Abstract
Purpose of review
Pulmonary vein stenosis (PVS) is an increasingly recognized disease in infants and young children. Controversy exists regarding the best management of this disease. The hallmarks of PVS include recurrence, upstream progression, and spread to previously unaffected pulmonary veins. This review highlights invasive and medical therapies for pediatric patients with primary and secondary PVS.
Recent findings
Catheter-based therapies for PVS are the mainstay of anatomic, large-vessel therapy. Angioplasty and stenting both have a role in the anatomic management of PVS. Repeated transcatheter interventions have been shown to improve survival. Surgical PVS intervention is an important element to PVS therapy. Primary (medical) therapy is increasingly recognized as an important element to PVS treatment. New anti-proliferative therapies have proven to be a useful adjunct to invasive therapies.
Summary
Congenital and acquired PVS are progressive diseases that pose a growing challenge for cardiologists. Surgical and transcatheter interventions in most cases have early success but are only temporizing solutions in the face of myofibroblastic proliferation. More recent studies focusing on benefits of immunomodulatory therapy have paved the way for the use of primary treatment alongside the anatomic therapy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pulmonary vein stenosis (PVS) in the pediatric population is a relatively rare condition making up 0.4% of congenital heart diseases [1]. The reported incidence of PVS, however, likely lags behind the current frequency seen at large pediatric centers. Some of this increase in frequency may reflect a growing awareness of PVS, while a true higher rate of PVS may be explained as well by growing rates of prematurity, chronic lung disease (CLD), and other unidentified environmental factors. PVS is often categorized as congenital (primary) or acquired (secondary), e.g., following surgical repair of partial or total anomalous pulmonary venous return. Secondary PVS is seen as a postoperative complication in ~ 10% of cases [2, 3]. Primary PVS is often associated with chronic lung disease of prematurity, and it is widely held that pulmonary parenchymal inflammation and maldevelopment lead to arterial as well as pulmonary venous vascular changes [4•].
The pathophysiology of PVS is becoming better described. Animal models of PVS have demonstrated that discrete narrowing at the junction of the PV and left atrium is enough of a disturbance to set off a cascade of cellular and molecular pathways. These signals can induce upstream changes in intimal cells, with myofibroblast and myocyte conversion [5]. The myofibroblasts are proliferative in nature and eventually lead to worsening PVS which, when aggressive or untreated, develops into pulmonary vein atresia (PVA).
PVS has proven to be a difficult disease to manage because of its progressive and proliferative nature. Patients with bilateral disease, prematurity, and early age at onset are known to have a high mortality rate [6]. Death typically occurs during infancy and the likelihood of survival significantly increases after 1 year of age [7••]. Surgical repair alone has not been an effective solution for many patients. In the last decade, many centers have introduced new anatomic and anti-proliferative therapies to fight this relentless disease. The focus of the current review will be to describe the surveillance, treatment options, and our algorithm for patients with PVS.
Clinical presentation
PVS is most commonly diagnosed within the first year of life—typically in premature infants with chronic lung disease. PVS can occur in isolation, but as many as half of patients will also have some other form of congenital heart disease [8].
The clinical presentation of patients with PVS is that of congestive heart failure and respiratory difficulty. Respiratory symptoms can range from mild tachypnea to severe respiratory distress. Recurrent viral or bacterial respiratory infections are common in PVS patients given their propensity for alveolar fluid retention and airway congestion. Hemoptysis is one of the later symptoms that can occur after the lungs have experienced chronic damage. If left untreated, symptoms will progress to involve severe pulmonary hypertension, right heart failure, and death.
Chest radiography in patients with pulmonary vein stenosis can provide clinical clues although the findings are non-specific. Pulmonary venous congestion can manifest on chest x-rays as Kerley B lines, pulmonary edema, or reticular opacities. Asymmetry in pulmonary vascularity or in lung volumes may be noted if PVS is isolated to one lung; however, this asymmetry is often a late finding.
Diagnosis and evaluation
Echocardiography
If there is suspicion for PVS, echocardiography has historically been the first step in making the diagnosis. Echocardiographic evaluation of pulmonary veins is notoriously fraught, however. Although echocardiography provides valuable information, there are still important limitations to note with its use. Obtaining adequate images to truly interrogate the pulmonary veins may be difficult in patients for a multitude of reasons including difficult windows, prominent lung artifact, and the posterior location of the veins within the chest. In addition, redistribution of pulmonary blood flow in the setting of regional PVS can result in lower Doppler gradients in affected veins and, unexpectedly, higher Doppler gradients in unaffected pulmonary veins [9]. Finally, many premature infants with CLD also have atrial septal defects (ASDs) or patent ductus arteriosus (PDA)—increased pulmonary blood flow due to these shunts can make interpretation of Doppler gradients even more challenging.
Cross-sectional imaging
Given the notorious limitations of echocardiography in defining pulmonary vein anatomy, cross-sectional imaging is usually necessary to diagnose PVS and guide interventions. Cardiac magnetic resonance imaging (cMR) can be considered as it often provides excellent anatomic detail with additional benefits of quantifying flows to affected and normal pulmonary veins. However, long acquisition times, need for anesthesia, and lower resolution make cMR less attractive than computed tomographic angiography (CTA).
Many infants and young children can undergo CTA without the need for anesthesia with feed and bundle protocols employed at many academic centers. CTAs also allow for evaluation of other cardiac structures, distal pulmonary vasculature as well as the underlying lung parenchyma. While CTAs are associated with exposure to ionizing radiation, the radiation doses from newer CT scanners with optimized imaging techniques has made the radiation dose during scans minimal [10]. At our center, CTA is a key component of the initial diagnosis. Importantly, serial CTA scans allow for grading of all pulmonary veins, allowing our team to track progression of PVS in each patient. Our institution uses a novel scheme to standardize grading severity and monitoring progression of disease [Fig. 1].
Novel pulmonary vein stenosis grading scheme based on cross-sectional imaging or angiography. Grade 1 veins are mildly stenotic with < 50% narrowing at the insertion of the pulmonary vein to the left atrium relative to the maximal caliber of the distal vessel. Grade 2 veins are moderate to severely stenotic with > 50% narrowing. Grade 3 veins are atretic. The PVS severity is further characterized as either grade A or grade B. Grade A denotes a normal caliber to the distal vessel, whereas grade B denotes diffuse hypoplasia (vein diameter < 2 mm).
Diagnostic cardiac catheterization
Cardiac catheterization remains the gold standard for definitive diagnosis of PVS, as catheterization provides angiographic as well as hemodynamic data. While much attention is appropriately paid to gradients across individual pulmonary veins in this disease, our center focuses more on right ventricular (RV) and pulmonary artery (PA) pressures. The rationale for this focus on RV and PA pressures is based upon the physiology expressed through the Gorlin equation: gradient across a stenosis is directly proportional to degree of stenosis, but also proportional to amount of flow [11]. As noted above, redistribution of blood flow to healthier lung segments can result in gradients which do not reflect actual PVS severity.
Angiography should be performed, whenever possible, using a large-caliber guide catheter or sheath, with retrograde venography [Fig. 2]. The value of retrograde venography is the ability to identify the upstream arborization, which is necessarily missed with angiography from the PA side. The presence of angiographic stenosis is pathognomonic for PVS but delayed transit time to the left atrium on a PA injection is a subtle sign of intraluminal PVS. The benefit of cardiac catheterization extends past the identification and grading of PVS. At our center, catheterization also allows for vasoreactivity testing in patients with PVS and pulmonary artery hypertension (PAH). Additionally, catheterization is the platform for the majority of anatomic therapy for patients with PVS.
Retrograde venography. In the frontal projection, an angiographic catheter is seen coursing from the inferior vena cava to the right atrium and then across an atrial septal defect into the left atrium and then to the left lower pulmonary vein. The panel on the left demonstrates retrograde venography highlighting important aspects of upstream arborization crucial for planning intervention. The white arrow denotes an area of stenosis in the posterior basal segmental branch of the left lower pulmonary vein that would not be identifiable by angiography from the pulmonary artery side. The right panel demonstrates improvement in stenosis after angioplasty. Of note, a prominent pulmonary vein drains the inferior segment of the left upper lobe (black arrows).
Anatomic therapy
Surgical technique
Conventional pulmonary vein stenosis repair was first described by Pacifico and colleagues in 1985 [12]. Minor modifications have been made to the original surgical technique which is still often used to this date for relief of congenital or acquired PVS. The technique involves using autologous atrial tissue to perform pulmonary venoplasty. It is important to note that with this technique the edges of the pulmonary veins themselves are directly anastomosed to patch material. Studies have demonstrated less than ideal results with 50% freedom from re-stenosis at 5 years [13, 14•]. Experts have postulated that the mixed results are related to myofibroblastic proliferation secondary to injury to anastomosed pulmonary veins.
Lacour-Gayet and colleagues described a different surgical approach in 1999 to combat the complications seen with conventional pulmonary vein repair. In this technique, the divided pulmonary veins are surrounded by an anastomosis made between the left atrium and pericardium [15]. There is no suture line directly on the pulmonary veins that could potentially propagate myofibroblastic proliferation and re-stenosis. This surgical technique has since been dubbed sutureless repair, or sutureless pericardial marsupialization. Mid-term results with the sutureless repair have been promising, demonstrating 90% freedom from re-stenosis at 5 years [16]. The external validity in translation of these results to the population with primary PVS has been limited. Conventional reviews of surgical outcomes for primary and secondary PVS continue to demonstrate recurrence [17,18,19].
Transcatheter interventions
A myriad of catheter-based approaches have been applied to PVS since the early 1980s, including conventional balloon dilation/angioplasty, cryo-balloon angioplasty, high frequency sonotherapy, novel cutting balloons, local drug delivery with drug-coated balloons and stents, and even bioresorbable scaffolds [20,21,22]. Unfortunately, despite the approach, PVS has proven a recalcitrant, recurring disease which progresses in many cases despite any single approach listed above.
Angioplasty
Balloon angioplasty as a therapy for PVS has been reported as early as 1982 in the literature by Driscoll and colleagues [23]. The procedure involves using a non-compliant balloon to serially dilate the stenotic region to mirror the size of the distal vessel. Prior to balloon angioplasty of an affected pulmonary vein, the operator should ensure that reliable angiography has been performed to allow for careful measurement of the minimal and maximal vein diameters. Serial balloon angioplasty should, in most circumstances, be chosen over going immediately to the goal diameter with a single large balloon catheter. Serial angioplasty allows for assessment of intimal tear, dissection, or vein rupture. Balloon angioplasty can be very effective in short-term relief of PVS. However, recurrence of PVS is expected.
Rarely, PVS is associated with resistant vascular stenotic lesions that are not amenable to simple balloon angioplasty. Resistant lesions are more commonly encountered in secondary PVS, such as following surgical repair of TAPVR. In these situations, cutting balloon angioplasty (CBA) has been used at our center and by others to relieve resistant lesions [24]. While CBA as primary therapy for PVS has been shown to be less effective than conventional balloon angioplasty, we continue to offer CBA for resistant lesions.
A novel balloon catheter which has recently emerged as a potential tool for PVS is the drug-coated balloon (DCB). DCBs were designed for peripheral artery disease angioplasty in adults with severe atherosclerosis. These balloons elute anti-proliferative agents such as paclitaxel, an mTOR-inhibitor-class anti-cancer agent. As described below, there has been enthusiasm for delivering high local concentrations of such agents to affected veins in patients with PVS [25]. However, data on DCBs in infants and children with PVS are currently lacking. Our center’s preliminary experience with DCBs suggests that there may in fact be a limited role for this tool for patients with recurrent PVS, particularly those with in-stent stenosis (ISS). Unfortunately, DCBs in our experience have also been associated with early vessel loss in multiple patients. The role of DCBs in PVS remains unproven [25].
Stenting
Intravascular stents have also been used to relieve venous and arterial stenoses since the early 1980s [26]. With advances in stent technologies, low-profile coronary stents can now be placed into neonatal pulmonary veins with minimal risk. The current generations of coronary stents include drug-eluting stents (DES) which elute anti-proliferative, anti-inflammatory, and anti-neoplastic agents. These agents are eluted at the local intimal level for up to 6 months post-implant [27]. Increasingly, DES are being employed to relieve PVS in infants and children [7••, 28•]. While DES have the advantages of being very low-profile and providing not only anatomic but anti-proliferative (primary) therapy, DES have limited future expansion capacity. Most DES cannot be dilated beyond 5–5.5 mm in diameter. As the adolescent child will have normally pulmonary veins of 10-12 mm diameter, these DES then can become woefully undersized in a relatively short period of time.
Other investigators have suggested that with implantation of larger diameter, bare-metal, stents (> 7 mm diameter), the late outcomes and overall survival are better [29]. Clearly, when distal vein diameter is larger, placement of a stent which matches this distal diameter is warranted. However, over-sizing of a stent to achieve an arbitrary diameter is seldom helpful and in fact, in most cases, promotes aggressive ISS and recurrence of PVS [30, 31]. Nonetheless, as most non-coronary bare-metal stents can be dilated up to 10–12 mm in diameter, these are more ideal for PVS because they can be considered durable anatomic therapy.
Reintervention
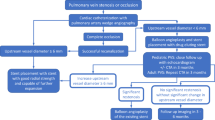
Because PVS is progressive both locally (recurring at the vein ostium, but also expanding upstream in an affected vein) and regionally (affecting previously normal pulmonary veins), surveillance and repeat interventions are the essential components to successful management of PVS. In the majority of cases, repeat interventions occur in the catheterization laboratory. The scope of repeat interventions includes conventional balloon angioplasty of affected veins, stent redilation to accommodate patient somatic growth, angioplasty of ISS, and placement of stents. Recent data suggest that patients who undergo repeat intervention have a significantly increased probability of survival. Cory and colleagues noted that in their cohort of PVS patients, survival was positively associated with greater frequency of catheterizations [7••]. The timing of repeat surveillance and reintervention varies. At our center, infants < 1 year of age are often brought back to the catheterization laboratory every 4–6 weeks, and the frequency gradually decreases. Our patients who are 3 years of age or older typically undergo catheterization annually. Our institution’s treatment algorithm is outlined below in greater detail [Fig. 3].
Pneumonectomy and lung transplant
PVS left untreated can lead to chronic changes predisposing patients to hemoptysis. Both pulmonary and bronchial circulations empty into the left atrium through the pulmonary veins. Chronic obstruction creates distended bronchial veins, endobronchial varices, and alveolar hemorrhage leading to the substrate for hemoptysis in PVS patients [32]. Recurrent hemoptysis secondary to unilateral pulmonary venous obstruction is considered an indication for pneumonectomy as this hemoptysis can be life-threatening. Some patients with severe bilateral PVS will develop hemoptysis and severe pulmonary hypertension. In some centers, lung transplantation is offered to children with severe PVS that has led to irreversible chronic lung disease, particularly when surgical and transcatheter therapies have proven to be unsuccessful [33].
Primary (medical) therapy
The angiographic and radiographic appearance of PVS belies the cellular and molecular basis of the disease. Histopathologic evaluation of PVS samples have revealed intimal hyperplasia, conversion of normal vascular layers to thickened, hypercellular vessels with proliferation of myofibroblasts—essentially, a regression of cellular differentiation to a more primordial, pluripotent, poorly differentiated cellular milieu. Reidlinger et al. were the first to demonstrate in 2006 an increased expression of smooth muscle antibodies and receptor tyrosine kinases in lesional cells consistent with myofibroblastic proliferation [5]. Kato et al. further elaborated on these upstream changes from pulmonary venous obstruction in a piglet model. They noted a similar endothelial to mesenchymal transition that was reversible when the obstruction was removed. Animal models of PVS have identified the involvement of specific pathways including receptor tyrosine kinases, mammalian target of rapamycin (mTOR), and TGF-훽 in myofibroblastic proliferation of PVS. These animal models suggest an interesting interplay between pulmonary vein anatomy and molecular signaling; in one study, stenting of proximal PVS ameliorated upstream abnormal cellular signaling [34]. Conversely, altering cellular signaling through medical therapies (see below) resulted in reduced anatomic PVS burden [35].
Vinblastine and methotrexate
The first chemotherapeutic trial for PVS in human subjects involved vinblastine and methotrexate [36]. Vinblastine is cell cycle specific and works by binding to mitotic spindle proteins preventing cell division. Methotrexate inhibits the enzyme dihydrofolate reductase preventing the formation of building blocks essential to DNA, RNA, and protein synthesis. These agents were chosen based on the treatment for desmoid tumors as their histology demonstrated similar myofibroblastic proliferation to PVS. One of the major setbacks during this study was medication toxicity leading to treatment delays and interruptions. Unfortunately, compared with controls, patients treated with vinblastine/methotrexate fared far worse.
Imatinib and bevacizumab
Two of the specific receptor tyrosine kinases upregulated in Reidlinger’s immunoreactivity study included Platelet Derived Growth Factor (PDGFR) and vascular endothelial growth factor receptor-2 (VEGF-2) [5]. Callahan and colleagues strategically selected imatinib with or without bevacizumab to target these receptors in their prospective trial to treat multivessel intraluminal PVS as adjuncts to anatomic interventions. Imatinib was administered orally as a once daily tablet with the dose of 340 mg/m2. Bevacizumb was administered intravenously with the dose of 10 mg/kg every 2 weeks. Kaplan-Meier analysis demonstrated 77% probability of survival at 72 weeks in the imatinib with or without bevacizumab group while the control group demonstrated a < 40% probability of survival. Adverse effects from drug toxicity were minimal [37••].
Sirolimus
Sirolimus inhibits mTOR similar to the compounds used in drug-eluting stents discussed earlier. Prior literature has established that mTOR is heavily involved in proliferation of a variety of cell types. Its use in PVS was first published in 2006 for adult iatrogenic PVS secondary to radiofrequency ablation for atrial fibrillation. Daily oral sirolimus was prescribed at a dose of 2 mg in conjunction with bare-metal stenting. The two patients followed in this study were noted to be asymptomatic without re-stenosis 1 year from interventions [38]. Systemic sirolimus use in the pediatric population for PVS has not been reported on in the literature although some centers have begun clinical trials. Systemic sirolimus has been used to prevent in-stent stenosis for peripheral pulmonary artery disease. Hallbergson and colleagues demonstrated reduction in in-stent stenosis with target serum sirolimus levels between 6 and 10 ng/mL. Medication toxicities were minimal in the cohort of 10 patients studied [39]. Extrapolating these results to pediatric patients with PVS demonstrates a positive outlook for the utility of systemic sirolimus but more research is needed to validate this claim.
Losartan
Losartan acts by selectively blocking the type 1 angiotensin II receptor, which is known to inhibit pathways downstream of TGF-훽 signaling. In 2014, Zhu and colleagues demonstrated in a piglet model of PVS that losartan treatment decreased intimal hyperplasia and pulmonary hypertension [35]. Losartan was initiated prophylactically prior to the development of significant myofibroblastic proliferation in contrast to how it would be used in human patients with PVS. Losartan has yet to be trialed in human subjects. Further clinical trials are needed to prove the utility of losartan in treating PVS in humans.
Conclusion
Congenital and acquired PVS represent a progressive disease with high rate of recurrence. This disease, increasingly seen in premature infants, has proven to be a challenge for cardiologists to manage. Surgical and transcatheter interventions in most cases have early success but are only temporizing solutions in the face of myofibroblastic proliferation. The physician should employ both anatomic as well as “primary” therapy (medical therapy). It is our center’s approach to utilize primary therapy in all patients with evidence of moderate PVS. Importantly, anatomic treatment should be continued during primary therapy, as the studies above indicate PVS proximally elicits upstream cellular signaling alterations.
More recent immunohistological studies have paved the way for a new type of therapy for patients with PVS. Signaling pathways allowing for myofibroblastic proliferation in PVS are now known and can specifically be targeted. Early investigations are already underway to determine whether anti-proliferative medications such as losartan, imatinib/bevacizumab, or sirolimus may be disease-modifying therapies that can change the outcomes for these patients. These therapies in conjunction with anatomic therapies have already demonstrated promising preliminary results.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Pazos-Lopez P, Garcia-Rodriguez C, Guitian-Gonzalez A, et al. Pulmonary vein stenosis: etiology, diagnosis and management. World J Cardiol. 2016;8:81–8.
Caldarone CA, Najm HK, Kadletz M, Smallhorn JF, Freedom RM, Williams WG, et al. Relentless pulmonary vein stenosis after repair of total anomalous pulmonary venous drainage. Ann Thorac Surg. 1998;66:1514–20.
Hancock Friesen CL, Zurakowski D, Thiagarajan RR, Forbess JM, del Nido PJ, Mayer JE, et al. Total anomalous pulmonary venous connection: an analysis of current management strategies in a single institution. Ann Thorac Surg. 2005;79:596–606 discussion 596-606.
DiLorenzo MP, Santo A, Rome JJ, et al. Pulmonary vein stenosis: outcomes in children with congenital heart disease and prematurity. Semin Thorac Cardiovasc Surg. 2019;31:266–73.
Riedlinger WF, Juraszek AL, Jenkins KJ, et al. Pulmonary vein stenosis: expression of receptor tyrosine kinases by lesional cells. Cardiovasc Pathol. 2006;15:91–9.
Balasubramanian S, Rehman M, Gauvreau K, Jenkins KJ. Bilateral disease and early age at presentation are associated with shorter survival in patients with congenital heart disease and intraluminal pulmonary vein stenosis. Congenit Heart Dis. 2012;7:378–86.
•• Cory MJ, Ooi YK, Kelleman MS, Vincent RN, Kim DW, Petit CJ. Reintervention is associated with improved survival in pediatric patients with pulmonary vein stenosis. JACC Cardiovasc Interv. 2017;10:1788–98 First study to demonstrate the significant impact of reintervention and frequent surveillance on patient survival and vein patency in pediatric pulmonary vein stenosis patients.
Drossner DM, Kim DW, Maher KO, Mahle WT. Pulmonary vein stenosis: prematurity and associated conditions. Pediatrics. 2008;122:e656–e61.
Roman KS, Kellenberger CJ, Macgowan CK, Coles J, Redington AN, Benson LN, et al. How is pulmonary arterial blood flow affected by pulmonary venous obstruction in children? A phase-contrast magnetic resonance study. Pediatr Radiol. 2005;35:580–6.
Ghoshhajra BB, Lee AM, Engel LC, Celeng C, Kalra MK, Brady TJ, et al. Radiation dose reduction in pediatric cardiac computed tomography: experience from a tertiary medical center. Pediatr Cardiol. 2014;35:171–9.
Gorlin R, Gorlin SG. Hydraulic formula for calculation of the area of the stenotic mitral valve, other cardiac valves, and central circulatory shunts. I Am Heart J. 1951;41:1–29.
Pacifico AD, Mandke NV, McGrath LB, Colvin EV, Bini RM, Bargeron LM Jr. Repair of congenital pulmonary venous stenosis with living autologous atrial tissue. J Thorac Cardiovasc Surg. 1985;89:604–9.
Lo Rito M, Gazzaz T, Wilder T, Saedi A, Chetan D, van Arsdell GS, et al. Repair type influences mode of pulmonary vein stenosis in total anomalous pulmonary venous drainage. Ann Thorac Surg. 2015;100:654–62.
Kalfa D, Belli E, Bacha E, et al. Outcomes and prognostic factors for postsurgical pulmonary vein stenosis in the current era. J Thorac Cardiovasc Surg. 2018;156:278–86.
Lacour-Gayet F, Zoghbi J, Serraf AE, Belli E, Piot D, Rey C, et al. Surgical management of progressive pulmonary venous obstruction after repair of total anomalous pulmonary venous connection. J Thorac Cardiovasc Surg. 1999;117:679–87.
Devaney EJ, Ohye RG, Bove EL. Pulmonary vein stenosis following repair of total anomalous pulmonary venous connection. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2006;9:51–5.
Alsoufi B, Cai S, Van Arsdell GS, Williams WG, Caldarone CA, Coles JG. Outcomes after surgical treatment of children with partial anomalous pulmonary venous connection. Ann Thorac Surg. 2007;84:2020–6 discussion 2020-6.
Quinonez LG, Gauvreau K, Borisuk M, Ireland C, Marshall AM, Mayer JE, et al. Outcomes of surgery for young children with multivessel pulmonary vein stenosis. J Thorac Cardiovasc Surg. 2015;150:911–7.
Rosenblum JM, Altin HF, Gillespie SE, et al. Management outcomes of primary pulmonary vein stenosis. J Thorac Cardiovasc Surg. 2020;159:1029–1036 e1.
McMahon CJ, Mullins CE, El Said HG. Intrastent sonotherapy in pulmonary vein restenosis: a new treatment for a recalcitrant problem. Heart. 2003;89:E6.
Peng LF, Lock JE, Nugent AW, Jenkins KJ, McElhinney DB. Comparison of conventional and cutting balloon angioplasty for congenital and postoperative pulmonary vein stenosis in infants and young children. Catheter Cardiovasc Interv. 2010;75:1084–90.
Bingler MA, Darst JR, Fagan TE. Cryo-balloon angioplasty for pulmonary vein stenosis in pediatric patients. Pediatr Cardiol. 2012;33:109–14.
Driscoll DJ, Hesslein PS, Mullins CE. Congenital stenosis of individual pulmonary veins: clinical spectrum and unsuccessful treatment by transvenous balloon dilation. Am J Cardiol. 1982;49:1767–72.
Seale AN, Daubeney PE, Magee AG, Rigby ML. Pulmonary vein stenosis: initial experience with cutting balloon angioplasty. Heart. 2006;92:815–20.
Mueller GC, Dodge-Khatami A, Weil J. First experience with a new drug-eluting balloon for the treatment of congenital pulmonary vein stenosis in a neonate. Cardiol Young. 2010;20:455–8.
Shaffer KM, Mullins CE, Grifka RG, O’Laughlin MP, McMahon W, Ing FF, et al. Intravascular stents in congenital heart disease: short- and long-term results from a large single-center experience. J Am Coll Cardiol. 1998;31:661–7.
Xi T, Gao R, Xu B, Chen L, Luo T, Liu J, et al. In vitro and in vivo changes to PLGA/sirolimus coating on drug eluting stents. Biomaterials. 2010;31:5151–8.
Khan A, Qureshi AM, Justino H. Comparison of drug eluting versus bare metal stents for pulmonary vein stenosis in childhood. Catheter Cardiovasc Interv. 2019;94:233–42.
Balasubramanian S, Marshall AC, Gauvreau K, Peng LF, Nugent AW, Lock JE, et al. Outcomes after stent implantation for the treatment of congenital and postoperative pulmonary vein stenosis in children. Circ Cardiovasc Interv. 2012;5:109–17.
Chen HY, Hermiller J, Sinha AK, Sturek M, Zhu L, Kassab GS. Effects of stent sizing on endothelial and vessel wall stress: potential mechanisms for in-stent restenosis. J Appl Physiol (1985). 2009;106:1686–91.
Zamora CA, Sugimoto K, Yamaguchi M, Sugimura K. Effect of stent oversizing on in-stent stenosis and lumen size in normal porcine veins. J Endovasc Ther. 2005;12:495–502.
Walsh A, Canny G, McMahon CJ, Redmond JM, McNally P. Hemoptysis from bronchial varices associated with pulmonary vein stenosis: role of surgical repair. Pediatr Pulmonol. 2013;48:838–40.
Bharat A, Epstein DJ, Grady M, Faro A, Michelson P, Sweet SC, et al. Lung transplant is a viable treatment option for patients with congenital and acquired pulmonary vein stenosis. J Heart Lung Transplant. 2013;32:621–5.
Kato H, Fu YY, Zhu J, Wang L, Aafaqi S, Rahkonen O, et al. Pulmonary vein stenosis and the pathophysiology of “upstream” pulmonary veins. J Thorac Cardiovasc Surg. 2014;148:245–53.
Zhu J, Ide H, Fu YY, Teichert AM, Kato H, Weisel RD, et al. Losartan ameliorates “upstream” pulmonary vein vasculopathy in a piglet model of pulmonary vein stenosis. J Thorac Cardiovasc Surg. 2014;148:2550–7.
Rehman M, Jenkins KJ, Juraszek AL, Connor JA, Gauvreau K, Muneeb M, et al. A prospective phase II trial of vinblastine and methotrexate in multivessel intraluminal pulmonary vein stenosis in infants and children. Congenit Heart Dis. 2011;6:608–23.
•• Callahan R, Kieran MW, Baird CW, et al. Adjunct targeted biologic inhibition agents to treat aggressive multivessel intraluminal pediatric pulmonary vein stenosis. J Pediatr. 2018;198:29–35 e5 First and only prospective study to demonstrate the benefits of primary (medical) therapy in children with multivessel pulmonary vein stenosis.
Bromberg-Marin G, Tsimikas S, Mahmud E. Treatment of recurrent pulmonary vein stenoses with endovascular stenting and adjuvant oral sirolimus. Catheter Cardiovasc Interv. 2007;69:362–8.
Hallbergson A, Esch JJ, Tran TX, Lock JE, Marshall AC. Systemic rapamycin to prevent in-stent stenosis in peripheral pulmonary arterial disease: early clinical experience. Cardiol Young. 2016;26:1319–26.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cardiology/CT Surgery
Rights and permissions
About this article
Cite this article
Patel, J.D., Briones, M., Jones, S. et al. Medical and Invasive Management of Congenital and Acquired Pulmonary Vein Stenosis. Curr Treat Options Peds 6, 170–181 (2020). https://doi.org/10.1007/s40746-020-00198-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40746-020-00198-0