001498 This study is the first study to investigate the optimal fluid management of pediatric acute pancreatitis. This study demonstrated that the use of lactated ringers compared to normal saline may reduce hospital length of stay. Further studies, including randomized trials, are needed to determine the optimal fluid management of acute pancreatitis in children.
Wu BU, Hwang JQ, Gardner TH, Repas K, Delee R, Yu S, et al. Lactated Ringer's solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol. 2011;9(8):710–7.e1. https://doi.org/10.1016/j.cgh.2011.04.026.
Thompson DR. Narcotic analgesic effects on the sphincter of Oddi: a review of the data and therapeutic implications in treating pancreatitis. Am J Gastroenterol. 2001;96(4):1266–72. https://doi.org/10.1111/j.1572-0241.2001.03536.x.
• Grover AS, Mitchell PD, Manzi SF, Fox VL. Initial pain management in pediatric acute pancreatitis: opioid versus non-opioid. J Pediatr Gastroenterol Nutr. 2018;66(2):295–8. https://doi.org/10.1097/MPG.0000000000001809 This is the first study investigating pain management of acute pancreatitis in children. Given that pain management is fundamental for treating acute pancreatitis, this study demonstrated the preferential use of opioids in children with acute pancreatitis and the need for future randomized trials to determine optimal pain management.
Banks PA. Acute pancreatitis: landmark studies, management decisions, and the future. Pancreas. 2016;45(5):633–40. https://doi.org/10.1097/mpa.0000000000000632.
Theodoridis X, Grammatikopoulou MG, Petalidou A, Stamouli EM, Fotiadou I, Gkiouras K, et al. Nutrition interventions in pediatric pancreatitis: guidelines we can trust. J Pediatr Gastroenterol Nutr. 2019;69(1):120–5. https://doi.org/10.1097/mpg.0000000000002364.
Kumar A, Singh N, Prakash S, Saraya A, Joshi YK. Early enteral nutrition in severe acute pancreatitis: a prospective randomized controlled trial comparing nasojejunal and nasogastric routes. J Clin Gastroenterol. 2006;40(5):431–4. https://doi.org/10.1097/00004836-200605000-00013.
• Szabo FK, Fei L, Cruz LA, Abu-El-Haija M. Early enteral nutrition and aggressive fluid resuscitation are associated with improved clinical outcomes in acute pancreatitis. J Pediatr. 2015;167(2):397–402.e1. https://doi.org/10.1016/j.jpeds.2015.05.030 This study is the first study in the pediatric population that demonstrated that the initiation of early enteral nutrition resulted in improved clinical outcomes (shorter hospital stay, decreased ICU admission, and decreased rates of SAP).
Abu-El-Haija M, Wilhelm R, Heinzman C, Siqueira BN, Zou Y, Fei L, et al. Early enteral nutrition in children with acute pancreatitis. J Pediatr Gastroenterol Nutr. 2016;62(3):453–6. https://doi.org/10.1097/mpg.0000000000001013.
Abu-El-Haija M, Uc A, Werlin SL, Freeman AJ, Georgieva M, Jojkić-Pavkov D, et al. Nutritional considerations in pediatric pancreatitis: a position paper from the NASPGHAN Pancreas Committee and ESPGHAN Cystic Fibrosis/Pancreas Working Group. J Pediatr Gastroenterol Nutr. 2018;67(1):131–43. https://doi.org/10.1097/mpg.0000000000002023.
Banks PA, Freeman ML. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101(10):2379–400. https://doi.org/10.1111/j.1572-0241.2006.00856.x.
Perez A, Whang EE, Brooks DC, Moore JFD, Hughes MD, Sica GT, et al. Is severity of necrotizing pancreatitis increased in extended necrosis and infected necrosis? Pancreas. 2002;25(3):229–33. https://doi.org/10.1097/00006676-200210000-00003.
Besselink MG, van Santvoort HC, Boermeester MA, Nieuwenhuijs VB, van Goor H, Dejong CHC, et al. Timing and impact of infections in acute pancreatitis. Br J Surg. 2009;96(3):267–73. https://doi.org/10.1002/bjs.6447.
Forsmark CE, Vege SS, Wilcox CM. Acute Pancreatitis. N Engl J Med. 2016;375(20):1972–81. https://doi.org/10.1056/NEJMra1505202.
Agarwal J, Nageshwar Reddy D, Talukdar R, Lakhtakia S, Ramchandani M, Tandan M, et al. ERCP in the management of pancreatic diseases in children. Gastrointest Endosc. 2014;79(2):271–8. https://doi.org/10.1016/j.gie.2013.07.060.
• da Costa DW, Bouwense SA, Schepers NJ, Besselink MG, van Santvoort HC, van Brunschot S, et al. Same-admission versus interval cholecystectomy for mild gallstone pancreatitis (PONCHO): a multicentre randomised controlled trial. Lancet. 2015;386(10000):1261–8. https://doi.org/10.1016/s0140-6736(15)00274-3 While this study was done in the adult population, this multicenter randomized clinical trial demonstrated that for patients with gallstone pancreatitis, same admission cholecystectomy reduced the risk of gallstone related complications in comparison to patients who underwent interval cholecystectomy. The results of this study have been extrapolated to the pediatric population and is regarded as standard practice to perform same admission cholecystectomy for mild acute pancreatitis.
Bolia R, Srivastava A, Yachha SK, Poddar U, Kumar S. Prevalence, natural history, and outcome of acute fluid collection and pseudocyst in children with acute pancreatitis. J Pediatr Gastroenterol Nutr. 2015;61(4):451–5. https://doi.org/10.1097/MPG.0000000000000800.
Tyberg A, Karia K, Gabr M, Desai A, Doshi R, Gaidhane M, et al. Management of pancreatic fluid collections: a comprehensive review of the literature. World J Gastroenterol. 2016;22(7):2256–70. https://doi.org/10.3748/wjg.v22.i7.2256.
Varadarajulu S, Bang JY, Sutton BS, Trevino JM, Christein JD, Wilcox CM. Equal efficacy of endoscopic and surgical cystogastrostomy for pancreatic pseudocyst drainage in a randomized trial. Gastroenterology. 2013;145(3):583–90.e1. https://doi.org/10.1053/j.gastro.2013.05.046.
Park DH, Lee SS, Moon SH, Choi SY, Jung SW, Seo DW, et al. Endoscopic ultrasound-guided versus conventional transmural drainage for pancreatic pseudocysts: a prospective randomized trial. Endoscopy. 2009;41(10):842–8. https://doi.org/10.1055/s-0029-1215133.
Baron TH, DiMaio CJ, Wang AY, Morgan KA. American gastroenterological association clinical practice update: management of pancreatic necrosis. Gastroenterology. 2020;158(1):67–75.e1. https://doi.org/10.1053/j.gastro.2019.07.064.
•• van Santvoort HC, Besselink MG, Bakker OJ, Hofker HS, Boermeester MA, Dejong CH, et al. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010;362(16):1491–502. https://doi.org/10.1056/NEJMoa0908821 This landmark study, also done in the adult population, compared open necrosectomy (standard of care) to a minimally invasive approach (including endoscopic or percutaneous drainage) for the management of necrotizing pancreatitis. This study demonstrated a reduction in mortality rates with a minimally invasive approach and is now regarded as standard of care for the management of necrotic (and infected necrotic) pancreatitis.
Wrobel PS, Kaplan J, Siddiqui AA. A new lumen-apposing metal stent for endoscopic transluminal drainage of peripancreatic fluid collections. Endosc Ultrasound. 2014;3(4):203–4. https://doi.org/10.4103/2303-9027.144508.
Siddiqui AA, Adler DG, Nieto J, Shah JN, Binmoeller KF, Kane S, et al. EUS-guided drainage of peripancreatic fluid collections and necrosis by using a novel lumen-apposing stent: a large retrospective, multicenter U.S. experience (with videos). Gastrointest Endosc. 2016;83(4):699–707. https://doi.org/10.1016/j.gie.2015.10.020.
Costa PA, Ho S, Manvar A, Rivas Y, Novak I. Use of lumen-apposing metal stents for endoscopic drainage of intra-abdominal fluid collections in pediatric patients. J Pediatr Gastroenterol Nutr. 2020;70(2):258–60. https://doi.org/10.1097/mpg.0000000000002538.
Lal SB, Venkatesh V, Rana SS, Anushree N, Bhatia A, Saxena A. Paediatric acute pancreatitis: clinical profile and natural history of collections. Pancreatology. 2020;20(4):659–64. https://doi.org/10.1016/j.pan.2020.03.007.
Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–11. https://doi.org/10.1136/gutjnl-2012-302779.
Sweeny KF, Lin TK, Nathan JD, Denson LA, Husain SZ, Hornung L, et al. Rapid progression of acute pancreatitis to acute recurrent pancreatitis in children. J Pediatr Gastroenterol Nutr. 2019;68(1):104–9. https://doi.org/10.1097/MPG.0000000000002145.
Hao F, Guo H, Luo Q, Guo C. Disease progression of acute pancreatitis in pediatric patients. J Surg Res. 2016;202(2):422–7. https://doi.org/10.1016/j.jss.2016.01.016.
Uc A, Husain SZ. Pancreatitis in children. Gastroenterology. 2019;156(7):1969–78. https://doi.org/10.1053/j.gastro.2018.12.043.
Kumar S, Ooi CY, Werlin S, Abu-El-Haija M, Barth B, Bellin MD, et al. Risk factors associated with pediatric acute recurrent and chronic pancreatitis: lessons from INSPPIRE. JAMA Pediatr. 2016;170(6):562–9. https://doi.org/10.1001/jamapediatrics.2015.4955.
Hornung L, Szabo FK, Kalkwarf HJ, Abu-El-Haija M. Increased burden of pediatric acute pancreatitis on the health care system. Pancreas. 2017;46(9):1111–4. https://doi.org/10.1097/mpa.0000000000000918.
Pant C, Deshpande A, Olyaee M, Anderson MP, Bitar A, Steele MI, et al. Epidemiology of acute pancreatitis in hospitalized children in the United States from 2000 to 2009. PLoS One. 2014;9(5):e95552. https://doi.org/10.1371/journal.pone.0095552.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pediatric Gastroenterology
Rights and permissions
About this article
Cite this article
Templeton, K., Grover, A.S. Acute Pancreatitis in Children. Curr Treat Options Peds 7, 46–59 (2021). https://doi.org/10.1007/s40746-021-00221-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40746-021-00221-y