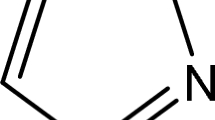
Abstract
Corrosion poses a significant challenge in various industries, leading to substantial economic losses and safety concerns. The electrochemical reactions between metallic metals and their surroundings result in corrosion, which deteriorates the metals and frequently cause environmental contamination, financial losses, and safety issues. The paper summarizes the various organic inhibitors for steel and copper in hydrochloric acid and sulphuric acid and the methodology that we can use to find the properties of inhibition. There are numerous factors that influence the corrosion inhibition potential of these compounds, including the substituents and heteroatoms present in their molecular structures. An analysis of the inhibition of copper and steel corrosion using organic compounds is presented in the present review article. This review provides insights into the various organic inhibitors that inhibit corrosion through heterocyclic compounds. The focus of this review paper is on the novel contributions to corrosion inhibition, demonstrating how organic compounds can be used to protect steel and copper surfaces from corrosion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
1.1 General Introduction to Corrosion
Electrochemical reactions between metallic metals and their surroundings result in corrosion, which deteriorates the metals and frequently endangers the environment, financial interests, and process safety. Serious problems like altered mechanical characteristics, physical appearance, and material resistance are also brought up by corrosion [1]. This is an extensive and considerable issue that affects many industry sectors, including oil and gas, chemical along manufacturing industries. The situation of corrosion can cause serious economic losses also endangering human safety [2]. The most common corrosion complications are caused by breakdown in pipelines, leakage of chemicals, and sometimes fire in industrial areas; mostly when electrical and corroded components are present. Most factories in various sectors suffer from a variety of corrosion, including galvanic corrosion, uniform corrosion, crack corrosion, intergranular corrosion pitting, and erosion [3]. Corrosion causes enormous economic losses which have been approximated to 1–5% of the country’s GDP, particularly in developed countries [4]. As a result, there is a constant demand for effective corrosion inhibitors to prevent or minimize corrosion damage.
1.2 Introduction of Corrosion Inhibitors
Corrosion inhibitors: the synthetic substances that in tiny amounts help prevent metals from corroding in harsh settings. Because of excessive corrosive rates in the mentioned areas, refinery units, pipelines, steam generators, ballast tanks, and many more industrial divisions use corrosion inhibitors [5]. Acidic solution mediums are employed in many industries as pickling and cleaning agents to remove unwanted particles from the surfaces of containers which cause corrosion of the metal surface [6]. The most dominant method of protecting metals from corrosion is to incorporate a compound that can act as inhibitors in aggressive media [7]. Here a number of inhibitors we can use in aggressive media. Figure 1 shows four types of corrosion inhibitors; Environmental inhibitors, green inhibitors, organic inhibitors, and inorganic inhibitors; which minimize the corrosion on the metallic surfaces. Effective inhibitors are chosen according to their mode of action mechanism. Inhibitors should be effective even at high concentrations of acidic medium and high temperatures also. Furthermore, the inhibitory capacity of the substance is supported by the existence of a structure of adsorption active sites with lone pairs and orbitals, heterocyclic rings comprising nitrogen, phosphorus, oxygen, and sulfur atoms. These chemicals can either form a strong coordination connection with a metal atom or a surface passive film [8]. The effective inhibitors are then adsorbed on top of the metal while blocking the active sites [9]. Organic inhibitors work by being adsorbed on the metal and hindering the active sites by replacing molecules of water and creating a dense bar hurdle coating to slow down the rate of corrosion [10].
All inhibitors shown in Fig. 1 are explained below and their advantages and disadvantages are discussed below:
1.2.1 Environmental Corrosion Inhibitors [11, 12]
Environmental corrosion inhibitors are substances that are added to environments to reduce the rate of corrosion of metals and other materials. The current trend for inhibitor usage is towards more environmentally friendly green chemicals. For environmental protection, the scientific efforts have increased to study the inhibiting power of natural products like peels, seeds, fruit-shells and leaves that contain different organic compounds which (e.g. amino acids, alkaloids, flavonoids, pigments, tannins, etc.) suppress the dissolution reaction of metals and prevent the environmental pollution. In 1930, plant extracts (dried stems, leaves and seeds) of Celandine (Chelidonium majus) and other plants were used in H2SO4 pickling baths. Animal proteins (by products of meat and milk industries) were also used for retarding acid corrosion. The additives used in acid, included flour, bran, yeast, a mixture of molasses and vegetable oil, starch and hydrocarbons (tars and oils).
Advantages and disadvantages of environmental corrosion inhibitors [13].
-
Advantages of environmental corrosion inhibitors
-
1.
These are environment-friendly and have less harmful effects.
-
2.
These are easy to apply and is time saving.
-
3.
These are renewable in nature.
-
4.
The organic compounds present in the natural extracts improve the corrosion inhibition efficiency in metal surfaces.
-
5.
These inhibitors breaks down themselves.
-
1.
-
Disadvantages of environmental corrosion inhibitors
-
1.
These are less effective as compared to other inhibitors.
-
2.
They can be more expensive to produce than traditional inhibitors, which can increase the overall cost for industries.
-
1.
1.2.2 Green Corrosion Inhibitors
Green corrosion inhibitors are extracted from natural products or prepared from natural products (raw materials). Some organic corrosion inhibitors and polymer corrosion inhibitors fall under this category. Leaves, flowers, fruits, seeds, pericarp, or other parts of plants can be the sources of effective inhibition substances [12]. The extraction methods include soaking, heating reflux, enzymatic hydrolysis, Soxhlet extraction, and ultrasonic extraction methods. The efficiency of extraction of the active components depends on the solvent used, extraction temperature (60–80 °C for most plants), drying temperature (60–80 °C), extraction time, liquid–solid ratio, etc. Different green corrosion inhibitors are isolated from different sources that help to minimize corrosion of various metals. A lot of research has been done on development of green corrosion inhibitors. The natural substances can serve as impressive corrosion inhibitors in the near future owing to their advantages which include easy accessibility, eco–friendliness, biodegradability on earth and non-toxicity [13].
Advantages and disadvantages of green corrosion inhibitors [14, 15].
-
Advantages of green corrosion inhibitors
-
1.
Green corrosion inhibitors are made up of natural resources like wood, seeds, leaves, etc.
-
2.
These are less toxic and biodegradable in nature.
-
3.
These are less costly and causes less pollution.
-
4.
The extracts have good inhibition efficiency for metal corrosion in various media.
-
1.
-
Disadvantages of green corrosion inhibitors
-
1.
These are less effective in some cases.
-
2.
These are not compatible with all types of metals or materials.
-
3.
These takes more time as compared to other types of corrosion inhibitors
-
1.
1.2.3 Inorganic Corrosion Inhibitors
Inorganic corrosion inhibitors are substances that are added to a metal surface to prevent or slow down the corrosion process. They work by forming a protective layer on the surface of the metal, which prevents or limits contact with corrosive agents. Inorganic corrosion inhibitors can be classified based on their chemical composition, mode of action, and application. Metal salts such as chromates, molybdates, and phosphates are commonly used as corrosion inhibitors. These inhibitors are commonly used in the oil and gas industry. The inorganic phosphate inhibitor forms a protective layer on the surface, while the organic inhibitor provides additional protection by inhibiting corrosion [16].
Advantages and disadvantages of inorganic corrosion inhibitors.
-
Advantages of inorganic corrosion inhibitors
-
1.
These are effective at blocking anodic and cathodic sites.
-
2.
These are effective in preventing corrosion especially in aggressive environments.
-
3.
These are less expensive as compared to organic inhibitors and are cost effective options for large scale.
-
4.
These are stable under a wide range of environmental conditions including high temperature.
-
1.
-
Disadvantages of inorganic corrosion inhibitors
-
1.
These are toxic in nature.
-
2.
The disposal and handling of these inhibitors requires stringent conditions.
-
3.
They can cause scaling or precipitation in some cases.
-
1.
1.2.4 Organic Corrosion Inhibitors
Synthetic organic corrosion inhibitors; are of two types as mentioned in Fig. 2 i.e. liquid-phase organic corrosion inhibitors and volatile organic corrosion inhibitors. Using organic chemicals, particularly heterocyclic compounds, provides the most effectual corrosion prevention techniques available today. Organic compounds with specific polar functional groups or coordination sites have effective corrosion inhibition properties [17]. It is one of the most popular and effective approaches because of its association with various advantageous qualities such as high inhibitory capacity, lesser cost, easier to apply, and recyclability [18]. During cleaning procedures in industries, heterocyclic compounds are introduced to aggressive solutions. Here heterocyclic compounds may adsorb on the surface of metal to reduce the risk of corrosion. Adsorption of heterocyclic compounds on metal creates a defensive bar that prevents aggressive electrolytes from corroding [19, 20]. The capacity of these inhibitors is because of the existence of many heteroatoms in the form of polar functional groups like, –NH2, –NO2, –COOC2H5, etc., as well as non-bonding or Π-electrons. During metal-inhibitor interactions, polar functional groups, non-bonding, and Π-electrons serve as adsorption center [21].
Advantages and disadvantages of organic corrosion inhibitors [22].
-
Advantages of organic corrosion inhibitors
-
1.
These seem to be highly effective and their cost is attractive.
-
2.
These kinds of inhibitors are widely used and possess higher efficiency than all other inhibitors.
-
3.
These inhibitors have highest corrosion inhibition efficiency.
-
1.
-
Disadvantages of organic corrosion inhibitors
-
1.
These inhibitors need higher dosages to exert better effects.
-
2.
The usage of these inhibitors was restricted due to high exploitation rates.
-
3.
These are least eco-friendly.
-
4.
The mechanism of these inhibitors to operate are often tedious.
-
1.
1.2.4.1 Liquid-Phase Organic Corrosion Inhibitors
The inhibitory efficiency of these organic compounds is related to the heterocyclic nature of the compounds with N, S, O and P heteroatoms in their molecules, which serve as reaction centers for adsorption on the metal surface. The inhibition efficiency of the heteroatoms in heterocyclic compounds follows the sequence O < N < S < P. The electron transfer from these inhibitors to the metal surface is facilitated by the availability of non-bonded (lone pairs) and π-electrons in the inhibitor molecules. Liquid-phase inhibitors are further classified into three types: simple organic molecules, synthetic organic polymers, and surfactant molecules [23, 24].
-
Simple organic molecules Simple organic compounds such as azoles (imidazole, benzimidazole, benzotriazole, triazole, tetrazole), amines, urea, mercaptobenzothiazole (MBT), aldehydes, heterocyclic nitrogen compounds, sulfur-containing compounds, acetylenic compounds and also ascorbic acid, succinic acid, and tryptamine have been reported to be effective organic corrosion inhibitors.
-
Synthetic organic polymers Organic polymeric materials can also be efficient corrosion inhibitors because they contain potent heteroatom functional groups that form inhibitor (polymer)–metal ions complexes; which can occupy large zone over metallic surface and protect it from the corrosive media.
-
Surfactant molecules Surfactants (Surface active agents) is another class of synthetic organic inhibitors, which have also been extensively used as commercial corrosion inhibitors and their inhibition efficiency may be attributed to their ability to affect the nature of metal surfaces and solution interfaces. It is generally assumed that adsorption of the surfactant molecules onto the metal surface is the first stage in the mechanism of surfactants as corrosion inhibitors in aggressive media. The adsorption process is influenced by the nature and the surface charge of the metal, the chemical structure of the surfactant, and the nature of the aggressive electrolyte.
1.2.4.2 Volatile Organic Corrosion Inhibitors
Volatile Organic Corrosion Inhibitors (VOCIs) are compounds used to protect metal surfaces from corrosion. These inhibitors work by vaporizing and forming a protective layer on the metal surface, preventing the interaction of the metal with corrosive agents such as moisture and oxygen. These are used in packaging materials to protect metal parts during storage and transportation and applied in marine applications to protect ship parts and offshore structures. Due to their volatile nature, VOCIs can protect complex geometries and internal surfaces. Some VOCIs can be harmful to the environment due to their volatile nature and potential toxicity. VOCIs contain organic and inorganic chemical compounds able to vaporize and condense in the presence of moisture-forming thin films on metallic surfaces [25, 26].
1.2.4.3 Shortcomings of Having Lack of Organic Corrosion Inhibitors [27,28,29]
Lack of organic corrosion inhibitors can lead to several significant shortcomings, particularly in industries where metal corrosion is a concern. Here are some of the main problems occuring due to lack of organic corrosion inhibitors:
-
Without organic corrosion inhibitors, metal structures and components are more prone to rust and degradation, leading to more frequent maintenance and replacement needs.
-
Corrosion can significantly reduce the lifespan of machinery, pipelines, and other metal structures, necessitating earlier-than-expected investments in replacements.
-
Equipment failure due to corrosion can lead to unexpected downtime, disrupting production schedules and affecting overall operational efficiency.
-
Corrosion can lead to leaks and spills, especially in the case of pipelines carrying hazardous materials. This can result in environmental contamination and regulatory fines.
-
Corroded surfaces can increase friction and resistance in mechanical systems, leading to reduced efficiency and higher energy consumption.
-
In the absence of organic inhibitors, alternative corrosion control measures, such as cathodic protection or the use of more expensive corrosion-resistant materials, may be required, leading to higher overall costs.
-
In industries like food and beverage, pharmaceuticals, and electronics, corrosion can contaminate products, affecting their quality and safety.
-
Many industries are subject to strict regulations regarding corrosion control. Lack of adequate inhibitors can lead to non-compliance, legal issues, and potential shutdowns.
Corrosion inhibitors are frequently useful in industrial sectors to slow down the corrosion of metallic compounds in contact with an acidic medium. Chemical and electrochemical methodologies combined with analytical methods are applied to properly select effective inhibitors; still, there is a demand for a standardized procedure to describe the interplay between inhibitors and the surface of the metal in the hope of exploring new and much more efficient inhibitors. The inhibitor’s activity is influenced by the structure of the molecule and electronic parameters; for instance; the highest occupied molecular orbital energy, the lowest unoccupied molecular orbital energy, and the HOMO–LUMO energy difference [30].
1.2.4.4 Some Applications of Organic Corrosion Inhibitors in Various Sectors
1. Organic corrosion inhibitors for Reinforced concrete
Reinforcement corrosion is one of the major causes of degradation in concrete structures. A wide variety of types of corrosion-inhibiting admixtures for reinforced concrete are known. These include organic corrosion inhibitors such as formulations based on amines and alkanolamines (amino alcohols) [31]. When concrete is exposed to carbonation and/or to chloride salts, the embedded steel may cease to be passivated and start corroding at a significant rate. Since the volume of the corrosion product is normally several times greater than that of the original metal, the associated stresses in the concrete can lead to cracking and eventually to spalling [32]. Some authors also report that organic inhibitors help to decrease the chloride content in concrete and to promote a decrease on the chloride ion diffusion. Jamil HE et al. studied that the organic inhibitors studied in this work (preventive and curative amino alcohol-based inhibitors) reduced the corrosion rate of the reinforcing steel in solutions simulating concrete interstitial electrolyte contaminated with 2 g/l of NaCl and 4% (v/v) of inhibitor [33].
2. Organic corrosion inhibitors used in oil and gas industry
In the petroleum industry, general and localized corrosion are the most common types of corrosion occurrences. The other large problem in operating pipe flow lines is internal corrosion, mainly due to stress corrosion cracking. The combination of corrosion and erosion is the main problem in pipe deterioration [34]. In the petroleum industry, one facet of the development of new oil and gas production is the stimulation process. Overall, the stimulation process involves many different aspects, including the acidizing portion utilized to stimulate the carbonate reservoir or for dissolving fines. Hydrochloric, hydrofluoric, acetic, or formic acids are injected into the well during the acidizing stimulation process which cause serious corrosion issues. In the absence of corrosion inhibitors (CIs), the general CR (corrosion rate) can be extremely high (> 100 mm/y) and can increase exponentially with increasing temperatures and acid concentrations [35]. To control the corrosion damage of well tubulars, mixing tanks, coiled tubings, and other metallic surfaces, acids need to be inhibited by the use of an effective organic corrosion inhibitor solution. For stimulation applications, inhibitors are added to the acid fluids in batch-wise fashion; batch-wise refers to the single addition of the CI into the holding tank of the acid before the acid is used in the stimulation process [36]. Abiola et al. [37], reported that 3-(4-amino-2-methyl-5-pyrimidyl methyl)-4-methyl thiazolium chloride effectively prevents hydrogen evolution and corrosion of MS in 0.5 mol/L and 5 mol/L HCl at 30 ℃.
3. Organic corrosion inhibitors used in hydraulic metal structures
The hydraulic metal structures are exposed to harsh and complex stresses (corrosive stresses, physical loads, biological stresses, etc.). Corrosive stresses depend largely on the location of a metal structure. Hydraulic metal structures have significant exposure in several zones. The most effective and common method of corrosion protection is to paint organic coatings on the surface of metal structures. The organic coatings on hydraulic metal structures can be exposed to various environmental factors, such as the temperature, humidity, ultraviolet radiation (UV), chloride ion and mechanical stress. The organic coatings deteriorate with the increase of exposure time, and periodic recoating is necessary for hydraulic metal structures to prevent environmental attack and to extend their service life [38].
4. Organic corrosion inhibitors used in electronics industry
Numerous field failure returns of electronics are marked as “no failure found”, yet many of these failures are likely due to corrosion. Electronic equipment components, computers, integrated circuits (IC) and microchips in indoor atmospheres are exposed to a variety of environmental conditions and frequently corrosion failure of these devices occurs. Corrosion is becoming an even more significant factor in the reliability of electrical and electronic equipment. Within the last decade, the electronic and electrical industries are increasingly applying more Vapour Phase Corrosion Inhibitors (VCI) for electronic components and devices. As electronics continue to shrink in size and grow in capacity, the importance of corrosion control increases. The corrosion of small and micro components of silver causes extensive losses to the electronics industry due to reject product, problems with soldering processes and production delays. The silver surface becomes tarnished by the effect of H2S and, depending on exterior pollution, the problem can occur at controlled indoor conditions in clean rooms too [39, 40].
1.3 Corrosion on Mild Steel
Mild steel (M-steel) is one of the most often used pipeline materials in the gas and oil industries. It is widely utilized as a building material for domestic and industrial purposes due to its abundance, low cost, and strong mechanical power. They do, however, dissolve rapidly in acid during the cleaning, pickling, and descaling procedures. However, corrosion of steel pipes is a significant issue that has resulted in several failures. Because of its corrosive nature, it should not be exposed to acids. Inhibitors can adsorb on the exterior of mild steel and hinder active sites, reducing the rate of corrosion. Mild steel degradation has been long a worry due to its extremely high corrosion rate in hostile solutions and atmospheres. As a result, the application of coatings is highly advised to preserve these alloys at moderate temperatures, even if it may cause minor flaws (tiny cracks and holes) on the substrate’s surface. Mineral solutions are comprehensively used in a diversity of sectors, including descaling, pickling along with many more. It is consequently critical to add inhibitors to mineral acid solutions used in various operations to reduce the rate of corrosion attack and the amount of acid consumed [41,42,43,44].
1.4 Corrosion on Copper
Copper, which has a reddish-orange color, is 5th most prevalent metal in the geosphere and also immensely useful in both pure and alloying forms. It is an essential non-ferrous things utilized in industry due to its qualities like high conductivity, mechanical adaptability, also feasibility [45]. Copper and its alloys have been employed in a wide range of manufacturing units, including aerospace, automobile manufacturing, and bridge construction. These are highly prized because they are widely used in the manufacture of cable pipelines in the electronic and power industries, as well as heat exchangers and cooling towers. They are, however, susceptible to corrosion from the surrounding media, which can cause considerable economic and energy losses, as well as serious accidents. Copper can be protected against corrosion through coatings, altering the solution environment, and other ways [46,47,48]. Copper is considered a noble metal because it produces a protective film that provides enough reluctance to corrosion in the atmosphere and under certain chemical circumstances [49]. However, depending on the environmental circumstances, corrosion on the copper surface may occur because of the existence of oxygen with some aggressive anions such as chloride and sulfate ions [50].
1.5 Literature Survey
It has been found from the literature that numerous synthetic compounds are reported that act as inhibitors of corrosion as shown in Tables 1 and 2.
The Table 1 provides an overview of the efficiency of various organic corrosion inhibitors, highlighting how their effectiveness changes with different concentrations in different acidic mediums for protecting the metals (mild steel) from being corroded. The concentration is the amount of inhibitor used. The effectiveness of the inhibitor in reducing corrosion, expressed as a percentage (efficiency of the inhibitor). Efficiency indicates the reduction in corrosion rate when the inhibitor is used compared to a control sample without the inhibitor. Higher percentages indicate greater efficiency. The type of acidic environment in which the inhibitor's efficiency is tested (e.g., hydrochloric acid (HCl), sulfuric acid (H2SO4). The table highlights how the efficiency of organic inhibitors varies depending on the type of metal, concentration of the inhibitor, and the specific acidic environment. This data is important for selecting the most effective inhibitor and concentration for specific applications, ensuring optimal protection of metals in acidic conditions [51].
The Table 2 Summarizes the use of different organic corrosion inhibitors used for the protection of copper from being corroded. As clear from the Table 2, different methods had been used to find the efficiency. The data helps in selecting the most effective inhibitor for specific applications to prolong the lifespan of metals (copper) and reduce maintenance costs.
1.6 Methodologies Used for the Detection of Corrosion Inhibitors
1.6.1 Weight Loss Technique
Weight loss is a typical way of determining the efficiency of inhibitors. This approach involves weighing a metal sample before and after it has been exposed to a corrosive environment, with or without an inhibitor. The steps involved in weight loss methods are represented in Fig. 3. The weight loss data was estimated using the equations below [52].
Srimathi et al. [52], achieved maximum corrosion inhibition efficiency of polyvinyl alcohol; which was 90.54% at 6000 ppm by using the weight loss method. Jasim et al. [53], achieved maximum corrosion inhibition efficiency of (3,5-dimethyl-1H-pyrazol-1-y1) (4-((3,4-dimethoxybenzylidene) amino) phenyl) methanone; 95.9% at 400 ppm at 60 ℃ by weight loss method.
1.6.2 Electrochemical Impedance Spectroscopy (EIS)
It’s a potent approach used in research to examine the electrochemical behavior of a system, particularly the interactivity between the metal surface, its surroundings, and the inhibitor. EIS technique comes up with information on reductive and capacitive behavior at the contact, allowing to evaluation the compound's performance as an inhibitor [54, 55]. The principle of electrochemical impedance spectroscopy involves applying small amplitude, alternating current (AC) signals across an electrochemical cell over a range of frequencies.
Rbaa et al. [56], achieved maximum corrosion inhibition efficiency of 8-hydroxyquinoline; 96.5% at 10−3 M by the EIS method.
Figure 4 shows the working of the EIS technique using a working electrode, counter electrode, and reference electrode. The sample to be studied for its corrosion inhibition properties is placed in the beaker containing all the electrodes which are further connected to the EIS frequency response analyzer. The analyzer is connected to the computer system or recorder which records the data or graphs for the technique.
1.6.3 Potentiodynamic Polarization
PDP is one of the most generally acknowledged methods for determining a specimen’s corrosion. The potential difference is determined between reference and working electrodes. Ddifference is applied between two electrodes, and the resulting current is determined. It is a widely used electrochemical technique for analyzing metal behavior in a specific environment and determining corrosion susceptibility. The principle of potentiodynamic polarization involves sweeping the potential of an electrode (metal sample) linearly or logarithmically over a certain voltage range while measuring the resulting current.
Rbaa et al. [56], show that the maximum corrosion inhibition efficiency of 8-hydroxyquinoline is 93.8% at 10–3 M by the PDP method.
Potential versus resulting current curve:
It involves two regions:
-
Cathodic region (where reduction reaction occurs)
-
Anodic region (where oxidation reaction occurs)
Figure 5 shows the basic principle and working of the PDP method. In this method, a beaker contains a magnetic bead, a digital thermometer, a reference electrode (standard calomel electrode), an auxiliary platinum electrode as well as a working electrode attached to a steel specimen; is placed over a magnetic stirrer. The working electrode, reference electrode, and auxiliary platinum electrode; are connected to potentiostatic/galvanostatic, which is further connected to a computer system that records the data.
1.6.4 Atomic Force Microscopy
AFM is a kind of scanning probe microscopy developed in 1985 to measure local attributes (e.g., surface topography) with a probe. AFM is one method for understanding metal corrosion properties. This approach is applied to study the properties of surface layers in various materials, including ceramics, metals, polymers, and organic/inorganic composites [57]. Several imaging modalities are now available, which provide information about the sample surfaces as well as full three-dimensional topographies. Some of these include contact mode, tapping (or intermittent touch), and non-contact mode [58]. This technique's surface morphology can be used to investigate corrosion phenomena and the inhibitory impact of corrosion inhibitors [59]. Surface roughness can be used to characterize the inhibitory capacity of corrosion inhibitors. The rougher surface is related to the deterioration of the metal surface, resulting in an increased corrosion rate [60].
1.6.5 SEM (Scanning Electron Microscope)
SEM is an electron microscope that results in high-resolution images of surfaces of bulk and nanomaterials at nanoscale levels. The images produced by SEM have two-dimensional and three-dimensional appearances, which are important for assessing the surface features of the specimens [61]. It is being employed in a variety of polymers as well as applications, surface roughness, including fiber-matrix adhesion and phase boundaries in blends [62].
1.6.6 UV Spectroscopy
UV spectroscopy is utilized to determine the absorbance of a substance in solution. It has been used extensively in a diversity of settings to aid in qualitative as well as quantitative analysis and reaction monitoring. UV absorption spectroscopy can help organic chemists monitor reactions and detect compounds online or offline. It can also be used for simple purposes like displaying pH [63]. UV spectroscopy is utilized to better understand corrosion and inhibitory mechanisms. It is used to identify the reactions taking place throughout the corrosion and inhibition processes [64].
Figure 6 shows the basic principle and working of the UV–Vis spectrophotometer. It includes mirrors, UV and visible light sources, slits, diffraction grating, filter, reference and sample cuvettes, lenses, detectors, reference and sample beams, and recorder.
A solution of sample is placed in the sample cuvette and the reference cuvette is filled with reference solvent used to dissolve the sample. A command is given to the system for recording the absorbance and graph for the sample. The absorbance and graph data are displayed over the recorder.
1.6.7 FTIR Spectroscopy
Infrared spectroscopy has long been an effective method for identifying organic compounds. FTIR spectroscopy has become a prominent tool for quantitative examination of complex mixtures, as well as the exploration of surface and interfacial phenomena [65]. IR spectrometer is a device that records both infrared spectroscopic and spatial information about a sample. Such images can reveal information about the sample's chemical and physical qualities [66].
Spectra of IR are determined by passing infrared rays through the sample and studying how much amount of incident rays are being absorbed at a particular frequency [67].
1.7 Mechanism of Corrosion Inhibition Process
There exist various kinds of mechanisms on which inhibitors of corrosion work on metal surfaces; one of which is the adsorption mechanism. Generally, adsorption can be distinguished as physical adsorption, chemical adsorption, or mixed type; conditional on the interplay between metal and heteroatoms or π-electrons of the inhibitor [68]. Some defined parameters define the type of mechanism; including the chemical structure and charge distribution, and also metal surface charge. Chemical adsorption occurs by producing coordination bonds by a chemical reaction between unshared free pairs of electrons and unoccupied d-orbital of the metal and in physical adsorption, the charges on inhibitor molecules bind with electrically charged metal surface [69]. When one of the hydrogen atoms at the carbon atom in a heterocyclic ring is substituted by aldehyde, nitrosyl, carboxyl, or amino substituent groups; the inhibition efficiency of organic inhibitor increases [70].
In HCl, while introducing the organic compounds, they form a thin layer on metal and reduce the corrosion rate, called substitution reaction which takes place between inhibitor and water molecules at the metal-solution interface; which can be represented as:
where Org(a) and Org(b) are inhibitor molecules dissolved in solution and inhibitor molecules adsorbed on the metal surface, respectively, and H2O(a) and H2O(b) are water molecules and adsorbed water molecules on the metal surface, respectively. x, is the size ratio, representing the number of water molecules replaced by one molecule of organic inhibitor [71].
In addition, the membrane’s stability of inhibitor molecules adsorbed on metal is based conditionally on active sites, steric hindrance, the electron density of molecules; acidic medium, and nature of the interaction between a π-orbital and d-orbitals of iron. Information regarding the adsorption mode of molecules on metal surfaces can be achieved from the adsorption isotherm [28]. Different isotherms i.e. Langmuir (1), Flory–Huggins (2), Temkin (3) and Freundlich (4) explain the anti-corrosion mechanisms.
where C is the concentration of inhibitor in use, θ is the degree of surface coverage, Kads is constant, f is the energy parameter, and a is water molecules on the metal surface [72].
Figure 7 shows the mechanism of corrosion inhibition process in physical and chemical adsorption. The charged inhibitor molecules and inhibitor molecules having donor atoms aromatic character pi-electrons are adsorbed on the metal surface. Chemisorption produces a coordination bonds in a chemical reaction whereas in physisorption, the charged inhibitor molecules bind up with the charge of the metal surface.
1.8 Current Research Status of Organic Corrosion Inhibitors
The research of organic corrosion inhibitors continues to be active and evolving. There is a growing emphasis on developing organic inhibitors that are environmentally friendly and sustainable. Researchers are exploring natural compounds, biodegradable polymers, and renewable resources as potential inhibitors. Researchers are developing inhibitors tailored for specific applications and environments, including different types of metals, temperatures, pH levels, and corrosive agents. There is ongoing research into understanding the mechanisms by which organic inhibitors adsorb onto metal surfaces and inhibit corrosion. organic corrosion inhibitors continue to be an active area of research aimed at enhancing corrosion protection, reducing maintenance costs, and extending the lifespan of metallic structures and equipment in various industrial sectors.
1.9 Future Scope of Corrosion Inhibition Studies
Corrosion inhibitors; are commonly used to stop or lessen corrosion in several domains, including industrial, construction, and cultural heritage. Conservation and restoration workers use corrosion inhibitors to protect and preserve metallic cultural material [73]. The future scope of corrosion inhibition research is promising; also affected by several components, including technical improvements, environmental concerns, also the changing needs of diverse businesses. Here are some potential areas of focus in the future:
-
Advanced Materials and Nanotechnology
-
Green Corrosion Inhibitors
-
Smart Coatings and Sensors
-
Computational Modelling and Simulation
-
Corrosion in Renewable Energy Systems
1.10 Research Gap
As from the literature study most of the work done in HCl and H2SO4, but these compounds should also be studied in basic mediums and saline mediums. As apart from acidic medium, basic and saline conditions are also responsible for the corrosion of the metals.
2 Conclusion
The above studies about synthetic organic compounds reveal effective anti-corrosive properties that show significant potential in protecting various materials from corrosion. From various research and developments, these compounds show remarkable effectiveness in forming protective barriers or films, inhibiting reactions, and extending the lifespan of metal surfaces. Their versatility and compatibility with different substrates make them valuable assets in various industries. The compounds containing heteroatoms as active materials for being adsorbed on the surface of metal reducing metal dissolution in HCl or H2SO4 medium. Each compound shows different inhibiting efficiency with different concentrations.
Data Availability
No datasets were generated or analyzed during the current study.
Abbreviations
- EIS:
-
Electrochemical impedance spectroscopy
- PDP:
-
Potentiodynamic polarization
- AFM:
-
Atomic force microscope
- DFT:
-
Density functional theory
- MD:
-
Molecular dynamic simulations
- SEM:
-
Scanning electron microscope
- EDS:
-
Energy dispersive spectroscopy
- EDX:
-
Energy dispersive X-ray spectroscopy
- XPS:
-
X-ray photoelectron spectroscopy
- RDF:
-
Radial distribution function
- MSD:
-
Mean square displacement
- ICP-OES:
-
Inductively coupled plasma-optical spectroscopy
References
Sedik A, Lerari D, Salci A, Athmani S, Bachari K, Gecibesler İH, Solmaz R (2020) Dardagan Fruit extract as eco-friendly corrosion inhibitor for mild steel in 1 M HCl: electrochemical and surface morphological studies. J Taiwan Inst Chem Eng 107:189–200
Al-Amiery AA, Al-Azzawi WK (2023) Mannich bases as corrosion inhibitors: an extensive review. J Mol Struct 1294:136421
Al Jahdaly BA, Maghraby YR, Ibrahim AH, Shouier KR, Alturki AM, El-Shabasy RM (2022) Role of green chemistry in sustainable corrosion inhibition: a review on recent developments. Mater Today Sustain 20:100242
Verma C, Ebenso EE, Quraishi MA (2017) Corrosion inhibitors for ferrous and non-ferrous metals and alloys in ionic sodium chloride solutions: a review. J Mol Liq 248:927–942
Kosari A, Momeni M, Parvizi R, Zakeri M, Moayed MH, Davoodi A, Eshghi H (2011) Theoretical and electrochemical assessment of inhibitive behavior of some thiophenol derivatives on mild steel in HCl. Corros Sci 53(10):3058–3067
Behpour M, Ghoreishi SM, Mohammadi N, Soltani N, Salavati-Niasari M (2010) Investigation of some Schiff base compounds containing disulfide bond as HCl corrosion inhibitors for mild steel. Corros Sci 52(12):4046–4057
Goel R, Siddiqi WA, Ahmed B, Khan MS, Chaubey VM (2010) Synthesis characterization and corrosion inhibition efficiency of N-C2 (2E)-2-[4-(dimethylamino) benzylidene] hydrazinyl 2-oxo ethyl benzamide on mild steel. Desalination 263(1–3):45–57
Alaoui K, El Kacimi Y, Galai M, Touir R, Dahmani K, Harfi A, Touhami ME (2016) Anti-corrosive properties of polyvinyl-alcohol for carbon steel in hydrochloric acid media: electrochemical and thermodynamic investigation. J Mater Environ Sci 7(7):2389–2489
Herrag L, Hammouti B, Elkadiri S, Aouniti A, Jama C, Vezin H, Bentiss F (2010) Adsorption properties and inhibition of mild steel corrosion in hydrochloric solution by some newly synthesized diamine derivatives: experimental and theoretical investigations. Corros Sci 52(9):3042–3051
Obot IB, Obi-Egbedi NO (2010) Adsorption properties and inhibition of mild steel corrosion in sulphuric acid solution by ketoconazole: experimental and theoretical investigation. Corros Sci 52(1):198–204
El-Enin SA, Amin A (2015) Review of corrosion inhibitors for industrial applications. Int J Eng Res Rev 3(2):127–145
Raja PB, Sethuraman MG (2008) Natural products as corrosion inhibitor for metals in corrosive media—a review. Mater Lett 62(1):113–116
Shang Z, Zhu J (2021) Overview on plant extracts as green corrosion inhibitors in the oil and gas fields. J Market Res 15:5078–5094
Verma C, Hussain CM, Ebenso EE (2021) Organic corrosion inhibitors: synthesis, characterization, mechanism, and applications. Wiley, Hoboken
Verma C, Ebenso EE, Bahadur I, Quraishi MA (2018) An overview on plant extracts as environmental sustainable and green corrosion inhibitors for metals and alloys in aggressive corrosive media. J Mol Liq 266:577–590
Al-Amiery AA, Yousif E, Isahak WN, Al-Azzawi WK (2023) A review of inorganic corrosion inhibitors: types, mechanisms, and applications. Tribol Ind 44(2):313
Verma C, Alfantazi A, Quraishi MA, Rhee KY (2023) Significance of Hammett and Taft substituent constants on bonding potential of organic corrosion inhibitors: tailoring of reactivity and performance. Coord Chem Rev 495:215385
Verma C, Ebenso EE, Quraishi MA (2020) Molecular structural aspects of organic corrosion inhibitors: influence of–CN and–NO2 substituents on designing of potential corrosion inhibitors for aqueous media. J Mol Liq 316:113874
Branzoi V, Golgovici F, Branzoi F (2003) Aluminium corrosion in hydrochloric acid solutions and the effect of some organic inhibitors. Mater Chem Phys 78(1):122–131
Bahrami MJ, Hosseini SM, Pilvar P (2010) Experimental and theoretical investigation of organic compounds as inhibitors for mild steel corrosion in sulfuric acid medium. Corros Sci 52(9):2793–2803
Goyal M, Kumar S, Bahadur I, Verma C, Ebenso EE (2018) Organic corrosion inhibitors for industrial cleaning of ferrous and non-ferrous metals in acidic solutions: a review. J Mol Liq 256:565–573
Ahmed ESJ, Ganesh GM (2022) A comprehensive overview on corrosion in RCC and its prevention using various green corrosion inhibitors. Buildings 12(10):1682
Olajire AA (2017) Corrosion inhibition of offshore oil and gas production facilities using organic compound inhibitors—a review. J Mol Liq 248:775–808
Al-Lamei AJ, Muftin NK, Hussein FM, Mahdi AS (2020) Organic compounds as corrosion inhibitors from natural products. Int J Adv Res Phys Sci 7:25–35
Bastidas DM, Cano E, Mora EM (2005) Volatile corrosion inhibitors: a review. Anti-Corros Methods Mater 52(2):71–77
Valdez B, Schorr M, Cheng N, Beltran E, Salinas R (2018) Technological applications of volatile corrosion inhibitors. Corros Rev 36(3):227–238
Palanisamy G (2019) Corrosion inhibitors. Corros Inhibition 2019:1–24
Kadhim A, Betti N, Al-Bahrani HA, Al-Ghezi MK, Gaaz T, Kadhum AH, Alamiery A (2021) A mini review on corrosion, inhibitors and mechanism types of mild steel inhibition in an acidic environment. Int J Corros Scale Inhibition 10(3):861–884
Li Y, Zhang Y, Jungwirth S, Seely N, Fang Y, Shi X (2014) Corrosion inhibitors for metals in maintenance equipment: introduction and recent developments. Corros Rev 32(5–6):163–181
Ahamad I, Prasad R, Quraishi MA (2010) Inhibition of mild steel corrosion in acid solution by Pheniramine drug: experimental and theoretical study. Corros Sci 52(9):3033–3041
Angst UM, Büchler M, Schlumpf J, Marazzani B, Bakalli M (2016) Long-term field performance of an organic corrosion inhibitor for reinforced concrete. Mater Perform 55:36–40
Page CL, Ngala VT, Page MM (2000) Corrosion inhibitors in concrete repair systems. Mag Concr Res 52(1):25–37
Jamil HE, Shriri A, Boulif R, Bastos C, Montemor MF, Ferreira MG (2004) Electrochemical behaviour of amino alcohol-based inhibitors used to control corrosion of reinforcing steel. Electrochim Acta 49(17–18):2753–2760
Ghareba S, Omanovic S (2010) Interaction of 12-aminododecanoic acid with a carbon steel surface: towards the development of ‘green’corrosion inhibitors. Corros Sci 52(6):2104–2113
Barmatov E, Geddes J, Hughes T, Nagl M (2012) Research on corrosion inhibitors for acid stimulation. In: NACE corrosion, pp NACE-2012. NACE
Finšgar M, Jackson J (2014) Application of corrosion inhibitors for steels in acidic media for the oil and gas industry: a review. Corros Sci 86:17–41
Abiola OK (2006) Adsorption of 3-(4-amino-2-methyl-5-pyrimidyl methyl)-4-methyl thiazolium chloride on mild steel. Corros Sci 48(10):3078–90
Zhang Z, Wu J, Zhao X, Zhang Y, Wu Y, Su T, Deng H (2020) Life evaluation of organic coatings on hydraulic metal structures. Prog Org Coat 148:105848
Rathish RJ, Prabha SS, Dorothy R, Jancyrani S, Rajendran S, Singh G, Kumaran SS (2019) Corrosion issues in electronic equipments—an overview. Int J Corros Scale Inhibition 8(4):799–815
Valdez B, Cheng J, Flores F, Schorr M, Veleva L (2003) Application of vapour phase corrosion inhibitors for silver corrosion control in the electronics industry. Corros Rev 21(5–6):445–458
Ahmed MH, Al-Amiery AA, Al-Majedy YK, Kadhum AA, Mohamad AB, Gaaz TS (2018) Synthesis and characterization of a novel organic corrosion inhibitor for mild steel in 1 M hydrochloric acid. Results Phys 8:728–733
Crespo MD, Murillo AG, Torres-Huerta AM, Yanez-Zamora C, Carrillo-Romo FD (2009) Electrochemical behaviour of ceramic yttria stabilized zirconia on carbon steel synthesized via sol–gel process. J Alloy Compd 483(1–2):437–441
Sayed AR, Saleh MM, Al-Omair MA, Abd Al-Lateef HM (2019) Efficient route synthesis of new polythiazoles and their inhibition characteristics of mild-steel corrosion in acidic chloride medium. J Mol Struct 1184:452–461
Dagdag O, Safi Z, Erramli H, Wazzan N, Obot IB, Akpan ED, Verma C, Ebenso EE, Hamed O, El Harfi A (2019) Anticorrosive property of heterocyclic based epoxy resins on carbon steel corrosion in acidic medium: electrochemical, surface morphology, DFT and Monte Carlo simulation studies. J Mol Liq 287:110977
Pareek S, Jain D, Hussain S, Biswas A, Shrivastava R, Parida SK, Kisan HK, Lgaz H, Chung IM, Behera D (2019) A new insight into corrosion inhibition mechanism of copper in aerated 3.5 wt.% NaCl solution by eco-friendly Imidazopyrimidine dye: experimental and theoretical approach. Chem Eng J 358:725–42
Feng L, Zheng S, Zhu H, Ma X, Hu Z (2023) Detection of corrosion inhibition by dithiane self-assembled monolayers (SAMs) on copper. J Taiwan Inst Chem Eng 142:104610
Guo X, Huang H, Liu D (2021) The inhibition mechanism and adsorption behavior of three purine derivatives on the corrosion of copper in alkaline artificial seawater: structure and performance. Colloids Surf A 622:126644
Duran B, Bereket G, Duran M (2012) Electrochemical synthesis and characterization of poly (m-phenylenediamine) films on copper for corrosion protection. Prog Org Coat 73(2–3):162–168
Adeloju SB, Hughes HC (1986) The corrosion of copper pipes in high chloride-low carbonate mains water. Corros Sci 26(10):851–870
Habib K (1998) In-situ monitoring of pitting corrosion of copper alloys by holographic interferometry. Corros Sci 40(8):1435–1440
Qiang Y, Zhang S, Wang L (2019) Understanding the adsorption and anticorrosive mechanism of DNA inhibitor for copper in sulfuric acid. Appl Surf Sci 492:228–238
Srimathi M, Rajalakshmi R, Subhashini S (2014) Polyvinyl alcohol–sulphanilic acid water soluble composite as corrosion inhibitor for mild steel in hydrochloric acid medium. Arab J Chem 7(5):647–656
Jasim AS, Khadom AA, Rashid KH, Al-Azawi KF (2022) (3, 5-dimethyl-1H-pyrazol-1-y1)(4-((3, 4-dimethoxybenzylidene) amino) phenyl) methanone as a novel corrosion inhibitor for low-carbon steel in hydrochloric acid: synthesis, diagnosis, and application. Results Chem 4:100569
Pearson P, Cousins A (2016) Assessment of corrosion in amine-based post-combustion capture of carbon dioxide systems. Absorption-based post-combustion capture of carbon dioxide. 439–463
Gadow HS, Motawea MM (2017) Investigation of the corrosion inhibition of carbon steel in hydrochloric acid solution by using ginger roots extract. RSC Adv 7(40):24576–24588
Rbaa M, Abousalem AS, Rouifi Z, Lakhrissi L, Galai M, Zarrouk A, Lakhrissi B, Lakhrissi Y (2020) Selective synthesis of new sugars based on 8-hydroxyquinoline as corrosion inhibitors for mild steel in HCl solution-effect of the saturated hydrocarbon chain: theoretical and experimental studies. Inorg Chem Commun 118:108019
Binnig G, Quate CF, Gerber C (1986) Atomic force microscope. Phys Rev Lett 56(9):930
Baykara MZ, Schwarz UD (2017) Atomic force microscopy: methods and applications. In: Encyclopedia of spectroscopy and spectrometry. Elsevier, Amsterdam
Singh A, Ansari KR, Chauhan DS, Quraishi MA, Kaya S (2020) Anti-corrosion investigation of pyrimidine derivatives as green and sustainable corrosion inhibitor for N80 steel in highly corrosive environment: experimental and AFM/XPS study. Sustain Chem Pharm 16:100257
Zhu Y, Poplawsky JD, Li S, Unocic RR, Bland LG, Taylor CD, Locke JS, Marquis EA, Frankel GS (2020) Localized corrosion at nm-scale hardening precipitates in Al-Cu-Li alloys. Acta Mater 189:204–213
Asmatulu R, Khan WS (2019) Chapter 13-Characterization of electrospun nanofibers. Synth Applf Electrospun Nanofibers 257–281
Khanam PN, Al-Maadeed MA, Khanam PN (2015) Silk as a reinforcement in polymer matrix composites. In: Advances in silk science and technology, pp 143–170. Woodhead Publishing.
Edwards AA, Alexander BD. UV-visible absorption spectroscopy, organic applications
Aribou Z, Ouakki M, Khemmou N, Sibous S, Ech-chihbi E, Kharbouch O, Galai M, Souizi A, Boukhris S, Touhami ME, AlObaid AA (2023) Exploring the adsorption and corrosion inhibition properties of indazole as a corrosion inhibitor for brass alloy in HCl medium: a theoretical and experimental study. Mater Today Commun 37:107061
Dutta A (2017) Fourier transform infrared spectroscopy. Spectrosc Methods Nanomater Charact 73–93
Tran CD (2005) Principles, instrumentation, and applications of infrared multispectral imaging, an overview. Anal Lett 38(5):735–752
Dole MN, Patel PA, Sawant SD, Shedpure PS (2011) Advance applications of Fourier transform infrared spectroscopy. Int J Pharm Sci Rev Res 7(2):159–166
Kadhim A, Al-Okbi AK, Jamil DM, Qussay A, Al-Amiery AA, Gaaz TS, Kadhum AA, Mohamad AB, Nassir MH (2017) Experimental and theoretical studies of benzoxazines corrosion inhibitors. Results Phys 7:4013–4019
Liu D, Qiu YB, Tomoe Y, Bando K, Guo XP (2011) Interaction of inhibitors with corrosion scale formed on N80 steel in CO2-saturated NaCl solution. Mater Corros 62(12):1153–1158
Khodyrev YP, Batyeva ES, Badeeva EK, Platova EV, Tiwari L, Sinyashin OG (2011) The inhibition action of ammonium salts of O, O′-dialkyldithiophosphoric acid on carbon dioxide corrosion of mild steel. Corros Sci 53(3):976–983
Chen L, Lu D, Zhang Y (2022) Organic compounds as corrosion inhibitors for carbon steel in HCl solution: a comprehensive review. Materials 15(6):2023
Al-Amiery AA, Kadhum AA, Alobaidy AH, Mohamad AB, Hoon PS (2014) Novel corrosion inhibitor for mild steel in HCl. Materials 7(2):662–672
Cano E, Lafuente D (2013) Corrosion inhibitors for the preservation of metallic heritage artefacts. Corrosion and conservation of cultural heritage metallic artefacts. 570–594
Ech-chebab A, Dahmani K, Hsissou R, El Khouja O, Verma DK, Berdimurodov E, Erdoğan Ş, Tüzün B, Lachhab R, Ejbouh A, Galai M (2023) Anticorrosion properties of the epoxy polymer TGETBAU for mild steel in a solution of HCl (1.0 M): experimental and computational approaches. J Mol Struct 1284:135441
Erramli H, Dagdag O, Safi Z (2020) Trifunctional epoxy resin as anticorrosive material for carbon steel in 1 M HCl: experimental and computational studies. Surf Interface 21:100707
Abbout S, Hsissou R, Louiza O, Bourzami R, Erramli H, Chebabe D, Hajjaji N (2023) Anticorrosion propriety of new resin epoxy derived from phosphorus as inhibitor of steel corrosion in 0.5 M H2SO4. J Mol Struct 1294:136491
Kumar H, Dhanda T (2021) 5-Aminotetrazole a highly efficient corrosion inhibitor for mild steel in 0. N sulphuric acid: experimental & theoretical study. Chem Data Collect 33:100721
Kahkesh H, Zargar B (2023) Estimating the anti-corrosive potency of 3-nitrophthalic acid as a novel and natural organic inhibitor on corrosion monitoring of mild steel in 1M HCl solution. Inorg Chem Commun 2:111533
Swathi NP, Samshuddin S, Alamri AH, Rasheeda K, Alva VD, Aljohani TA (2022) Experimental and theoretical investigation of a new triazole derivative for the corrosion inhibition of carbon steel in acid medium. Egypt J Pet 31(2):15–21
Arrousse N, Fernine Y, Haldhar R, Berdimurodov E, Ichou H, Al-Zaqri N, Koudad M, Kim SC, Taleb M (2023) Corrosion protection studies of different alloys in 1 M HCl by benzimidazole derivative: combined molecular dynamic simulations/DFT. J Environ Chem Eng 11(3):109642
Obot IB, Obi-Egbedi NO, Odozi NW (2010) Acenaphtho [1, 2-b] quinoxaline as a novel corrosion inhibitor for mild steel in 0.5 M H2SO4. Corros Sci 52(3):923–6
Obot IB, Obi-Egbedi NO (2010) Indeno-1-one [2, 3-b] quinoxaline as an effective inhibitor for the corrosion of mild steel in 0.5 M H2SO4 solution. Mater Chem Phys 122(2–3):325–8
Senthilkumar G, Umarani C, Ramachandran A (2021) Investigation on corrosion inhibition effect of N-[4-(1, 3-benzo [d] thiazol-2-ylcarbamoyl) phenyl] quinoline-6-carboxamide as a novel organic inhibitor on mild steel in 1N HCl at different temperatures: experimental and theoretical study. J Indian Chem Soc 98(6):100079
Shkoor M, Jalab R, Khaled M, Shawkat TS, Korashy HM, Saad M, Su HL, Bani-Yaseen AD (2023) Experimental and theoretical investigations of the effect of bis-phenylurea-based aliphatic amine derivative as an efficient green corrosion inhibitor for carbon steel in HCl solution. Heliyon 9(10):e20254
Xu S, Li W, Zuo X, Zheng D, Zheng X, Zhang S (2019) Structural origin of corrosion inhibition effect over 2-(2-Hydroxyphenyl) benzothiazole on steel in HCl medium. Int J Electrochem Sci 14(6):5777–5793
Raviprabha K, Bhat RS (2023) Corrosion inhibition of mild steel in 0.5 M HCL by substituted 1, 3, 4-oxadiazole. Egypt J Petrol 32(2):1
Alamiery A (2021) Corrosion inhibition effect of 2-N-phenylamino-5-(3-phenyl-3-oxo-1-propyl)-1, 3, 4-oxadiazole on mild steel in 1 M hydrochloric acid medium: insight from gravimetric and DFT investigations. Mater Sci Energy Technol 4:398–406
Wang C, Lai C, Xie B, Guo X, Fu D, Li B, Zhu S (2018) Corrosion inhibition of mild steel in HCl medium by S-benzyl-O, O’-bis (2-naphthyl) dithiophosphate with ultra-long lifespan. Results Phys 10:558–567
Ech-Chihbi E, Nahlé A, Salim R, Benhiba F, Moussaif A, El-Hajjaji F, Oudda H, Guenbour A, Taleb M, Warad I, Zarrouk A (2020) Computational, MD simulation, SEM/EDX and experimental studies for understanding adsorption of benzimidazole derivatives as corrosion inhibitors in 1.0 M HCl solution. J Alloys Compds 844:155842
Gadow HS, Fawzy A, Khairy M, Sanad MM, Toghan A (2023) Experimental and theoretical approaches to the inhibition of carbon steel corrosion by thiophene derivative in 1 M HCl. Int J Electrochem Sci 18(7):100174
Benabdellah M, Yahyi A, Dafali A, Aouniti A, Hammouti B, Ettouhami A (2011) Corrosion inhibition of steel in molar HCl by triphenyltin2–thiophene carboxylate. Arab J Chem 4(3):243–247
Benzbiria N, Thoume A, Echihi S, Belghiti ME, Elmakssoudi A, Zarrouk A, Azzi M, Zertoubi M (2023) Coupling of experimental and theoretical studies to apprehend the action of benzodiazepine derivative as a corrosion inhibitor of carbon steel in 1M HCl. J Mol Struct 1281:135139
Al Garadi W, Jrajri K, El Faydy M, Benhiba F, El Ghayati L, Sebbar NK, Essassi EM, Warad I, Guenbour A, Bellaouchou A, Jama C (2022) 4-phenyl-decahydro-1H-1, 5-benzodiazepin-2-one as novel and effective corrosion inhibitor for carbon steel in 1 M HCl solution: a combined experimental and empirical studies. J Indian Chem Soc 99(11):100742
Azgaou K, Damej M, El Hajjaji S, Sebbar NK, Elmsellem H, El Ibrahimi B, Benmessaoud M (2022) Synthesis and characterization of N-(2-aminophenyl)-2-(5-methyl-1H-pyrazol-3-yl) acetamide (AMPA) and its use as a corrosion inhibitor for C38 steel in 1 M HCl. Experimental and theoretical study. J Mol Struct 1266:133451
Lu Y, Feng H, Xia H, Xia WH (2022) Carboxymethyl cellulose-polyaniline composites as efficient corrosion inhibitor for Q235 Steel in 1 M HCl solution. Int J Electrochem Sci 17(11):221180
El-Aouni N, Hsissou R, Safi Z, Abbout S, Benhiba F, El Azzaoui J, Haldhar R, Wazzan N, Guo L, Erramli H, Elharfi A (2021) Performance of two new epoxy resins as potential corrosion inhibitors for carbon steel in 1MHCl medium: combining experimental and computational approaches. Colloids Surf A 626:127066
Chahmout H, Ouakki M, Sibous S, Galai M, Arrousse N, Ech-chihbi E, Benzekri Z, Boukhris S, Souizi A, Cherkaoui M (2023) New pyrazole compounds as a corrosion inhibitor of stainless steel in 2.0 M H2SO4 medium: electrochemical and theoretical insights. Inorg Chem Commun 147:110150
Gupta SK, Mehta RK, Yadav M, Dagdag O, Mehmeti V, Berisha A, Ebenso EE (2023) Diazenyl derivatives as efficient corrosion inhibitors for mild steel in HCl medium: gravimetric, electrochemical and computational approach. J Mol Liq 382:121976
Fouda AE, Etaiw SE, Ismail MA, Abd El-Aziz DM, Eladl MM (2022) Novel naphthybithiophene derivatives as corrosion inhibitors for carbon steel in 1 M HCl: electrochemical, surface characterization and computational approaches. J Mol Liq 367:120394
Ech-chebab A, Missioui M, Guo L, El Khouja O, Lachhab R, Kharbouch O, Galai M, Ouakki M, Ejbouh A, Dahmani K, Dkhireche N (2022) Evaluation of quinoxaline-2 (1H)-one, derivatives as corrosion inhibitors for mild steel in 1.0 M acidic media: electrochemistry, quantum calculations, dynamic simulations, and surface analysis. Chem Phys Lett 809:140156
Benhiba F, Hsissou R, Benzekri Z, Belghiti ME, Lamhamdi A, Bellaouchou A, Guenbour A, Boukhris S, Oudda H, Warad I, Zarrouk A (2020) Nitro substituent effect on the electronic behavior and inhibitory performance of two quinoxaline derivatives in relation to the corrosion of mild steel in 1M HCl. J Mol Liq 312:113367
Benhiba F, Benzekri Z, Guenbour A, Tabyaoui M, Bellaouchou A, Boukhris S, Oudda H, Warad I, Zarrouk A (2020) Combined electronic/atomic level computational, surface (SEM/EDS), chemical and electrochemical studies of the mild steel surface by quinoxalines derivatives anti-corrosion properties in 1 mol⋅ L-1 HCl solution. Chin J Chem Eng 28(5):1436–1458
Ouakki M, Galai M, Benzekri Z, Verma C, Ech-chihbi E, Kaya SA, Boukhris S, Ebenso EE, Touhami ME, Cherkaoui M (2021) Insights into corrosion inhibition mechanism of mild steel in 1 M HCl solution by quinoxaline derivatives: electrochemical, SEM/EDAX, UV-visible, FT-IR and theoretical approaches. Colloids Surf A 611:125810
Laabaissi T, Benhiba F, Missioui M, Rouifi Z, Rbaa M, Oudda H, Ramli Y, Guenbour A, Warad I, Zarrouk A (2020) Coupling of chemical, electrochemical and theoretical approach to study the corrosion inhibition of mild steel by new quinoxaline compounds in 1 M HCl. Heliyon 6(5)
Abdelhadi RA, Ahmed ZE, Abouzeid AM (2023) Synthesis, spectroscopic analysis and electrochemical studies of novel organic compound based on N-alkylphthalazinone chemistry as corrosion inhibitor for carbon steel in 1M HCl. Int J Electrochem Sci 18(5):100121
Verma C, Quraishi MA, Lgaz H, Olasunkanmi LO, Sherif ES, Salghi R, Ebenso EE (2019) Adsorption and anticorrosion behaviour of mild steel treated with 2-((1H-indol-2-yl) thio)-6-amino-4-phenylpyridine-3, 5-dicarbonitriles in a hydrochloric acid solution: experimental and computational studies. J Mol Liq 283:491–506
Sharma D, Thakur A, Sharma MK, Sharma R, Kumar S, Sihmar A, Dahiya H, Jhaa G, Kumar A, Sharma AK, Om H (2023) Effective corrosion inhibition of mild steel using novel 1, 3, 4-oxadiazole-pyridine hybrids: synthesis, electrochemical, morphological, and computational insights. Environ Res 234:116555
Kalia V, Kumar P, Kumar S, Goyal M, Pahuja P, Jhaa G, Lata S, Dahiya H, Kumar S, Kumari A, Verma C (2022) Synthesis, characterization and corrosion inhibition potential of oxadiazole derivatives for mild steel in 1M HCl: electrochemical and computational studies. J Mol Liq 348:118021
Prashanth MK, Kumar CP, Prathibha BS, Raghu MS, Kumar KY, Jagadeesha MB, Mohana KN, Krishna H (2021) Effect of OH, NH2 and OCH3 groups on the corrosion inhibition efficacy of three new 2, 4, 5-trisubstituted imidazole derivatives on mild steel in acidic solutions: experimental, surface and DFT explorations. J Mol Liq 329:115587
Rbaa M, Ouakki M, Galai M, Berisha A, Lakhrissi B, Jama C, Warad I, Zarrouk A (2020) Simple preparation and characterization of novel 8-Hydroxyquinoline derivatives as effective acid corrosion inhibitor for mild steel: experimental and theoretical studies. Colloids Surf A 602:125094
Zeng J, Tan B, Zhang S, Li W (2022) The behavior of two indazole derivatives on the copper/sulfuric acid interface in terms of adsorption and corrosion inhibition. J Taiwan Inst Chem Eng 140:104567
Gong W, Xu B, Yin X, Liu Y, Chen Y, Yang W (2019) Halogen-substituted thiazole derivatives as corrosion inhibitors for mild steel in 0.5 M sulfuric acid at high temperature. J Taiwan Inst Chem Eng 97:466–79
Döner A, Solmaz R, Özcan M, Kardaş G (2011) Experimental and theoretical studies of thiazoles as corrosion inhibitors for mild steel in sulphuric acid solution. Corros Sci 53(9):2902–2913
Kumar S, Kalia V, Goyal M, Jhaa G, Kumar S, Vashisht H, Dahiya H, Quraishi MA, Verma C (2022) Newly synthesized oxadiazole derivatives as corrosion inhibitors for mild steel in acidic medium: experimental and theoretical approaches. J Mol Liq 357:119077
Ettahiri W, Al Ati G, Salim R, Chkirate K, Hammouti B, Achour R, Rais Z, Baouid A, Essassi EM, Taleb M (2024) Synthesis and characterization of pyrazole-acetamide schiff bases as highly effective inhibitors for mild steel in 1 M HCl. J Ind Eng Chem
Al Kiey SA, El-Sayed AA, Khalil AM (2024) Controlling corrosion protection of mild steel in acidic environment via environmentally benign organic inhibitor. Colloids Surf A 20(683):133089
Belghiti ME, El Ouadi Y, Echihi S, Elmelouky A, Outada H, Karzazi Y, Bakasse M, Jama C, Bentiss F, Dafali A (2020) Anticorrosive properties of two 3, 5-disubstituted-4-amino-1, 2, 4-triazole derivatives on copper in hydrochloric acid environment: Ac impedance, thermodynamic and computational investigations. Surf Interfaces 21:100692
Ech-chihbi E, Salim R, Ouakki M, Koudad M, Guo L, Azam M, Benchat N, Rais Z, Taleb M (2023) Corrosion resistance assessment of copper, mild steel, and aluminum alloy 2024–T3 in acidic solution by a novel imidazothiazole derivative. Mater Today Sustain 24:100524
Farahati R, Ghaffarinejad A, Mousavi-Khoshdel SM, Rezania J, Behzadi H, Shockravi A (2019) Synthesis and potential applications of some thiazoles as corrosion inhibitor of copper in 1 M HCl: experimental and theoretical studies. Prog Org Coat 132:417–428
Long W, Xiao W, Liu Q, Ge P, Liang T (2022) 2-(4-Morpholinothio) benzothiazole as a high-efficiency corrosion inhibitor for copper in 0.5 M H2SO4 solution. Int J Electrochem Sci 17(11):221111
Zeng W, Li W, Tan B, Liu J, Chen J (2021) A research combined theory with experiment of 2-Amino-6-(Methylsulfonyl) Benzothiazole as an excellent corrosion inhibitor for copper in H2SO4 medium. J Taiwan Inst Chem Eng 128:417–429
Yan T, Zhang S, Feng L, Qiang Y, Lu L, Fu D, Wen Y, Chen J, Li W, Tan B (2020) Investigation of imidazole derivatives as corrosion inhibitors of copper in sulfuric acid: combination of experimental and theoretical researches. J Taiwan Inst Chem Eng 106:118–129
Sherif EM, Park SM (2006) Effects of 2-amino-5-ethylthio-1, 3, 4-thiadiazole on copper corrosion as a corrosion inhibitor in aerated acidic pickling solutions. Electrochim Acta 51(28):6556–6562
Zhang J, Li H (2020) 2-(2-chlorophenyl)-1H-benzimidazole as a new corrosion inhibitor for copper in sulfuric acid. Int J Electrochem Sci 15(6):5362–5372
Salim R, Ech-chihbi E, Fernine Y, Koudad M, Guo L, Berdimurodov E, Azam M, Rais Z, Taleb M (2024) Inhibition behavior of new ecological corrosion inhibitors for mild steel, copper and aluminum in acidic environment: theoretical and experimental investigation. J Mol Liq 393:123579
Broch L, Crespo JS, Beltrami LV, Giovanela M (2024) Copper corrosion inhibition in acidic aqueous media through tolyltriazole application: performance analysis. Chem Eng Commun 19:1–6
Funding
The author does not have any financial assistance for the research.
Author information
Authors and Affiliations
Contributions
Anshula Sharma wrote the introduction and literature survey. Dr. Jasdeep Kaur compiled the methodologies that can be used to find the inhibition. Dr. Akhil Saxena reviewed the whole manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, A., Kaur, J. & Saxena, A. Anti-corrosive Properties of Synthetic Organic Compounds: A Review. J Bio Tribo Corros 10, 80 (2024). https://doi.org/10.1007/s40735-024-00884-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-024-00884-8