Abstract
Joint replacement surgeries are progressing at an increased rate in the USA, with around 1 million surgeries being performed annually. While these orthopedic implants provide a solution for a damaged joint, debris such as metal ions generated due to the wear and corrosion of the implants prove to be of considerable risk of systemic and local toxicity. As an effective diagnostic tool, there is a need to develop a patient-friendly, cost-effective, and rapid mode of metal ion detection in orthopedic patients with various implants. In this study, an electrochemical biosensor chip (Dropsens) is proposed as a diagnostic tool to detect metal ions through changes in impedance. In vivo studies were conducted using CoCrMo particles intraarticularly injected into mice. Then, blood was collected on 0th day, 14th day, and 28th day after injection and was centrifuged to obtain serum. The serum was subject to various electrochemical techniques, such as Electrochemical Impedance Spectroscopy (EIS) and cyclic voltammetry (CV). Additionally, standardization experiments were performed using different concentrations of CoCrMo wear particles suspended in PBS and mice serum. EIS results reveal a negative correlation between impedance (Rp) and the metal ion concentration in the standardization experiments. EIS and CV standardization data were used to obtain a regression equation which were later used to estimate the metal ion concentrations in experimental samples. Here, the positive control group shows the highest impedance at any frequency followed by the 28th-day experimental mice serum as seen on the bode plot. CV experimental data reveal a possible correlation between CV area and the metal ion concentrations. In this study, experimental mice models were established to mimic the possible diffusion of metal particles from implants. Dropsens electrochemical biosensors were efficiently employed to detect metal ions in serum through changes in impedance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Joint replacement surgeries of orthopedic implants are progressing at an increased rate in the USA, with around 1 million surgeries being performed annually [1]. These implants are made of materials such as metals, ceramic, or polyethylene and are biocompatible as well as safe to use [2]. While orthopedic implants provide a solution for a damaged joint, metal debris generated due to the wear and corrosion of the implants proves to be of considerable risk of systemic and local toxicity [3]. CoCrMo implants are widely used metal-on-metal implants due to their increased resistance to wear compared to the metal on polyethylene implants [4]. But recent clinical reports suggested that the failure rate of these implants is steadily increasing [5]. It was observed that metal ions generated from the wear of CoCrMo alloys diffuse into the surrounding tissues as well as to other locations, thereby leading to implant failure owing to inflammatory immune responses [6].
Previously, efforts have been made to detect metal ions released from implants from the serum, urine, and synovial fluid. While the metal ion content in the blood can be an indicator of osteolysis of bones [7], this also helps monitor implant activity in patients with metal-on-metal implants. In 2008, a translational study was conducted on the serum of twenty-six patients with existing metal-on-metal implants who had to undergo revision surgeries, revealing increased metal ion levels in their serum. This suggests a possible correlation between the metal ion content and implant failure [8]. With the development of technology, inductively coupled plasma mass spectroscopy (ICP-MS) has been adapted to measure the metal ion concentration in solution. Many studies report the quantification of metal ions by ICP-MS from simulated body fluids to study tribocorrosion and corrosion kinetics [9, 10]. Showing that the method is one of the most sensitive and powerful tools to measure metal ion content in biological fluids [9]. However, this method’s requirement of large sample volumes is a major shortcoming. In the previously translational study, around 10 mL of blood was collected from each patient through intravenous catheters due to a minimum requirement of 5 mL needed for ICP-MS analysis [8, 11]. Moreover, other disadvantages of this method include economic unsuitability and unavailability of resources while also requiring trained personnel to operate the machines [12]. Therefore, there is a need for an economical, accurate, and rapid metal ion detection method requiring lesser sample volumes from patients.
Electrochemical tests have been employed to detect heavy metal ions in environmental samples and water to determine the detrimental effects of these metal ions in humans [13]. These tests can also be used to detect the concentration of heavy metals in serum and other biological fluids by passing a current through a solution and measuring the response signal [14]. The presence of heavy metal ions in the solution (electrolyte) causes various changes in electrochemical parameters, such as impedance, conductivity, current, and voltage. Electrochemical signals can be used to analyze and correlate using existing equivalent circuits to obtain quantitative data. [15, 16]
Screen-printed electrodes were first introduced in 1997 and are currently gaining use as a cheap, affordable, and convenient method to study electrochemical reactions [13, 16, 19]. They offer multiple advantages over general clinical diagnostic methods, such as miniaturization and easy sample preparation [20]. Our previous study developed a screen-printed electrode system to examine metal release kinetics in BCS solution. After the estimation of the metal ions by ICP-MS, the Dropsens biosensor technique was compared and concluded to estimate metal ions in biological solutions effectively. The study revealed preliminary data that could aid in the establishment of an affordable, patient-friendly point-of-care (POC) device akin to a glucometer to monitor implant performance [21].
In this study, mice models are designed to validate the sensor as a diagnostic tool further. The electrochemical Dropsens biosensor is used to detect electrical impedance and cyclic voltammetry in the mice serum that could directly correlate to the presence and concentration of metal ions [22]. Therefore the results can provide a rapid method of detection for metal ions requiring a small volume of biological serum. This can further be developed into a hand-held device through the technologically developing field of portable potentiostats [23, 24].
2 Materials and Methods
2.1 In Vivo Experimental Group
C3H/HeJ mice (Jackson Laboratory, ME) were used in this study and injected with either phosphate buffer solution (PBS, control group) or CoCrMo < 200-nm wear particles (Co-67%, Cr-26%, Mo-7%) from Bioengineering solutions (Chicago, IL, tested group). The IRB team approved IRB paperwork at the UIC, College of Medicine Rockford campus (IRB ID#: 1,684,850–3, dated. 02/18/2021). The experiments in each group were conducted in triplicate (N = 3). Each mouse was tagged using an ear punch for identification. Prior to experiments, CoCrMo wear particles were dispersed using a Probe Sonicator (Branson Ultrasonics, CT, USA) and the size distribution was estimated using Dynamic light scatter (DLS) to obtain particles < 200 nm in size (Fig. 1a). ½-ml syringe with 27G needle was used for injection of samples and 3-ml disposable syringe Luer slip was used in blood sample collection. Also used in blood collection are a heparinized micro-hematocrit capillary tube, 0.5-M Ethylenediaminetetraacetic acid (EDTA) Solution, and BD Vacutainer lithium heparin additive.
On the 0th day, after collection of blood through tail clip assay, the CoCrMo particles suspended in PBS (50 ug/mL concentration) were injected into the knee capsule of C3h/HEJ mice (intraarticularly) for the treatment group (N = 3) The mice in the control group were injected with just PBS (N = 3). On the 14th day, the blood was collected through tail clip assay. On the 28th day, the mice were euthanized, and the blood was collected through cardiac puncture from the treatment group mice. After centrifugation at 800 (rpm) for 5 min, serum was isolated from the supernatant, and various electrochemical tests were performed.
For the experimental models, 4 groups were compared and studied: (I) 0th day (negative control), (II) 14th day, (III) 28th day, and (IV) 50-ug/mL particles in mice serum.
2.2 Electrochemical Tests
All electrochemical studies were carried out using Gamry Interface 1000 potentiostat and Dropsens screen-printed electrode (Fig. 1b), which consists of a gold working electrode (WE), platinum control electrode (CE), and silver reference electrode (RE). Electrochemical tests that were employed are Open-Circuit Potential (OCP), Electrical impedance spectroscopy (EIS), and Cyclic Voltammetry (CV). In addition, a calibration curve of current vs. concentration was generated using the cyclic voltammetry data [25].
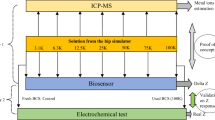
All experiments were conducted at room temperature, and 100 µl of each sample was used. EIS was conducted with a frequency range from 100 kHz to 0.01 Hz and an amplitude of 10 mV. Cyclic voltammetry was conducted at a scan rate of 100 mV/s, ranging from –1.5 V to + 1.5 V. After the electrochemical studies, the obtained data (intermediate values) was used to plot the Bode and Nyquist plots. The cyclic voltammetry graph was plotted as current vs. voltage, and the oxidation peaks and area of the curves were estimated. Overall, the tests were divided into three stages, standardization of CoCrMo particles in PBS/serum, blind study, and in vivo experimental mice model validation.
2.3 Standardization
(A) Standardization with PBS: For standardization, initially 10 ug/mL, 20 ug/mL, 30 ug/mL, 40 ug/mL, and 50 ug/mL of CoCrMo particles suspended in PBS were used to obtain cyclic voltammetry data (concentration vs current I) and EIS data (concentration vs impedance). The samples were diluted and sonicated, after which the size distribution was studied using Dynamic Light Scatter (DLS).
(B) Standardization with serum: To mimic the biological conditions of CoCrMo particles in blood serum, an effort has been made with second standardization with mice serum. BALB/c mice were selected for this step. After the collection of blood through the cardiac puncture, serum was isolated, and CoCrMo particles at different concentrations (10, 20, 30, 40, and 50 ug/mL) were suspended in mice serum, and the electrochemical tests were performed.
(C) Blind study: To further validate the standard regression equation, a blind study was conducted with a pre-set concentration of CoCrMo particles (unknown to the author) suspended in collected BALB/c mice serum. The resulting Rp (impedance) value after fitting using the CPE circuit in Gamry was inserted into the standard regression equation to obtain the final concentration.
3 Results
3.1 Standardization- PBS
3.1.1 Electrical Impedance Spectroscopy (EIS)
The fitted EIS data were visualized as Bode and Nyquist plots. Nyquist plot shows the real and imaginary parts of the vector quantity of impedance (Fig. 2a) and Bode plot exhibits the impedance and phase angle with frequency (Fig. 2b). As there is only one time constant in both Bode and Nyquist plots, the Randles Constant Phase Element (CPE) equivalent circuit is used (Fig. 1c) to estimate the Rp and capacitance using Gamry Electrochem software. The decrease in impedance was observed for the increasing concentrations in Bode and Nyquist plots, indicating the current methodology’s capability to measure the concentration of metal particles in the solution.
3.1.2 Cyclic Voltammetry (CV)
In the cyclic voltammetry of particles suspended in PBS, the oxidative peaks provided a relation between the concentration and the current (A). As the concentration increased from 10 to 50 ug/mL, the oxidative peaks reduced in intensity, i.e., the current decreased (Fig. 2c). While this provided a negative correlation, the next standardization with the mice serum was expected to show a better correlation as it could precisely recapitulate human blood and serum.
3.2 Standardization-Serum
3.2.1 Electrochemical Impedance Spectroscopy
The Nyquist plot again shows the imaginary and real parts of the vector quantity impedance (Fig. 3a). The Bode plot also reveals decreasing impedance for increasing concentrations in mice serum (Fig. 3b). After being fitted with the CPE equivalent circuit, polarization resistance (Rp) values can be obtained. Rp exhibits the linear relationship to the concentration of CoCrMo particles after being fitted with the linear regression equation (Fig. 3c). The similar linear relationship between Rp and particle concentrations supports the methodology that can also be employed in obtained serum.
Another approach to analyze the data is the analysis of impedance in the Bode plot. The percentage change in impedance between the standards is calculated using the impedance z value at frequency 0.0125 Hz using the formula: %ΔZ = (ΔZ/Z0) × 100 where ΔZ =|(Zf – Z0)|. Zf and Z0 are impedances of solution with and without CrCoMo particles, respectively. The reduction of impedance increases as CoCrMo concentration increases (Fig. 3d). CoCrMo particles' presence reduces the biosensor’s surface impedance, which also demonstrates the ability of metal ion detection.
3.2.2 Cyclic Voltammetry
Contrary to the expected results, no oxidation or reduction peaks were seen in any of the concentrations for particles suspended in mice serum. Figure 4a shows the cyclic voltammetry (Potential vs. Current) for 50 ug/mL concentration in PBS and serum samples. On the other hand, the CV area divulged a possible correlation with increasing concentration (Fig. 4b exhibits the linear relationship between the hysteresis areas of cyclic voltammetry to the concentrations of CoCrMo particles. The linear equation was also used to validate the blind study).
3.3 Blind Study
After performing the electrochemical tests on the blind study solution with an unknown concentration of metal particles, three parameters were collected to validate the obtained model from standardization. Impedance data were acquired from the Bode plot at the frequency of 0.0125 (Hz), Rp was obtained after fitting using the CPE circuit in the Gamry software, and the hysteresis areas were attained from cyclic voltammetry. Every test was repeated three times (N = 3), and the results were averaged.
The following calculation is performed using the linear equation from Sect. 3.2.1 (Fig. 3c.
a Nyquist plot showing Z real vs Z imaginary for CoCrMo particles in mice serum. b Bode plot showing phase angle and impedance at different frequencies for CoCrMo particles in mice serum. c Standard graph showing the correlation between Rp (impedance) and concentration of CoCrMo particles in mice serum. d %change in impedance for differing concentrations of CoCrMo particles in mice serum
Since the average Rp is equal to 3.69 × 106 (Ohms),
Concentration = 44.01 ug/mL.
The obtained concentration was in accordance with the pre-set concentration of 45 ug/mL; hence, the standardization was validated. Similarly, the value of impedance at 0.0125 Hz frequency for the blind study was inserted into the obtained equation (See Fig. 4c), and the following calculation was done,
Since the average of impedance at 0.0125 Hz is 1.20 × 10.6 , Concentration = 30.7692 ug/mL
Figure 4b exhibits the linear relationship between the hysteresis area of cyclic voltammetry to the concentrations of CoCrMo particles. The linear equation was also used to validate the blind study.
Since the average of hysteresis area is 8.17 × 10–4 , Concentration = 35.5 ug/mL
A comparison of the predicted value for the blind study is provided in Table 1. Hence, the Rp standard curve demonstrates the most accurate estimation of the concentration of CoCrMo particles in mice serum.
3.4 In Vivo Mice Model
3.4.1 Electrochemical Impedance Spectroscopy (EIS)
The Bode and Nyquist plots from the four groups studied reveal important information that coincides with the study’s hypothesis (Fig. 5a, b). The 28th-day serum revealed close changes in impedance to the positive control. In contrast, the negative control and 14th-day serum showed some difference, suggesting impedance’s role in a possible increase in metal concentration in mice’s bloodstream. After fitting with the CPE circuit, the Rp was inserted into the standard equation, and the concentration was found to be 50.9 ug/mL, which is similar to the injected concentration of particles. The %change in impedance between the experimental model samples was also observed (Fig. 5c). Similarly, inserting the raw Zmod value into the standard equation obtained from the mice serum standard Zmod values, the concentration was estimated as 36.42 ug/mL.
a Nyquist plot showing Z real vs Z imaginary for the four experimental groups under study. b Bode plot showing phase angle and impedance at different frequencies for the four experimental groups. c %change in impedance between the four different experimental groups. d Cyclic voltammetry graph between negative control and 28th-day experimental group
3.4.2 Cyclic Voltammetry
The cyclic voltammetry graph represented as current vs. potential did not reveal any expected oxidation or reduction peaks (Fig. 5d) in the experimental groups. But since the standardization revealed a possible correlation between the CV area and the concentration, the standard equation was used to calculate the concentration in the 28th-day final mice serum. This was revealed to be about 25 ug/mL and was not in accordance with the concentration obtained through EIS. So, cyclic voltammetry needs to be further employed and studied to obtain reliable and consistent results.
4 Discussion
Many experiments have been conducted to study metal ions in biological fluids. In an in vitro experiment to study tribocorrosion, PBS and albumin at the optimal human body temperature of 37 degrees Celsius were studied using ICP-MS. Albumin was seen to increase the metal ion release [9]. The interaction of the implant with the proteins and other biological substances in the environment could contribute to the metal ion release [26]. In our previous study, Bovine Calf Serum (BCS) was used to study the presence of metal ion concentration in accordance with the change in impedance. While this successful study failed to accurately simulate the biological (human) blood and the proteins in the serum. Various follow-up translational study reports using patient serum have been published [5, 27], but the use of ICP-MS to determine the metal ion content has had limitations [8, 11, 12]. In this study, the mice models are used to imitate patient blood serum and diffusing particles that travel in the bloodstream. By injecting the CoCrMo particles into the knee capsule, the released metal particles from a patient’s implant are modeled in mice, thereby mimicking the implant microenvironment.
Many techniques have been used to detect metal ions in solutions. But electrochemical analysis can be considered a forerunner due to its ease of access and compact instrumentation. Dropsens biosensors are screen-printed electrodes of length 3.4 cm. These are powerful tools requiring only 100 µL of the solution to cover the working electrode (4 mm diameter), a counter electrode, and a reference electrode. They can be subject to electrical tests when connected to a potentiostat. These electrode chips are also disposable and would reduce the risk of contamination between different analyte solutions. Therefore, they are a suitable candidate for studying biological samples. In this study, multiple electrochemical tests were carried out on mice serum. One limitation in using the Dropsens biosensor chip could be the small working surface area of the working electrode [28].
4.1 Detection of Metal Particles in Serum from Screen-Printed Electrode
The screen-printed electrodes have been widely used in biomedical applications to detect different biomarkers through electrochemical techniques [17]. Based on the surface, antibodies or aptamers can be fixed on the electrodes. Depending on the specificity of antibodies and aptamers, only the intended biomarkers can be attached to the electrodes, reflecting the change in the electrochemical signals [29]. In one such study, an aptamer immobilized on the graphite screen-printed electrode coated with gold nanoparticle sensors was used to detect the MUC1 tumor biomarker. With the higher concentration of MUC1 protein, EIS and differential pulse voltammetry results exhibited qualitative trends. [30] Another study proposed a screen-printed electrode modified with N-(4-amino-3-methoxyphenyl)-methanesulfonamide to detect Δ9-tetrahydrocannabinol (Δ9-THC) in saliva through chronoamperometric reduction. The study indicated high specificity to detect Δ9-THC in the methodology, but further research was suggested to increase the accuracy of the testing. Compared to previous research, no surface modification is required in the current study. Since the metal particles are conductive, their sediments change the electrical properties on the surfaces of the electrodes, affecting the results in EIS.
The EIS results reveal a negative correlation with our study’s increasing metal ion and particle concentration. The results presented here agree with the notion that higher concentrations of metal ions assist in the easier flow of electric current and hence lesser impedance Rp in a Randles CPE circuit. This result is also following our previous work with BCS [21]. Figures 6 &a show that the serum proteins, in the absence of metal ions, crowd the working surface, thereby resisting the flow of current and increasing impedance. On the other hand, the metal ions assist the current flow, thereby decreasing the impedance. While the 28th-day serum showed the same concentration as the injected particle concentration, the result is counterintuitive. When the particles/ions enter the mice’s bloodstream, they might get settled in the liver, spleen, and other organs; therefore, the final concentration obtained here may not be conclusive, and further studies need to be conducted to arrive at tangible deductions [31,32,33].
4.2 Cyclic Voltammetry of Serum with Metal Particles
Cyclic voltammetry is typically used to study solutions’ oxidative and reductive capabilities. It has been employed mainly to study redox status in correlation with disease progression in biological samples [34, 35]. Free metal ions produced due to the wear of implants can actively undergo redox reactions and cause oxidative stress to the localized cells [36, 37]. Considering this, cyclic voltammetry can further be used to establish a possible relation with the metal ion concentration based on Fenton reaction [37]. While the EIS data exhibited evidence for the correlation between impedance and metal ion/particle content, cyclic voltammetry failed to reveal solid inferences [25]. The serum standardization did not show any oxidation or reduction peaks. This could be due to the various proteins dampening the particle’s reactivity. Proteins such as albumin in the blood serum could interfere with the Fenton reaction involved in the oxidative stress reactions by chelation to metals [36] thereby reducing the number of redox reactions. This was further validated when the initial standardization with the particles suspended in PBS (with no presence of metal chelating proteins) revealed distinct oxidation and reduction peaks. CoCrMo wear particles generated in an in vitro study under tribological conditions suggested that the particles may be surrounded by the organic residues in solution and would therefore prevent further oxidation of the particles. This could explain the absence of oxidation peaks in cyclic voltammetry [38]. In conclusion, while CV area revealed some correlation, blind study and experimental data did not confirm with the expected concentrations. Hence, definite conclusions could not be made. This could be extensively analyzed in future experiments.
4.3 Limitations of the Work
The CV area seemed to divulge a possible correlation, albeit weak, with the different concentrations of CoCrMo particles in the standard experiments. But when using the standard equation to obtain the concentration in the 28th-day collected serum, the results were far lower than the injected particle concentration. Hence, this needs to be further validated with a more extensive study group. Furthermore, the study also consisted of a few animals and proved to be a limitation, as observed in the cyclic voltammetry data. This study can further be expanded with a larger sample size to arrive at better conclusive results. Other areas of limitations where future work can be imparted include the concentrations of the CoCrMo alloy used. While the concentrations of the metal alloy used were compliant with the American Society for Testing and Materials (ASTM-F75) standards for medical implants, exploring other concentrations may reveal important insights [39]. The Rp difference measured is assumed to be from metal ions, and free radicals may also cause the change in impedance. This would need further analysis.
4.4 Clinical Application and Future Directions
The Dropsens biosensor can be developed as a hand-held device to monitor implant performance using small amounts of patient serum [20]. Regular analysis of the metal ion content in patients with the implant could help in earlier diagnosis of implant corrosion and mitigates the chances of metal ion toxicity in the liver and other susceptible organs. Through the developing technology of wireless potentiostats and software applications, mobile phones can be used to keep track of the metal ions and also provide easy access to statistical reports to physicians [40]. Through further advancements, implantable biosensors for real-time detection of metal ions in serum could be developed [23]. Typical blood levels of Co and Cr in patients with unilateral, well-functioning hip implants are 30 and 45 nmol/L (1.7 and 2.3 μg/L), respectively. Hence, determining the sensitivity and detection limit of the proposed biosensor would help in the establishment of the point-of-care device.
5 Conclusion
The main conclusions from this study are as follows.
-
In this study, experimental mice models to study metal ion in serum were established through intra-articular injection of CoCrMo particles.
-
Dropsens biosensors were used to detect metal ion concentration through EIS and CV.
-
Further, compared to the existing method of metal ion detection in a biological sample, such as the ICP-MS method of analysis, this biosensor would require a smaller sample size and shorter response time.
-
This biosensor would be an economically affordable point-of-care device that patients can regularly use to monitor implant performance [41].
-
The necessity for any future revision surgery can be closely tracked using this biosensor in orthopedic patients with metal-on-metal implants [42].
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Maradit Kremers H et al (2015) Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am 97:1386–1397
Shayesteh Moghaddam N et al (2016) Metals for bone implants: safety, design, and efficacy. Biomanufact Rev 1:1
Bijukumar DR et al (2018) Systemic and local toxicity of metal debris released from hip prostheses: a review of experimental approaches. Nanomed Nanotechnol Biol Med 14:951–963
Buford A, Goswami T (2004) Overview of metal-on-polyethylene, metal-on-metal, and ceramic hip wear mechanisms. JMBM. https://doi.org/10.1515/JMBM.2004.15.1-2.73
Neumann DRP et al (2010) Long-term results of a contemporary metal-on-metal total hip arthroplasty: a 10-year follow-up study. J Arthroplasty 25:700–708
Koronfel MA et al (2018) Understanding the reactivity of CoCrMo-implant wear particles. Npj Mater Degrad 2:1–5
Wynn-Jones H et al (2011) Silent soft tissue pathology is common with a modern metal-on-metal hip arthroplasty. Acta Orthop 82:301–307
De Smet K et al (2008) Metal ion measurement as a diagnostic tool to identify problems with metal-on-metal hip resurfacing. J Bone Jt Surg 90:202–208
Espallargas N, Torres C, Muñoz AI (2015) A metal ion release study of CoCrMo exposed to corrosion and tribocorrosion conditions in simulated body fluids. Wear 332–333:669–678
Radice S et al (2019) Investigation of CoCrMo material loss in a novel bio-tribometer designed to study direct cell reaction to wear and corrosion products. Biotribol Oxf 18:100090
Sarmiento-González A, Marchante-Gayón JM, Tejerina-Lobo JM, Paz-Jiménez J, Sanz-Medel A (2008) High-resolution ICP–MS determination of Ti, V, Cr Co, Ni, and Mo in human blood and urine of patients implanted with a hip or knee prosthesis. Anal Bioanal Chem 391:2583–2589
Bansod B, Kumar T, Thakur R, Rana S, Singh I (2017) A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens Bioelectron 94:443–455
Barton J et al (2016) Screen-printed electrodes for environmental monitoring of heavy metal ions: a review. Microchim Acta 183:503–517
Yantasee W et al (2007) Electrochemical sensors for the detection of lead and other toxic heavy metals: the next generation of personal exposure biomonitors. Environ Health Perspect 115:1683–1690
Zhang JXJ, Hoshino K (2019) Electrical transducers: electrochemical sensors and semiconductor molecular sensors. Mol Sens Nanodev. https://doi.org/10.1016/B978-0-12-814862-4.00004-1
Su L, Liao X, Huang Z (2019) A theoretical study on resistance of electrolytic solution: measurement of electrolytic conductivity. Results Phys 13:102274
Mincu N-B et al (2020) Screen-printed electrodes (SPE) for in vitro diagnostic purpose. Diagnostics 10:517
García-Miranda Ferrari A, Rowley-Neale SJ, Banks CE (2021) Screen-printed electrodes: transitioning the laboratory in-to-the field. Talanta Open 3:100032
Hayat A, Marty JL (2014) Disposable screen printed electrochemical sensors: tools for environmental monitoring. Sensors 14:10432–10453
Zhang W, Wang R, Luo F, Wang P, Lin Z (2020) Miniaturized electrochemical sensors and their point-of-care applications. Chin Chem Lett 31:589–600
Mathew MT et al (2018) SMART biosensor for early diagnostic detection of metal ion release in orthopedic patients: initial outcome. J Bio- Tribo-Corros 4:74
Kerr E et al (2020) A comparison of commercially available screen-printed electrodes for electrogenerated chemiluminescence applications. Front Chem 8:628483
Beach RD, Conlan RW, Godwin MC, Moussy F (2005) Towards a miniature implantable in vivo telemetry monitoring system dynamically configurable as a potentiostat or galvanostat for two- and three-electrode biosensors. IEEE Trans Instrum Meas 54:61–72
Rowe AA et al (2011) CheapStat: an open-source, “do-it-yourself” potentiostat for analytical and educational applications. PLoS ONE 6:e23783
Chen L-D, Wang W-J, Wang G-J (2021) Electrochemical detection of electrolytes using a solid-state ion-selective electrode of single-piece type membrane. Biosensors 11:109
Yan Y, Neville A, Dowson D, Williams S (2006) Tribocorrosion in implants—assessing high carbon and low carbon Co–Cr–Mo alloys by in situ electrochemical measurements. Tribol Int 39:1509–1517
Levine BR et al (2013) Ten-year outcome of serum metal ion levels after primary total hip arthroplasty: a concise follow-up of a previous report*. J Bone Joint Surg Am 95:512–518
Zhao L, Han H, Ma Z (2018) Improved screen-printed carbon electrode for multiplexed label-free amperometric immuniosensor: addressing its conductivity and reproducibility challenges. Biosens Bioelectron 101:304–310
Florea A, Taleat Z, Cristea C, Mazloum-Ardakani M, Săndulescu R (2013) Label free MUC1 aptasensors based on electrodeposition of gold nanoparticles on screen printed electrodes. Electrochem Commun 33:127–130
Wanklyn C et al (2016) Disposable screen printed sensor for the electrochemical detection of delta-9-tetrahydrocannabinol in undiluted saliva. Chem Cent J 10:1
Keegan GM, Learmonth ID, Case CP (2007) Orthopaedic metals and their potential toxicity in the arthroplasty patient: a review of current knowledge and future strategies. J Bone Joint Surg Br 89B:567–573
Urban RM et al (2000) Dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with hip or knee replacement. J Bone Joint Surg Am 82:457–476
Merritt K, Brown SA (1996) Distribution of cobalt chromium wear and corrosion products and biologic reactions. Clin Orthop. https://doi.org/10.1097/00003086-199608001-00020
Mantovani G et al (2002) Quantitative evaluation of oxidative stress, chronic inflammatory indices and leptin in cancer patients: correlation with stage and performance status. Int J Cancer 98:84–91
Mittal A et al (2010) The redox status of experimental hemorrhagic shock as measured by cyclic voltammetry. Shock Augusta Ga 33:460–466
Primožič J et al (2021) Risk assessment of oxidative stress induced by metal ions released from fixed orthodontic appliances during treatment and indications for supportive antioxidant therapy: a narrative review. Antioxidants 10:1359
Kanti Das T, Wati MR, Fatima-Shad K (2015) Oxidative stress gated by Fenton and Haber Weiss reactions and its association with Alzheimer’s disease. Arch Neurosci 2:1
Pourzal R, Catelas I, Theissmann R, Kaddick C, Fischer A (2011) Characterization of Wear Particles Generated from CoCrMo Alloy under Sliding Wear Conditions. Wear Int J Sci Technol Frict Lubr Wear 271:1658–1666
Liao Y et al (2013) CoCrMo metal-on-metal hip replacements. Phys Chem Chem Phys. https://doi.org/10.1039/c2cp42968c
Ainla A et al (2018) Open-source potentiostat for wireless electrochemical detection with smartphones. Anal Chem 90:6240–6246
Sidaginamale RP et al (2013) Blood metal ion testing is an effective screening tool to identify poorly performing metal-on-metal bearing surfaces. Bone Jt Res 2:84–95
Morlock MM, Kunze J, Wimmer MA, Lohmann CH (2012) Metal-on-metal: ion levels as an intervention strategy. Semin Arthroplasty JSES 23:283–285
Acknowledgements
The authors would like to acknowledge the financial support from Blazer foundation and scientific contributions from Dr. Markus Wimmer, Dr. Nadim Hallab, Dr. Joshua Jacobs (Department of Orthopedics, Rush University Medical Center, Chicago), and Dr. Shalini Prasad (Department of Biomedical Engineering, UTD, Dallas, TX), the help with animal studies from Dr. Sam Pope and Dr. Divya Bijukumar (UIC College of Medicine, Rockford), and the Animal facility at UIC College of Medicine at Rockford (Jessica Gilles and Valentina Ascitto).
Funding
Blazer foundation.
Author information
Authors and Affiliations
Contributions
DK contributed to lab-scale work and preparation of the first draft, EB contributed to animal model and blood collection, KC contributed to corrosion analysis and mentoring, MTM contributed to funding and supervision
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
There is no competing interest between authors on this submitted work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gokul, D., Catton, E., Cheng, K.Y. et al. Electrochemical Biosensor to Detect Implant-Derived Metal Ions: A Mice Model. J Bio Tribo Corros 9, 28 (2023). https://doi.org/10.1007/s40735-023-00743-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-023-00743-y