Abstract
Purpose of review
The purpose of this review is to present the epidemiology of Clostridium difficile infection in children and to present a number of clinical challenges in diagnosis and therapy that are unique to the pediatric population.
Recent findings
Current research has focused on novel methods of prevention and treatment of C. difficile infection in children. Fecal microbial transplantation (FMT) for recurrent C. difficile is increasingly prescribed for children, and recent data includes comparisons of effectiveness and tolerability between methods of delivery.
Summary
The incidence of C. difficile infection in children has risen in recent decades both in healthy children and those with underlying comorbidities. Antibiotic use, acid suppressive medication use, and the presence of enteric feeding tubes are well-documented risk factors. There is no standard method of testing for C. difficile infection in children, which complicates epidemiological tracking and detection of active disease versus asymptomatic colonization. First-line therapy for initial infection in children is metronidazole, while vancomycin may be reserved for those with severe infection or high risk of complications. Recurrence of infection is frequent, and while repeat courses of antibiotics may be effective, fecal microbial transplant should be considered a safe and efficacious alternative therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clostridium difficile is a gram-positive, toxin-producing bacillus that may reside in a healthy pediatric intestinal microbiome. However, the microbial balance can tip such that C. difficile causes diarrheal illness ranging from mild, self-limited disease to pseudomembranous colitis, toxic megacolon, septic shock, and even death. The incidence of C. difficile infection (CDI) in children in the United States (US) has risen over the last two decades, particularly among high risk groups such as those with inflammatory bowel disease (IBD) or cancer [1, 2]. However, the pathogenesis of CDI in children, risk factors, and optimal prevention and treatment strategies remain up for debate. The purpose of this review is to present the epidemiology of and national trends in CDI among pediatric patients and to present a number of clinical challenges in diagnosis and therapy that are unique to the pediatric population. Specifically, we will address diagnostic challenges including differentiation of active infection versus colonization, the role of probiotics, and optimal treatment of both healthy and high risk pediatric populations.
Epidemiology of infection
C. difficile is the most common cause of health care-associated diarrhea in the US, affecting nearly 500,000 individuals annually [3•]. Incomplete reporting and surveillance as well as variability in diagnostic testing limits our understanding of true incidence of CDI. CDI is categorized as community acquired or nosocomial, which is subtyped into healthcare onset (during hospitalization) or healthcare-associated (within 4 weeks of discharge) [4].
The Centers for Disease Control began active surveillance of CDI for 10 US locations in their Emerging Infections Program in 2009. Using these data, Lessa et al. estimated the national incidence of CDI across age groups. Children ages 1–17 years had an incidence of community acquired CDI of 17.9/100,000 and nosocomial CDI incidence of 6.23/100,000 [3•]. Annual reports from this program have indicated rising incidence of CDI. In 2015, the incidence among children aged 1–17 years for community-acquired, and nosocomial CDI were 23.45/100,000 and 7.45/100,000 persons, respectively [5].
Recently, Pant et al. reviewed 7,070,485 discharges from the Healthcare Cost and Utilization Project Kids’ Inpatient Database (HCUP-KID). They reported an increase in CDI diagnosis from 24/10,000 discharges in 2003 to 58/10,000 in 2012. The largest percent increase was 181% in the > 15-year group and the highest overall prevalence was in the 1–4-year group [6, 7]. C. difficile testing rates were not reported in this study.
The BI/NAP1/027 strain of C. difficile has been responsible for outbreaks and severe disease in adults and has been cited as a reason for the observed increased CDI incidence in the US [8]. It has been reported in at least two North American cancer center’s pediatric populations [9, 10], but not necessarily with more severe or outbreak disease patterns. Other reports have investigated and not found the strain in pediatric populations [10, 11]. The exact etiology of the increased incidence remains unclear.
It is important to recognize the associated cost of CDI. A retrospective review using the Kids’ Inpatient Database (KID) reviewed 313,664 children undergoing thoracic or abdominal surgery in the years 2003, 2006, 2009, and 2012. The estimated mean excess duration of stay associated with CDI was 5.8 days and $12,801 per patient [12].
Epidemiology of colonization
To better understand risk of CDI, asymptomatic pediatric colonization rates are worth review. C. difficile carriage rates have been reported since the 1980s with 2–90% of neonates and infants testing positive for C. difficile depending on duration of inpatient nursery stay and method of testing [13,14,15]. Colonization is likely due to environmental exposure, as higher incidence correlated with duration of nursery stay, and there was no evidence of maternal-fetal transmission [13].
Recent studies report C. difficile carriage rates in children 0–2 years ranging from 16 to 33.7%, with subsequent declines after age 2 years to < 5%, the estimated adult carriage rate [16,17,18,19]. Protective factors against colonization in early life include breastfeeding, while risk factors include the presence of a pet dog [16].
Despite high carriage rates, there are few reports of symptomatic CDI in infants < 12 months [20]. The host defense mechanism preventing CDI in infants is not well understood. It has been proposed that the absence of receptors to toxin A and toxin B precludes active infection [21, 22].
Pathogenesis
C. difficile is a resilient, spore-forming organism resistant to heat and most common disinfectants. It is transmitted via fecal-oral route from environmental exposure. Exposure may come from contaminated food products, though no food-borne outbreaks have been reported [23].
Following colonization of the lower gastrointestinal tract, C. difficile causes disease by producing toxin proteins, toxin A (TcdA) and/or toxin B (TcdB). These toxins bind to receptors on the plasma membrane of the colonocyte. The toxins are then internalized and inactivate Rho guanine triphosphatases, which results in cytokine production, disruption of the epithelial barrier, and cell death [8]. The resulting inflammation and cellular destruction cause the symptoms of CDI including diarrhea with or without pseudomembranous colitis, the classic endoscopic finding with diffuse erythema and purulent exudate.
Response to C. difficile is influenced by the virulence of the strain as well as host defense mechanisms. Interestingly, infantile asymptomatic colonization by C. difficile has been shown to produce a robust, durable Immunoglobulin G antitoxin to TcdA and TcdB that may ameliorate risk of infection later in life [24].
Risk factors
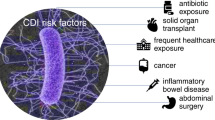
CDI risk factors in adults are well established and include advanced age, antibiotic use, acid suppression, and severe underlying chronic disease [25]. Similarly, pediatric risk factors include antibiotic use, proton pump inhibitor (PPI) use and to a lesser extent histamine 2 (H2) blocker use, the presence of an enteric feeding tube, chronic conditions such as IBD, cancer, and history of solid organ transplant [26].
Community acquired CDI risk factors
A large retrospective case control study from 2001 to 2013 using the military health system database (MHS) found 1331 children with CA-CDI. This study demonstrated significant increased risk with antibiotic use, as well as PPI and H2 receptor antagonist use. There was a slightly increased risk (adjusted OR 1.22) with corticosteroid use and no increased risk of CA-CDI in those with a sibling < 1 year of age. Recent outpatient health care visits had an adjusted OR of 1.35 [27]. As expected, there was increased risk associated with increased number of antibiotic class exposures (6.07 for 1 class, 38.53 for 3 classes). Importantly, of the 1331 cases of CA-CDI identified, approximately 40% had no recent antibiotic exposure. This percent closely aligns with previous data in an emergency room setting reporting 43% of CA-CDI cases identified in the ED with no history of antibiotic exposure [28].
These data highlight two points. First, clinicians must carefully weigh the risks and benefits of antibiotics and acid suppression. Acid suppressing medications are over the counter, but they are not without long-term consequence and should not be used with abandon. Antimicrobial stewardship programs have increasingly grown in number and influence in pediatric hospitals, but the burden of judicious use of acid suppressing medications in children lies in the hands of the general pediatrician, hospitalist, and gastroenterologist. To this end, the American Academy of Pediatrics (AAP) specifically recommends education and reassurance, and not acid suppression, for physiological reflux that is effortless, painless, and not affecting growth [29]. Implementation of evidenced-based guidelines for PPI and H2 blocker use in the Neonatal Intensive Care Unit setting have also been shown to be effective at decreasing acid suppression use [30]. Further work is needed in this area.
Special populations
While CDI in healthy children has risen in the past two decades, certain populations are particularly susceptible.
Pediatric patients with newly diagnosed IBD have a baseline prevalence of CDI of 8–47% [31, 32]. Children with IBD also suffer from a greater risk of recurrence. A retrospective case control study of pediatric inpatients reported a 34% CDI recurrence rate for those with CDI and IBD as compared with a recurrence rate of 7.5% in those with CDI alone [33].
Why are those with IBD at increased risk of C. difficile infection? IBD is associated with significant gastrointestinal dysbiosis, or microbial imbalance, as measured by decreased diversity and richness. Decreases in beneficial Bacteroidetes and concurrent increases in detrimental Proteobacteria may persist despite clinical remission and mucosal healing [34]. A similar pattern of dysbiosis is also seen in non-IBD patients with recurrent CDI [35]. Host factors such as genetic susceptibility [36], immune suppression, and neutrophil dysfunction may also play a role in increased susceptibility. Further research is needed in this area.
Another population of interest includes patients under significant immunosuppression without underlying enteritis. This includes the pediatric cancer population as well as the solid organ transplant population. A multicenter study reviewed first time hospitalizations for malignancy at 43 centers. Antibiotic exposure, PPI, and chemotherapy within the past 2 weeks were noted to confer increased risk of CDI [27].
A retrospective study from 2010 to 2013 reported single center data on CDI in pediatric solid organ transplant (SOT) recipients [37]. Overall, they found that 104/202 SOT recipients were tested, and of those 25 were positive for C. difficile. Liver transplant recipients had the highest incidence of CDI at 18.4%. Notably, they did not find a correlation of increased C. difficile with antibiotic exposure, prior hospitalization, or calcineurin inhibitor type. Steroid dose was not reported. PPI use was found to have a modest protective effect in this population.
These studies highlight at-risk populations that may have frequent exposures to the health care system. Further, large scale prospective data is needed to define the interplay between immunosuppression, antimicrobial use, the microbiome, and C. difficile susceptibility.
Who to test
The general pediatrician will often encounter a healthy child with acute diarrhea, defined as greater than three unformed stools daily. CDI testing should be considered in a child or adolescent with diarrhea not clearly explained by alternative diagnoses, especially if risk factors are present. The AAP recommends testing infants < 12 months only in outbreak situations or situations of severely altered gut motility, such as in Hirschsprung’s disease [38]. Testing between ages 1 and 3 years should be done judiciously to avoid ascribing CDI to a patient with an alternative cause of diarrhea and C. difficile colonization. Patients with underlying conditions which may result in higher morbidity and mortality deserve a higher index of suspicion. Because C. difficile can be detected in the stool for many weeks after effective treatment, test of cure is not recommended [38].
How to test (Fig. 1, Table 1)
C. difficile testing in the last decade has undergone a major shift. The gold standards for CDI diagnosis are toxigenic cell culture to detect the organism, or cell cytotoxicity assay to detect presence of toxin A or toxin B [39]. These tests are labor intensive and time consuming, thus limiting their clinical applications. Currently, most laboratories perform either toxin detecting enzyme immune assay (EIA) or toxin gene detecting nucleic acid amplification testing (NAAT) using polymerase chain reaction (PCR). The utility of EIA for toxin(s) is limited by variable sensitivity (0.73–0.87) but high specificity (0.98–0.99) [39]. Toxin gene NAAT has excellent sensitivity (0.87–0.92) and specificity, (0.94–0.97) [39] but may detect asymptomatic colonization as it detects toxin gene presence and the potential for toxin production only. EIA for glutamate dehydrogenase (GDH) is an additional test that can be performed to detect antigen present on all strains of C. difficile. Because GDH is present on toxigenic and non-toxigenic strains alike, it cannot be used to diagnose CDI, but can be used to confirm the absence of C. difficile [39].
Testing modality has implications for CDI detection and treatment on a patient level, but also broad implications for CDI surveillance. The shift from EIA-based testing to NAAT testing makes it difficult to compare incidence year to year, as some changes are likely the result of NAAT detection of colonization (false positive). For example, a large European study published in 2016 evaluated the CDI positivity rate at 60 hospitals across France, Italy, and the UK. The CDI positivity rate was 2.5-fold higher when methods not directly detecting toxin were used [40].
Many labs try to prevent false positives by accepting only liquid stool samples—an indication the patient is actively experiencing diarrhea. Other labs employ a two-step process beginning with EIA for GDH followed by EIA for toxin(s) or NAAT for toxin gene(s) if the GDH test is positive. This has been shown to be an effective testing strategy, with perhaps reduced cost compared to universal NAAT testing [41]. To date, there is no standard process for CDI testing in children.
Some adult and pediatric hospitals have opted to implement testing algorithms or computerized decision support tools to help care providers order testing on appropriate patients. For example, one such decision support tool in an adult hospital flagged patients currently on laxatives for whom C. difficile testing had been ordered [42]. In pediatrics, an age-based alert system which notified providers of the AAP testing guidelines for any child < 36 months was implemented in a US emergency room from 2012 to 2013. This clinical alert decreased testing in the < 12 months age group from 11.5 to 1 per 10,000 patient days [43].
The question of true positive versus false positive (i.e., active infection versus colonization) is particularly challenging in pediatrics. As discussed, age, symptoms, and risk factors should guide testing. To augment the ability to discern true infection, a variety of biomarkers have been studied in the setting of CDI.
Fecal calprotectin is a neutrophil product that can be measured in stool as a marker of intestinal inflammation. Multiple adult studies have shown that elevated fecal calprotectin correlates with increased CDI severity and may assist in detecting colonization versus infection [44, 45], but data is limited in pediatrics. In children, fecal levels of lactoferrin, another widely used biomarker of intestinal inflammation, and cytokines IL-8 and CXCL-5 have been shown to be elevated in the setting of diarrhea, but they are not specific to CDI. Further, C. difficile bacterial load seems to play no role in the pathogenesis [46]. Currently, these adjunct tests are not considered reliable for differentiating colonization from CDI.
Prevention
A recent large Cochrane meta-analysis of both adult and pediatric data suggests the use of probiotics to prevent C. difficile in patients receiving antibiotic therapy may be beneficial, with the most benefit in high risk groups (baseline risk > 5%) [47•]. This group had a risk reduction of 70%. However, the overall pooled risk in those receiving probiotic was not significantly decreased (risk ratio of 0.86 (95% CI .67 to 1.10). In this report, Saccharomyces boulardii or Lactobacillus Acidophilus + Lactobacillus Casei at a dose of 10–50 billion CFUs per day were reported as most effective [47•]. Small studies have indicated some promise for cholestyramine in preventing infection for adults on chronic antibiotics, and Kefir, a liquid yogurt drink, combined with staggered antibiotics, helped a small number of patients avoid recurrence of CDI [48, 49]. Further prospective pediatric studies are needed to fully explore the risk and benefits of probiotic use to prevent CDI.
Treatment (Table 2)
The treatment of CDI is an evolving topic, particularly in children where US Food and Drug Administration (FDA) approval and clinician familiarity may lag behind adult practice. Given the rising incidence and awareness of CDI, the menu of treatment options is expanding.
Currently, the AAP recommends discontinuation of antimicrobials as the first step in treatment of CDI [38]. Initial treatment for mild CDI is metronidazole 30 mg/kg/day in four divided doses for 10–14 days [38]. Metronidazole is approved for anaerobic bacterial infections at any age, not specifically for CDI, but it is the first-line choice because of low cost, ease of acquisition, and the emergence of vancomycin resistant enterococcus (VRE). If CDI recurs after effective treatment, retreatment with metronidazole is recommended. If metronidazole is ineffective, CDI recurs a second time, or if there is severe disease at any time, oral vancomycin is the treatment of choice [38]. In children with underlying neutropenia, bowel stasis such as that found in Hirschsprung’s disease, or IBD, vancomycin may be considered as first line treatment [55]. Oral vancomycin is typically administered 40 mg/kg/day in four divided doses (maximum 2 g/day) for 10–14 days. In adults, the standard dose is 125 mg four times per day, but weight-based recommendations can be used in more severe infections. In the setting of inability to tolerate oral antibiotics, intravenous (IV) metronidazole may be used with the addition of vancomycin via enema [4]. Vancomycin should not be administered IV for CDI. In adults, recent guidelines now recommend vancomycin as first line treatment for initial CDI, but this recommendation has not been made specifically in pediatrics [56].
In cases of multiple recurrences, there are several options for treatment. One option is a vancomycin pulsed taper, which includes reducing the number of doses per day after a standard course of vancomycin each week and then giving “pulse” doses separated by 1–3 days. This regimen, somewhat lacking in evidence, is thought to allow C. difficile spores to convert to vegetative state in between doses, and then these non-spore organisms are killed with “pulse” doses. For example, for an adolescent, one could prescribe a standard course of vancomycin, 125 mg four times daily for 14 days, then 125 mg twice daily for 7 days, then 125 mg once daily for 7 days, then 125 mg every other day for 7 days, then 125 mg every 3 days for 14 days.
Several newer drugs are being studied in the pediatric population. One such drug is fidaxomicin, a narrow spectrum macrolide antibiotic approved for treatment of CDI in adults in 2011. In adults, fidaxomicin has been reported to have lower rates of CDI recurrence [57, 58]. Recently, a small phase 2a multicenter pediatric trial reported on the safety and efficacy of fidaxomicin in children ages 11 months to 17 years. The drug, dosed at 16 mg/kg/day (max dose 200 mg) twice daily for 10 days was overall well tolerated and showed a response rate of 92% and sustained response (no recurrence within 28 days) of 65.8% [59•].
Following a course of vancomycin, pulse-tapered fidaxomicin with daily doses for 1 week and every other day doses for 1–4 weeks increased symptom free interval and outperformed a “chaser” course of fidaxomicin using standard dosing for 10 days [60]. Notably, both groups still had significant recurrence rates, around 17% for the group receiving pulse-tapered dosing and 38% for the chaser cohort.
Nitazoxanide and rifaximin are other antibiotics that have also been used to treat CDI with promising evidence of effectiveness in adults [61], but limited data in children. Novel treatments in the pipeline of development include new CDI-directed antibiotics, monoclonal antibodies to C. difficile toxins, and vaccines that would incite production of antitoxin antibodies [61, 62].
Fecal microbiota transplantation
Despite increasing antibiotic options for CDI, there still remains a high rate of recurrence in children and a need for additional safe, efficacious therapies. Fecal microbiota transplantation (FMT) has been a topic of great interest in recent years with speculation that FMT may be a panacea for all disease. While broad FMT applications are at a nascent stage, there is clear benefit in recurrent CDI [63]. The premise of FMT is to restore the intestinal microbiome to a healthy state (that of the donor), such that C. difficile does not regain or expand its foothold.
To date, the majority of literature reports on FMT outcome and experiences in adults, though there are several series reporting experience with children, and many centers now offer FMT [53]. In a recent review that compiled 45 reported cases of pediatric FMT for CDI, including in 13 patients with IBD, 89% of children had symptom improvement after administration, with relapse seen in 4% [63].
Donor stool is typically obtained from a known donor (family or friend) or a donor stool bank. The American Gastroenterological Association provides a guide for screening and testing potential donors to prevent transmission of communicable disease [54]. Both institutional and commercial stool banks may be used. The largest commercial stool bank is a non-profit stool bank called OpenBiome. Cost of commercial stool for FMT is approximately $485–$635 per dose, depending on liquid versus capsule formulation (www.openbiome.org). The treatment is currently indicated in the setting of second or third C. difficile recurrence.
FMT can be administered via many routes [54]. Upper GI tract delivery can be accomplished with oral, frozen capsules given as 15 capsules daily for 2 days, via feeding tube or during upper endoscopy. Lower GI tract administration is typically done via colonoscope into the cecum or most proximal portion of the colon reached safely, but may also be effective via retention enema [64]. FMT is preceded by a course of vancomycin, which is stopped 1–2 days prior to transplant.
Oral, frozen capsule use led to cure, defined as resolution of diarrhea without need for repeat treatment or surgery for 8 weeks, in 82% after 1 treatment and 91% after a second treatment. [65]. One benefit to this route of administration is the avoidance of anesthesia and the discomfort associated with nasogastric (NG) tube placement. However, in children, the ability to swallow capsules, let alone 15 capsules per dose, may limit the application of this FMT method to older, highly motivated children.
Alternatively, donor stool can be instilled via NG, gastrostomy, or gastrojejunostomy tube. Approximately 30 mL of liquid stool is delivered in one dose. This is typically done in clinic, again avoiding the risks of anesthesia. Treatment efficacy in adults has been reported at 85.3% with a recurrence rate of 5.9% [66]. In children, a nurse led FMT program in children described FMT in 42 children with recurrent CDI with and without underlying comorbidities. One patient ingested oral capsules and was treated successfully. Of the other 41 patients, cure was reached in 94% of healthy children, 75% of those with underlying chronic conditions, and 54% in those with IBD [67]. Donor stools were obtained through donor stool banks. This method of delivery was noted to be low cost compared to colonoscopy.
Lastly, FMT material can be delivered endoscopically, via gastroscope into the stomach or duodenum, or more typically, via colonoscope. Colonoscopic delivery entails the child undergoing a bowel prep followed by colonoscopic delivery of approximately 200–300 mL to the cecum or throughout the colon. This method allows the patient to have no awareness of the procedure as it takes place, but requires significantly more time and preparation. Efficacy of colonoscopic delivery is somewhat better than that of NG and oral administration with reported cure rate of 93.2% and recurrence rate of 5.4% [66].
Adverse events reported with FMT include vomiting, diarrhea, nausea, and fever [63, 67]. No serious adverse outcomes have been reported in children. Serious adverse outcomes in adults include IBD flare, infection, the development of autoimmune disease, and procedural related complications including one death due to aspiration during colonoscopy [68].
Given the favorable side effect profile and efficacy comparable to or superior to antibiotic treatment, FMT is likely to emerge as increasingly used therapy.
Conclusion
CDI is an increasingly common entity in children with and without underlying risk factors. Physicians should play an active role in decreasing risk through judicious prescription of acid suppressants and antibiotics. CDI testing should be done only when the clinician has a high index of suspicion for symptomatic disease, and clinical decision support tools and/or testing algorithms can help identify and test appropriate patients. Testing methods are hospital-dependent, but should be interpreted based on pre-test probability. Lastly, many patients will be successfully treated with an initial course of antibiotics, while others may develop a recurrent or refractory course. When available, FMT should be considered a safe and efficacious treatment option for recurrent CDI. No doubt the next decade will bring further insight into disease pathogenesis, risk factors, and treatment options.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance, •• Of major importance
Hourigan SK, Oliva-Hemker M, Hutfless S. The prevalence of Clostridium difficile infection in pediatric and adult patients with inflammatory bowel disease. Dig Dis Sci. 2014;59(9):2222–7. https://doi.org/10.1007/s10620-014-3169-4.
de Blank P, Zaoutis T, Fisher B, Troxel A, Kim J, Aplenc R. Trends in Clostridium difficile infection and risk factors for hospital acquisition of Clostridium difficile among children with cancer. J Pediatr. 2013;163(3):699–705 e1. https://doi.org/10.1016/j.jpeds.2013.01.062.
• Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(9):825–34. https://doi.org/10.1056/NEJMoa1408913. This is the largest, most recent, and most comprehensive epidemiological study of C. difficile trends in the Unites States.
Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431–55. https://doi.org/10.1086/651706.
2015 Annual Report for the Emerging Infections Program for Clostridium difficile Infection, The Centers for Disease Control and Prevention. 2015.
Pant C, Deshpande A, Gilroy R, Olyaee M, Donskey CJ. Rising incidence of Clostridium difficile related discharges among hospitalized children in the United States. Infect Control Hosp Epidemiol. 2016;37(1):104–6. https://doi.org/10.1017/ice.2015.234.
Zilberberg MD, Tillotson GS, McDonald C. Clostridium difficile infections among hospitalized children, United States, 1997-2006. Emerg Infect Dis. 2010;16(4):604–9. https://doi.org/10.3201/eid1604.090680.
Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015;372(16):1539–48. https://doi.org/10.1056/NEJMra1403772.
Kamboj M, Xiao K, Kaltsas A, Huang YT, Sun J, Chung D, et al. Clostridium difficile infection after allogeneic hematopoietic stem cell transplant: strain diversity and outcomes associated with NAP1/027. Biol Blood and Marrow Transpl: J Am Soc Blood Marrow Transpl. 2014;20(10):1626–33. https://doi.org/10.1016/j.bbmt.2014.06.025.
Kociolek LK, Gerding DN. Clinical utility of laboratory detection of Clostridium difficile strain BI/NAP1/027. J Clin Microbiol. 2016;54(1):19–24. https://doi.org/10.1128/JCM.02340-15.
McFarland LV, Ozen M, Dinleyici EC, Goh S. Comparison of pediatric and adult antibiotic-associated diarrhea and Clostridium difficile infections. World J Gastroenterol. 2016;22(11):3078–104. https://doi.org/10.3748/wjg.v22.i11.3078.
Mehrotra P, Jang J, Gidengil C, Sandora TJ. Attributable cost of Clostridium difficile infection in pediatric patients. Infect Control Hosp Epidemiol. 2017;38(12):1472–7. https://doi.org/10.1017/ice.2017.240.
Al-Jumaili IJ, Shibley M, Lishman AH, Record CO. Incidence and origin of Clostridium difficile in neonates. J Clin Microbiol. 1984;19(1):77–8.
Larson HE, Barclay FE, Honour P, Hill ID. Epidemiology of Clostridium difficile in infants. J Infect Dis. 1982;146(6):727–33.
Holst E, Helin I, Mardh PA. Recovery of Clostridium difficile from children. Scand J Infect Dis. 1981;13(1):41–5.
Stoesser N, Eyre DW, Quan TP, Godwin H, Pill G, Mbuvi E, et al. Epidemiology of Clostridium difficile in infants in Oxfordshire, UK: risk factors for colonization and carriage, and genetic overlap with regional C. difficile infection strains. PLoS One. 2017;12(8):e0182307. https://doi.org/10.1371/journal.pone.0182307.
Rousseau C, Lemee L, Le Monnier A, Poilane I, Pons JL, Collignon A. Prevalence and diversity of Clostridium difficile strains in infants. J Med Microbiol. 2011;60(Pt 8):1112–8. https://doi.org/10.1099/jmm.0.029736-0.
Kubota H, Makino H, Gawad A, Kushiro A, Ishikawa E, Sakai T, et al. Longitudinal investigation of carriage rates, counts, and genotypes of toxigenic Clostridium difficile in early infancy. Appl Environ Microbiol. 2016;82(19):5806–14. https://doi.org/10.1128/aem.01540-16.
Svenungsson B, Lagergren Å, Ekwall E, Evengård B, Hedlund KO, Kärnell A, et al. Enteropathogens in adult patients with diarrhea and healthy control subjects: a 1-year prospective study in a Swedish clinic for infectious diseases. Clin Infect Dis. 2000;30(5):770–8. https://doi.org/10.1086/313770.
Kuiper GA, van Prehn J, Ang W, Kneepkens F, van der Schoor S, de Meij T. Clostridium difficile infections in young infants: case presentations and literature review. IDCases. 2017;10:7–11. https://doi.org/10.1016/j.idcr.2017.07.005.
Keel MK, Songer JG. The distribution and density of Clostridium difficile toxin receptors on the intestinal mucosa of neonatal pigs. Vet Pathol. 2007;44(6):814–22. https://doi.org/10.1354/vp.44-6-814.
Eglow R, Pothoulakis C, Itzkowitz S, Israel EJ, O’Keane CJ, Gong D, et al. Diminished Clostridium difficile toxin A sensitivity in newborn rabbit ileum is associated with decreased toxin A receptor. J Clin Invest. 1992;90(3):822–9. https://doi.org/10.1172/jci115957.
Bauer MP, Kuijper EJ. Potential sources of Clostridium difficile in human infection. Infect Dis Clin N Am. 2015;29(1):29–35. https://doi.org/10.1016/j.idc.2014.11.010.
Viscidi R, Laughon BE, Yolken R, Bo-Linn P, Moench T, Ryder RW, et al. Serum antibody response to toxins A and B of Clostridium difficile. J Infect Dis. 1983;148(1):93–100.
Loo VG, Bourgault AM, Poirier L, Lamothe F, Michaud S, Turgeon N, et al. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med. 2011;365(18):1693–703. https://doi.org/10.1056/NEJMoa1012413.
Sandora TJ, Fung M, Flaherty K, Helsing L, Scanlon P, Potter-Bynoe G, et al. Epidemiology and risk factors for Clostridium difficile infection in children. Pediatr Infect Dis J. 2011;30(7):580–4. https://doi.org/10.1097/INF.0b013e31820bfb29.
Adams DJ, Eberly MD, Rajnik M, Nylund CM. Risk factors for community-associated Clostridium difficile infection in children. J Pediatr. 2017;186:105–9. https://doi.org/10.1016/j.jpeds.2017.03.032.
Benson L, Song X, Campos J, Singh N. Changing epidemiology of Clostridium difficile-associated disease in children. Infect Control Hosp Epidemiol. 2007;28(11):1233–5. https://doi.org/10.1086/520732.
Lightdale JR, Gremse DA. Gastroesophageal reflux: management guidance for the pediatrician. Pediatrics. 2013;131(5):e1684–95. https://doi.org/10.1542/peds.2013-0421.
Angelidou A, Bell K, Gupta M, Tropea Leeman K, Hansen A. Implementation of a guideline to decrease use of acid-suppressing medications in the NICU. Pediatrics. 2017;140(6):e20171715. https://doi.org/10.1542/peds.2017-1715.
Wultanska D, Banaszkiewicz A, Radzikowski A, Obuch-Woszczatynski P, Mlynarczyk G, Brazier JS, et al. Clostridium difficile infection in polish pediatric outpatients with inflammatory bowel disease. Eur J Clin Microbiol Infect Dis. 2010;29(10):1265–70. https://doi.org/10.1007/s10096-010-0997-9.
Mir SA, Kellermayer R. Clostridium difficile infection in newly diagnosed pediatric inflammatory bowel disease in the mid-southern United States. J Pediatr Gastroenterol Nutr. 2013;57(4):487–8. https://doi.org/10.1097/MPG.0b013e3182a027c5.
Kelsen JR, Kim J, Latta D, Smathers S, McGowan KL, Zaoutis T, et al. Recurrence rate of clostridium difficile infection in hospitalized pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2011;17(1):50–5. https://doi.org/10.1002/ibd.21421.
Lewis JD, Chen EZ, Baldassano RN, Otley AR, Griffiths AM, Lee D, et al. Inflammation, antibiotics, and diet as environmental stressors of the gut microbiome in pediatric Crohn’s disease. Cell Host Microbe. 2017;22(2):247. https://doi.org/10.1016/j.chom.2017.07.011.
Hourigan SK, Chen LA, Grigoryan Z, Laroche G, Weidner M, Sears CL, et al. Microbiome changes associated with sustained eradication of Clostridium difficile after single faecal microbiota transplantation in children with and without inflammatory bowel disease. Aliment Pharmacol Ther. 2015;42(6):741–52. https://doi.org/10.1111/apt.13326.
Ananthakrishnan AN, Oxford EC, Nguyen DD, Sauk J, Yajnik V, Xavier RJ. Genetic risk factors for Clostridium difficile infection in ulcerative colitis. Aliment Pharmacol Ther. 2013;38(5):522–30. https://doi.org/10.1111/apt.12425.
Ciricillo J, Haslam D, Blum S, Kim MO, Liu C, Paulsen G, et al. Frequency and risks associated with Clostridium difficile-associated diarrhea after pediatric solid organ transplantation: a single-center retrospective review. Transplant Infectious Dis: Off J Transpl Soc. 2016;18(5):706–13. https://doi.org/10.1111/tid.12584.
Schutze GE, Willoughby RE. Committee on infectious D, American Academy of P. Clostridium difficile infection in infants and children. Pediatrics. 2013;131(1):196–200. https://doi.org/10.1542/peds.2012-2992.
Bagdasarian N, Rao K, Malani PN. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA. 2015;313(4):398–408. https://doi.org/10.1001/jama.2014.17103.
Davies K, Davis G, Barbut F, Eckert C, Petrosillo N, Wilcox MH. Variability in testing policies and impact on reported Clostridium difficile infection rates: results from the pilot Longitudinal European Clostridium difficile infection diagnosis surveillance study (LuCID). Eur J Clin Microbiol Infect Dis. 2016;35(12):1949–56. https://doi.org/10.1007/s10096-016-2746-1.
Goldenberg SD, Cliff PR, Smith S, Milner M, French GL. Two-step glutamate dehydrogenase antigen real-time polymerase chain reaction assay for detection of toxigenic Clostridium difficile. J Hospital Infection. 2010;74(1):48–54. https://doi.org/10.1016/j.jhin.2009.08.014.
White DR, Hamilton KW, Pegues DA, Hanish A, Umscheid CA. The impact of a computerized clinical decision support tool on inappropriate Clostridium difficile testing. Infect Control Hosp Epidemiol. 2017;38(10):1204–8. https://doi.org/10.1017/ice.2017.161.
Nicholson MR, Freswick PN, Di Pentima MC, Wang L, Edwards KM, Wilson GJ, et al. The use of a computerized provider order entry alert to decrease rates of Clostridium difficile testing in young pediatric patients. Infect Control Hosp Epidemiol. 2017;38(5):542–6. https://doi.org/10.1017/ice.2017.16.
Kim J, Kim H, Oh HJ, Kim HS, Hwang YJ, Yong D, et al. Fecal calprotectin level reflects the severity of Clostridium difficile infection. Annals of laboratory medicine. 2017;37(1):53–7. https://doi.org/10.3343/alm.2017.37.1.53.
Peretz A, Tkhawkho L, Pastukh N, Brodsky D, Halevi CN, Nitzan O. Correlation between fecal calprotectin levels, disease severity and the hypervirulent ribotype 027 strain in patients with Clostridium difficile infection. BMC Infect Dis. 2016;16:309. https://doi.org/10.1186/s12879-016-1618-8.
El Feghaly RE, Stauber JL, Tarr PI, Haslam DB. Intestinal inflammatory biomarkers and outcome in pediatric Clostridium difficile infections. J Pediatr. 2013;163(6):1697–704.e2. https://doi.org/10.1016/j.jpeds.2013.07.029.
• Goldenberg JZ, Yap C, Lytvyn L, Lo CK, Beardsley J, Mertz D, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12:CD006095. https://doi.org/10.1002/14651858.CD006095.pub4. This is a large meta-analysis addressing the role of probiotics in the prevention of C. difficile infection.
Bakken JS. Resolution of recurrent Clostridium difficile-associated diarrhea using staggered antibiotic withdrawal and kefir. Minn Med. 2009;92(7):38–40.
Puri BK, Hakkarainen-Smith JS, Monro JA. The potential use of cholestyramine to reduce the risk of developing Clostridium difficile-associated diarrhoea in patients receiving long-term intravenous ceftriaxone. Med Hypotheses. 2015;84(1):78–80. https://doi.org/10.1016/j.mehy.2014.11.020.
Johnson S, Louie TJ, Gerding DN, Cornely OA, Chasan-Taber S, Fitts D, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014;59(3):345–54.
Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45(3):302–7.
Cornely OA, Miller MA, Louie TJ, Crook DW, Gorbach SL. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis. 2012;55(Suppl 2):S154–61.
Panchal P, Budree S, Tu E, Kahn SA, Allegretti JR, Fischer M, et al. Pediatric access to fecal microbiota transplantation for recurrent <em>clostridium difficile</em> infection in the United States and the impact of stool banks: a geospatial analysis. Gastroenterology. 2017;152(5):S849–S50. https://doi.org/10.1016/S0016-5085(17)32926-8.
Kelly CR, Kahn S, Kashyap P, Laine L, Rubin D, Atreja A, et al. Update on fecal microbiota transplantation 2015: indications, methodologies, mechanisms, and outlook. Gastroenterology. 2015;149(1):223–37. https://doi.org/10.1053/j.gastro.2015.05.008.
Khanna S, Shin A, Kelly CP. Management of <em>Clostridium difficile</em> infection in inflammatory bowel disease: expert review from the clinical practice updates committee of the AGA Institute. Clin Gastroenterol Hepatol. 2017;15(2):166–74. https://doi.org/10.1016/j.cgh.2016.10.024.
Shane AL, Mody RK, Crump JA, Tarr PI, Steiner TS, Kotloff K, et al. 2017 Infectious Diseases Society of America Clinical Practice Guidelines for the Diagnosis and Management of Infectious Diarrhea. Clin Infect Dis. 2017;65(12):e45–80. https://doi.org/10.1093/cid/cix669.
Louie TJ, Miller MA, Mullane KM, Weiss K, Lentnek A, Golan Y, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364(5):422–31. https://doi.org/10.1056/NEJMoa0910812.
Spiceland CM, Khanna S, Pardi DS. Outcomes with fidaxomicin therapy in Clostridium difficile infection. J Clin Gastroenterol. 2018;52(2):151–4. https://doi.org/10.1097/mcg.0000000000000769.
• O'Gorman MA, Michaels MG, Kaplan SL, Otley A, Kociolek LK, Hoffenberg EJ et al. Safety and pharmacokinetic study of fidaxomicin in children with Clostridium difficile-associated diarrhea: a phase 2a multicenter clinical trial. J Pediatric Infect Dis Soc 2017. https://doi.org/10.1093/jpids/pix037. This study specifically addresses the use and safety of fidaxomicin in pediatric patients with C. difficile.
Soriano MM, Danziger LH, Gerding DN, Johnson S. Novel fidaxomicin treatment regimens for patients with multiple Clostridium difficile infection recurrences that are refractory to standard therapies. Open Forum Infect Dis. 2014;1(2):ofu069. https://doi.org/10.1093/ofid/ofu069.
Soriano MM, Johnson S. Treatment of Clostridium difficile infections. Infect Dis Clin N Am. 2015;29(1):93–108. https://doi.org/10.1016/j.idc.2014.11.005.
Fehér C, Soriano A, Mensa J. A review of experimental and off-label therapies for Clostridium difficile infection. Infect Dis Ther. 2017;6(1):1–35. https://doi.org/10.1007/s40121-016-0140-z.
Chen B, Avinashi V, Dobson S. Fecal microbiota transplantation for recurrent Clostridium difficile infection in children. J Infect. 2017;74:S120–S7. https://doi.org/10.1016/s0163-4453(17)30202-5.
Lee CH, Belanger JE, Kassam Z, Smieja M, Higgins D, Broukhanski G, et al. The outcome and long-term follow-up of 94 patients with recurrent and refractory Clostridium difficile infection using single to multiple fecal microbiota transplantation via retention enema. Eur J Clin Microbiol Infect Dis. 2014;33(8):1425–8. https://doi.org/10.1007/s10096-014-2088-9.
Youngster I, Mahabamunuge J, Systrom HK, Sauk J, Khalili H, Levin J, et al. Oral, frozen fecal microbiota transplant (FMT) capsules for recurrent Clostridium difficile infection. BMC Med. 2016;14(1):134. https://doi.org/10.1186/s12916-016-0680-9.
Postigo R, Kim JH. Colonoscopic versus nasogastric fecal transplantation for the treatment of Clostridium difficile infection: a review and pooled analysis. Infection. 2012;40(6):643–8. https://doi.org/10.1007/s15010-012-0307-9.
Brumbaugh DE, De Zoeten EF, Pyo-Twist A, Fidanza S, Hughes S, Dolan SA, et al. An intragastric fecal microbiota transplantation program for treatment of recurrent Clostridium difficile in children is efficacious, safe, and inexpensive. J Pediatr. 2017;194:123–127.e1. https://doi.org/10.1016/j.jpeds.2017.10.016.
Wang S, Xu M, Wang W, Cao X, Piao M, Khan S, et al. Systematic review: adverse events of fecal microbiota transplantation. PLoS One. 2016;11(8):e0161174. https://doi.org/10.1371/journal.pone.0161174.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Jennifer Hellmann declares that she has no conflict of interest and Daniel Mallon declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Hospital Medicine
Rights and permissions
About this article
Cite this article
Hellmann, J., Mallon, D. C. difficile Infection in Children: What’s New?. Curr Treat Options Peds 4, 255–269 (2018). https://doi.org/10.1007/s40746-018-0124-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40746-018-0124-1