Abstract
In this paper, the rutile phase of titanium dioxide (TiO2) nanosheets are synthesized utilizing rectangular titanium plates as both electrodes (cathode and anode) with a current density of 9.2 × 10–3 mA/cm2 for 3 h, using the deposition electrochemical method. The rutile nanosheets TiO2 are examined using the X-ray diffraction technique (XRD), scanning electron microscopy (SEM), Fourier Transform Infrared ray (FTIR), and electron transmission microscopy (TEM). Characteristics and morphological studies showed the formation of nanoparticles with an average size of 13 nm. The nano-synthesized rutile TiO2 nanosheets have been used for mild steel coating. Thermometric, weight loss, polarization measurements, and electrochemical impedance microscopy were used to investigate the corrosion protection of mild steel in 1 M HCl in the presence and absence of TiO2. The results showed that the presence of nanoparticles performs well as an anticorrosion material. The greater protection efficiency was 79.1, 80.1, 78.5, and 79.8% using thermometric, weight loss, polarization measurements, and electrochemical impedance microscopy, respectively, at 200 ppm of nanoparticles and 20 °C. Reaction kinetics showed that the corrosion process was performed according to a zero-order model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Metal structures’ deterioration is caused by their interaction with the environment, known as corrosion [1,2,3,4,5]. A broad variety of items, including pipelines, buildings, and other objects, are made from metal [6,7,8,9,10]. A key concern in the chemical industry is corrosion, which causes various problems in production lines and is generally the source of faults and production interruption [11,12,13,14,15,16]. Damage from corrosive units pollutes the environment and puts human health at risk. Gas, oil, and chemical industries employ steel alloys frequently because of its mechanical properties [17,18,19,20,21,22]. Steel is also used in industry because it is less expensive than anticorrosive metals or alloys [23,24,25,26,27,28]. Today, mild steel is utilized in various technical applications, including manufacturing vehicle components, structural forms, and sheets used in pipelines, buildings, plants, bridges, etc. Because of its resistance to general corrosion, mild steel is one of the most frequently utilized metals in corrosive settings [29,30,31,32,33,34,35]. However, some environments, such as hydrochloric acid, are very aggressive to steel alloys. Hydrochloric acid is used widely in the processes of pickling, acid cleaning, and many other industrial applications. The corrosion rate of metallurgy and alloys is an essential property of the ease with which these metals react to a particular environment [36,37,38,39,40]. Sintering activity in nanoparticles is high, and they are significant because of their unique characteristics, which might lead to new and interesting uses [41,42,43,44,45]. In high-temperature settings, nanoparticle coatings have strong thermal and electrical characteristics and are resistant to oxidation, corrosion, erosion, and wear [46,47,48,49,50,51,52]. These characteristics are critical in applications, such as pipes, castings, and the automobile sector.
Any substance with unique or innovative characteristics due to its nanoscale is referred to as a nanomaterial, NPs, (1–100 nm). In addition to strong catalytic activity, structuring nanomaterials have intriguing electrical, optical, and magnetic characteristics [53,54,55,56,57,58,59]. The structural dimensionality of nanomaterials has a major impact on photocatalytic activity. However, the structural dimensionality of nanomaterials has a major influence on their characteristics. Spherical NPs with zero dimensionality, for example, have a large specific surface area, leading to a faster photocatalytic breakdown of organic contaminants [60,61,62,63,64,65,66]. Surface characteristics like adhesion, wettability, corrosion resistance, and wear resistance can all be improved by functional coatings. In other situations, such as semiconductor device production (when the substrate is a wafer), the coating contributes a new attribute to the completed product, such as magnetic response or electrical conductivity, and is an integral component. In the context of corrosion, coating refers to thin layers of a covering substance deposited or put on the surface of any item to improve critical qualities and create a protective barrier against the surface deteriorating owing to its reaction with its environment [67,68,69]. Titanium dioxide (TiO2) is now a popular material for adsorbents, catalytic, super capacitors, lithium-ion batteries, and gas sensors due to its exceptional optical, electrical, photocatalytic, and thermal properties. The hue of pure TiO2 is almost white. Due to the presence of other metals, it appears in different hues, such as yellow and brown [70, 71]. The three main phases of TiO2 crystals are anatase (tetragonal), rutile (tetragonal), and brookite (orthorhombic). The physical characteristics of each polymorph are very special. The rutile phase is, under environmental conditions, the most stable of the three polymorphs. Anatase and brookite may, however, be created and become rutile if the temperature increases. The scale of particles affects the stability of successive TiO2 phases. Anatase is thermodynamically stable when the nanoparticles are less than 11 nm. The enhanced surface characteristics of TiO2 nanosheets (NSs) are thought to make them more active than nanoparticles. This distinctive nanostructure included a significantly larger surface area, a greater number of open access sites, and enhanced durability, all of which are necessary for an effective coating [72, 73].
Many studies in the field of coating science are focused either on synthesis and diagnosis or on the testing of coating performance. Limited works take into considerations a comprehensive views of the synthesis, diagnosis, and testing via different techniques. The purpose of this study is to synthesize and characterize rutile TiO2 phase nanosheets. The synthesis process is achieved using the deposition electrochemical method. The characterization is carried out using XRD, SEM, TEM, and FTIR. The nano-synthesized rutile TiO2 nanosheets have been used for mild steel coating. Thermometric, weight loss, polarization measurements, and electrochemical impedance microscopy were used to investigate the corrosion protection of mild steel in 1 M HCl in the presence and absence of TiO2.
2 Experimental Work
2.1 Materials and Preparations
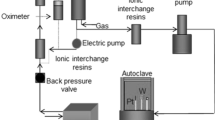
Mild steel was used as a test sample. It has the following chemical compositions (wt%): 0.4 C, 0.44 Mn, 0.038 S, 0.34 Si, 0.041 P, 0.35 Cr, 0.37 Ni, 0.09 Mo, and the balance is Fe. An electrolysis method was used to synthesize pure rutile TiO2 nanosheets using 120 mL of 0.1 M sodium hydroxide (NaOH) at 293 K as the electrolyte. A rectangular titanium sheet (40 mm × 20 mm × 1 mm) is utilized as the anode and counter electrode cathode. Before installing the substrates in the cell, they were cleaned acoustically using ethyl alcohol and deionized water in a 10-min sequence. For 3 h, the applied direct current voltage between the electrodes was 3 V with a current density of 9.2 × 10–3 mA/cm2. A white powder was formed, and the producing material was filtered with deionized water before drying for subsequent analysis overnights. The following is a proposal for an electrodeposition reaction route to produce TiO2 [24]:
Here, Ti* is an active form of Ti
Emulsion solution was prepared by adding different pure rutile TiO2 nanosheets powder concentrations to 100 mL of (acetic acid\water 1:4) as solvent. Then, the solutions were mixed using the ultrasonic technique method. A deposition cell device has been used to coat pure rutile TiO2 nanosheets on mild steel surface. The working electrode (mild steel) and small platinum sheet were inserted in the electrolyte as a counter electrode. The electrophoretic deposition of TiO2 nanosheet on the mild steel was carried out at room temperature with a direct current (DC) of 10 V and 45 min. Then, specimens were then cleaned and dried.
2.2 Methods
In the mylius tube, a piece of mild steel with a dimension of (2 cm × 0.5 cm × 0.01 cm) was employed with 15 mL of HCl solutions. Then the mylius tube was installed in a Dewar flask and sealed with a stopper and then drilled to insert the digital thermometer sensor. First, the temperature of the HCl solution was gradually raised until it reached a constant value. Next, the experiment was repeated with several concentrations of TiO2 nanosheet (25–200 ppm) [1,2,3,4,5].
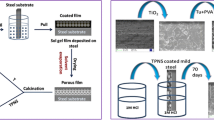
For weight loss method, in a beaker, 50 mL of 1 M HCl solution was added, and mild steel (M.S) with a dimension of 2.5 cm × 0.8 cm × 0.03 cm (about 4.2 cm2) was suspended before being submerged in HCl solution. The weight of the sample was determined before and after immersion at different periods. Weight losses were determined for all samples of mild steel (25–200 ppm) and uncoated mild steel. All samples were washed with deionized water and acetone before being weighed and then, the weight was assessed. For purposes of accuracy, all tests were repeated twice and the average value was taken. The whole experimental work is explained in a flowchart as shown in Fig. 1.
In electrochemical analysis, a three-electrode assembly of 1 cm2 area mild steel as the working electrode, Pt as the counter electrode, and saturated calomel electrode (SCE) as the reference electrode were utilized. During potentiodynamic polarization measurements, the Vertex One–Netherlands equipment was used. The Tafel graphs were recorded at a scanning rate of 0.5 mV/sec at a potential range of 2.5 V relative to OCP. From the liner region of the Tafel, its parameters are determined. Electrochemical Impedance Spectroscopy (EIS) measurements were taken in the 2500–1-Hz frequency range at OCP and a 10-mV AC potential. All electrochemical experiments for corrosion of mild steel in corrosive environments were conducted with and without the coating.
3 Result and Discussion
3.1 Diagnosis and Morphological Studies
XRD diagnosed the nature of produced powders. In Fig. 2, the TiO2 crystal structure was observed at diffraction angles between 20° and 80°, by XRD. Rutile TiO2 can be ascribed to the high peaks at the Muller values of 27.46°, 36.09°, and 54.34°, respectively, corresponding to 110, 101, and 211. With the formula of Scherrer (Eq. 1) [24], the dimensions of the crystallite were obtained. The predicted crystal size is approximately 24.6 nm.
where κ is the shape factor, λ represents the wavelength of the X-ray falling on the sample, β is the full width at half maximum, and θB is the Bragg’s diffraction angle.
SEM was used to determine the shape and size of the main nanosheets [11, 14], as illustrated in Fig. 3. The results reveal that the nanosheets are highly crystalline and exhibit distinct two-dimensional properties. They appear to have a rectangular form and nanoscale thickness. A few quantities of TiO2 nanoparticles are also shown with the produced nanosheets in the pictures. Their average size was estimated to be around 13 nm. In addition, a TEM examination of produced powders was performed. Aggregation occurs during the creation of nanosheets, as seen in Fig. 4. At the nanoscale, the flakes are revealed to have a thin thickness. The nanosheets’ large surface area necessitates research into their coating behavior [12].
Figure 5 shows the FTIR range of TiO2 nanosheets. The band, 500–600 cm−1, is a portion of the vibration bending (Ti–O–Ti) in the TiO2 matrix. The peak 1653 cm−1 concerning Ti–OH water bending modes. The broadest band, seen with 3373–3500 cm−1, is the extended vibration of the hydroxyl group O–H in TiO2 nanosheets [24].
3.2 Thermometric Studies
This method is considered one of the most delicate corrosion monitoring techniques. However, this approach is quite useful in analyzing the corrosion behavior of different metals and alloys in various conditions of corrosion mediums. Results of this technique were corroborated by well-established procedures, including weight loss and hydrogen evolution measurements. Mylius early documented and detailed the reaction vessel and process for assessing corrosion behavior using this method. Uncoated and coated mild steel surface with different concentrations of TiO2 nanosheets are shown in Fig. 6 at different time intervals (0–40 min). For the sample as uncoated (blank), the temperature was 17.9 °C at 40 min. Then, when coating the sample with 25-ppm TiO2 nanosheets, the temperature was decreased to 16.8 °C at 40 min. Finally, as a 50 ppm, when adding TiO2 nanosheets, the temperature dropped further to 15.0 °C at 40 min. However, these results agree with many researchers [9,10,11,12,13,14,15,16,17,18,19,20,21,22]. Temperature against time plots for blank and 25–200 ppm indicates the effect of (HCl) on corrosion of mild steel. The results can be evaluated as a function of reaction number (RN). It can be calculated and illustrated as shown in Eq. 2 and Fig. 7, respectively [12,13,14,15,16,17,18,19,20,21,22,23,24,25].
Tm and Ti denote the highest and starting temperatures, respectively, while t denotes the time to achieve Tm. Furthermore, corrosion protection percent can be determined by thermometric method (%Pth) using the following equation [15,16,17,18,19,20,21,22,23,24,25].
It can be observed when adding TiO2 nanosheets, the RN decreases, and the corrosion protection %Pth increases to approach 78% at 200 ppm. However, this result emphasizes that metal surface is protected and isolated from corrosive environments. This result is in good agreement with many researchers [13,14,15,16,17,18,19,20].
3.3 Weight Loss Method and Kinetics Studies
It is clear from these experiments that weight loss increases with increasing exposure duration time and decreases when metal is coated in both the presence and absence of nanosheets. The weight loss of coated and uncoated mild steel in HCl solutions can be determined and shown in Fig. 8A. Furthermore, the relationship between the percentage inhibition efficiency of TiO2 nanosheet and time is shown in Fig. 8B. The inhibition efficiency was calculated by dividing the weight difference before and after immersion divided by the original weight. It is clear that the inhibition efficiency was high during the first hour, approaching 97% in the presence of 200-ppm TiO2 nanosheet and then it decreased with time. This indicates a weak stability of TiO2 nanosheet in corrosive HCl solution during the long term of exposure (more than 3 h). However, corrosion inhibitors and coating materials are used to protect steel alloys against corrosion in aggressive solutions, especially during pickling and acid cleaning processes. Most pickling processes using HCl are achieved within 10–20 min, which makes TiO2 nanosheet a practical inhibition material within this period [23].
The weight loss of mild steel per samples surface area (m2) per time (days) is shown in Fig. 9, which represents the corrosion rate of mild steel. The corrosion response was greater for uncoated mild steel. The lowest corrosion rate was at 200 ppm, where a dissolving piece of metal decreased. However, this is in good agreement with many researchers [23,24,25,26,27,28,29,30,31,32,33,34,35,36]. The corrosion protection percentage (%PCR) is determined using the corrosion rate of coated and uncoated metal surface using the following equation [34,35,36].
The results showed that in the sample with a concentration of 200 ppm, the coating protection reached 75.93%. This is in good agreement with many researchers [48,49,50,51,52,53,54,55,56, 66,67,68,69,70,71,72,73].
Three models were suggested according to reaction order. Zero, first, and second order of reaction are shown in Eqs. 5 to 8.
where weight loss represents the difference between initial and final weight loss of mild steel after specific time. ko, k1, and k2 represent the rate constant of zero, first, and second order of reaction, respectively. After graphical analysis of these three models, the best fitting was obtained via zero-order model. Table 1 collects all kinetics data. The zero-order reaction provided the higher correlation coefficients (R2).
Kindly consider rephrasing the sentence ‘The variation of weight loss with time may...’ for better clarity. I deleted the sentence
3.4 Electrochemical Studies
An efficient dynamic polarization technique was conducted for 30 min using a mild steel sample immersed in a 1 M hydrochloric acid medium and various inhibitor doses (25–200 ppm). Mild steel, standard calomel electrode (SCE), and platinum electrode make up the electrode fitting. Polarization investigations were used to identify polarization curvatures. Cathodic and anodic curves can be used to determine current corrosion density (icorr) and corrosion potential (Ecorr). The following equation is used to calculate the protection efficiency from polarization data (%PPO) [25,26,27,28,29,30,31,32,33,34,35].
where (icorr)w and (icorr)i denote the corrosion current densities without and with inhibitor, respectively. Figure 10A shows the changes in the anodic and cathodic polarization curves. As the concentration of the nano-inhibitor increases, the polarization curve changes and through it, the corrosion current and corrosion voltage decrease at a temperature of 20 °C. Therefore, the titanium oxide nano-inhibitor has a good effect on coating processes (Table 2) [30,31,32,33,34,35,36,37,38,39,40]. The cathodic and anodic slopes of the linear Tafel reign are evaluated and listed in Table 2. The slight changes in the values of ba besides the displacement in Ecorr (maximum 196 mV at 200-ppm TiO2), which is higher than 85 mV, confirm that the used TiO2 nanosheet is considered as an anodic-type inhibitor [74]. The values of bc are approximately constant in the absence and presence of the inhibitor. Figure 10B displays the results of open-circuit potential (OCP) experiments on mild steel corrosion in 1 M HCl in the absence and presence of various concentrations of TiO2 nanosheet. In all cases, a relatively stable potential was established after approximately one thousand seconds of immersion. The potential for mild steel corrosion initially increased in the blank system. This was attributed to the production of insoluble iron oxides or corrosion products on the surface of mild steel, which rendered the steel more passive [34]. In the presence of TiO2 nanosheets, the nature of the OCP was comparable to that of the blank. In general, the OCP values of coated systems were greater than those of uncoated systems. This can be due to the protective layer formed by the TiO2 nanosheets on the surface of the steel and suggests that anodic dissolution of the steel is inhibited. In addition, the OCP values increase when TiO2 nanosheet concentrations rise [36].
A.C impedance measurements for mild steel corrosion in 1 M HCl after a half-hour immersion were taken at 20 °C. Figure 11 shows the Nyquist plots for mild steel in unrestrained acid, as well as the inhibitor concentrations, and data are collected in Table 2. Electrochemical impedance spectroscopy analysis reveals the kinetics of electrode activities and the electrochemical system’s surface characteristics. The observed rise in impedance characteristics in inhibited solutions is connected to the inhibitor’s corrosion-inhibiting effect. The Nyquist plots for all systems generally assume the shape of one depressed semicircle, corresponding to one time constant, despite the presence of a tiny hint of low-frequency inductive activity. The capability of the semicircle dips with its center below the actual axis to show that capacitance is scattered across the electrode surface inhomogeneity. As a result, Nyquist plots were fitted to the equivalent circuit model, which consist of solution resistance (Rs), capacitance of the double layer (Cdl), and charge transfer resistance (Rct). It was previously used to study mild steel/acid solution interaction. When an inhibitor is added to an acid, Rct rises, and Cdl falls, indicating that the corrosion process is delayed. Cdl values decline due to inhibitor adsorption on the metal/electrolyte interface, caused by a reduction in the dielectric constant and a rise in the double-layer thickness. This indicates that the inhibitors reduce the mild steel specimen’s corrosion rate by adsorption on the already formed metal/electrolyte contact. To quantify the protective effect from electrochemical impedance data (%PEI), the charge transfer resistance values in the inhibitor’s absence (Rct) and presence (Rctinh) may be compared [75,76,77].
where Rct and Rctinh are the charge transfer resistances for inhibited and uninhibited systems, respectively. Charge transfer resistance is calculated using the difference in impedance on the real axis at high and low frequencies. At the frequency where the imaginary component of the impedance is highest, the capacity Cdl is determined using Eqs. 10 and 11 [74,75,76,77].
Because the bigger adsorption layer acts as a dielectric constant, low double-layer capacitance suggests that less electric charge is stored. The increase in values in inhibited systems that correspond to an increase in the diameter of the Nyquist semicircle confirms that the corrosion-inhibiting effect is much stronger in the latter system, implying that the inhibitors’ corrosion-inhibiting effect is synergistically enhanced in the latter system. The Simplified Randles Cell is one of the most used cell models. A charge transfer, a solution resistance, and a double-layer capacitor are all incorporated (or polarization resistance). The double-layer capacitance is parallel to the charge transfer resistance. The Simplified Randles Cell is a useful model in and of itself and a foundation for more complex models. The comparable circuit for a Simplified Randles Cell is shown in Fig. 11. The charge transfer resistance (Rct) of mild steel in uninhibited HCl changes dramatically with the presence of an inhibitor. The value of charge transfer resistance grows as concentration rises. The fact that inhibitor efficiency has risen backs up this conclusion. The semicircular form of all Nyquist graphs implies that the charge transfer mechanism controls mild steel corrosion [74,75,76,77].
4 Conclusion
In conclusion, the findings of XRD, SEM, FTIR, and TEM techniques showed the formation of rutile nanosheets of TiO2 with an average size of 13 nm. Nanoparticles were used as a coating for steel surface in aggressive solution. The performance of nano-titanium oxide was 79.1, 80.1, 78.5, and 79.8% using thermometric, weight loss, polarization measurements, and electrochemical impedance microscopy, respectively, at 200 ppm of nanoparticles and 20 °C. Furthermore, polarization outcomes showed that the magnitude of corrosion current densities was reduced with the addition of nanosheets TiO2. While electrochemical impedance microscopy outcomes showed that the metal resistance was enhanced due to the formation of a coating layer on the surface. Reaction kinetics showed that the corrosion process was according to the zero-order model. However, more work may be recommended for the doping of TiO2 coating with traces of inorganic metal ions, such as zinc, samarium, strontium, and barium, which may enhance the performance of the protective layer.
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Shams El Din AM (2006) The mylius thermometric technique: a review of a simple corrosion testing method of wide potentialities. Egypt J Chem 49(1):1–18
Sanyal B, Srivastava K (2013) Determination of corrosion and inhibition of mild steel in HNO3 by the thermometric (mylius) technique. Br Corros J 8(1):28–32
Hussein EA, Fanfoon DY, Al-Uqaily RA, Salman AM, Kadhim MM, Salman AW, Abbas ZM (2021) 1-Isoquinolinyl phenyl ketone as a corrosion inhibitor: a theoretical study. Mater Today: Proc 42:2241–2246
AL-Uqaily RAH (2015) Inhibition by 4-chloro-2-fluorobenzylamine hydrochloride for corrosion for mild steel in HCl media. Res J Sci IT Manage 5(2):1–7
Obot IB, Obi-Egbedi NO (2010) Inhibition of aluminium corrosion in hydrochloric acid using nizoral and the effect of iodide ion addition. E-J Chem 7(3):837–843
Abd-El-Nabey BA, El-Housseiny S, El-Naggar GA, Matter EA, Esmail G (2016) Green inhibitors for the acidic corrosion of steel. Phys Chem 6(3):57–66
Bhawsar J, Jain P (2018) Investigation of mentha spicata extract as green corrosion inhibitor for mild steel in 2M sulphuric acid medium. Res J Pharm Technol 11(10):4627
Abd El Wanees S, Bukharic AAH, Alatawid NS, Khalile SA, Noohf S, Mustafac SK, Elyang SS (2021) Thermodynamic and adsorption studies on the corrosion inhibition of Zn by 2, 2’-dithiobis(2,3-dihydro-1,3-benzothiazole) in HCl solutions. Egypt J Chem 64(2):547–559
Rheima AM (2019) Synthesis of silver nanoparticles using the uv-irradiation technique in an antibacterial application. J Southwest Jiaotong Univ 54(5):1–5
Rheima AM, Anber AA, Abdullah HI, Ismail AH (2021) Synthesis of alpha-gamma aluminum oxide nanocomposite via electrochemical method for antibacterial activity. Nano Biomed Eng 13(1):1–5
Khajuria A, Kumar R, Bedi R, Swaminanthan J, Kumar D (2018) Impression creep studies on simulated reheated HAZ of P91 and P91B steels. Int J Mod Manuf Tech 10(1):50–56
Khajuria A, Bedi R, Kumar R (2019) Investigation of impression creep deformation behavior of boron-modified P91 steel by high-end characterization techniques. In: Manufacturing engineering. Springer, Singapore, pp 137–150
Khadom AA, Yaro AS (2011) Modeling of corrosion inhibition of copper-nickel alloy in hydrochloric acid by benzotriazole. Russ J Phys Chem A 85(11):2005–2012
Khajuria A, Kumar R, Bedi R (2018) Characterizing creep behaviour of modified 9Cr1Mo steel by using small punch impression technique for thermal powerplants. J Mech Mech Eng. https://doi.org/10.5281/zenodo.1453768
Al-Uqaily RAH, Al-bayaty SA, Abdulameer EA (2019) Inhibition by 4-phenylpyridine N-oxide as organic substance for corrosion for carbon steel in 1 M HCl media. J Adv Res Dyn Control Syst 11(11):1013–1018
Ali AI, Megahed HE, Elsayed M, El-Etre AY (2015) Inhibition of acid corrosion of aluminum using Salvadore persica. J Basic Environ Sci 2:110–122
Sethi T, Chaturvedi A (2019) Corrosion inhibition of aluminium by schiff base in HNO3 with and without additive (KNO3). Int J Res Appl Sci Eng Technol (IJRASET). https://doi.org/10.22214/ijraset.2019.6178
Jasim, A. S., Rashid, K. H., AL-Azawi, K. F., & Khadom, A. A. (2022). Synthesis of a novel pyrazole heterocyclic derivative as corrosion inhibitor for low-carbon steel in 1M HCl: Characterization, gravimetrical, electrochemical, mathematical, and quantum chemical investigations. Results in Engineering, 15, 100573
Verma C, Chauhan DS, Quraishi MA (2017) Drugs as environmentally benign corrosion inhibitors for ferrous and nonferrous materials in acid environment: an overview. J Mater Environ Sci, JMES 8(11):4040–4051
Mabrouk EM, Shokry H, AL-Naja KMABU (2011) Inhibition of aluminum corrosion in acid solution by mono- and bis-azo naphthylamine dyes. Part 1. Chem Met Alloys 4:98–106
Rheima AM, Mohammed MA, Jaber SH, Hameed SA (2019) Synthesis of silver nanoparticles using the UV-irradiation technique in an antibacterial application. J Southwest Jiaotong Univ. https://doi.org/10.35741/issn.0258-2724.54.5.34
Hussain DH, Rheima AM, Jaber SH, Kadhim MM (2020) Cadmium ions pollution treatments in aqueous solution using electrochemically synthesized gamma aluminum oxide nanoparticles with DFT study. Egypt J Chem 63(2):417–424
Agrawal KC (2001) Switchgear and controlgear assemblies. In: Agrawal KC (ed) Newnes power engineering series, industrial power engineering handbook. Butterworth-Heinemann, Oxford, pp 13333–13418
Rheima AM, Mohammed MA, Jaber SH, Hameed SA (2020) Adsorption of selenium (Se4+) ions pollution by pure rutile titanium dioxide nanosheets electrochemically synthesized. Desalin Water Treat 1(194):187–193
Jasim NA, Al-Gasha’a FA, Al-Marjani MF, Al-Rahal AH, Abid HA, Al-Kadhmi NA, Jakaria M, Rheima AM (2020) ZnO nanoparticles inhibit growth and biofilm formation of vancomycin-resistant S. aureus (VRSA). Biocatal Agric Biotechnol 29:101745
Al-uqaily RAH (2015) Inhibition by 1-methyl isoquinoline for mild steel corrosion in 1 M HCl media. Am Sci Res J Eng Technol Sci (ASRJETS) 14(1):55–63
Al-Uqaily RAH, Al-Bayaty SA, Maiws Al-Mayyahi SO (2020) Study of kinetics and inhibition efficiency by “ isoquinoline-5-carboxaldehyde” for corrosion for carbon steel in HCl acid. J Southwest Jiaotong Univ. https://doi.org/10.35741/issn.0258-2724.55.3.31
Jabber SH, Hussain DH, Rheima AM et al (2019) Comparing study of CuO synthesized by biological and electrochemical methods for biological activity. Al-Mustansiriyah J Sci 30(1):94–98
Mohammed MA, Rheima AM, Jaber SH, Hameed SA (2020) The removal of zinc ions from their aqueous solutions by Cr2O3 nanoparticles synthesized via the UV-irradiation method. Egypt J Chem 63(2):425–431
Ismail AH, Al-Bairmani HK, Abbas ZS et al (2020) Nanoscale synthesis of metal (II) theophylline complexes and assessment of their biological activity. Nano Biomed Eng 12(2):139–147
Abdulah HI, Hussain DH, Rheima AM (2016) Synthesis of α-Fe2O3, γ-Fe2O3 and Fe3O4 nanoparticles by electrochemical method. J Chem Biol Phys Sci 6(4):1288–1296
Rheima AM, Mohammed MA, Jaber SH et al (2019) Inhibition effect of silver-calcium nanocomposite on alanine transaminase enzyme activity in human serum of Iraqi patients with chronic liver disease. Drug Invent Today 12(11):2818–2821
Al-Uqaily RAH (2015) Using ethylthiazole-4-carboxylate as inhibitor for copper corrosion in 0.5 M HCL acid. Int J Recent Res Phys Chem Sci 2(1):1–7
El Adnani Z, Mcharfi M, Sfaira M, Benzakour M, Benjelloun AT, Ebn Touhami M, Hammouti B, Taleb M (2012) DFT study of 7-R-3methylquinoxalin-2(1H)-ones (R=H; CH3; Cl) as corrosion inhibitors in hydrochloric acid. Int J Electrochem Sci 7:6738–6751
Hussain DH, Abdulah HI, Rheima AM (2016) Synthesis and characterization of ɣ-Fe2O3 nanoparticles photo anode by novel method for dye sensitized solar cell. Int J Sci Res Publ 6(10):26–31
Sudheer, Quraishi MA (2014) 2-Amino-3,5-dicarbonitrile-6-thio-pyridines: New and effective corrosion inhibitors for mild steel in 1 M HCl. Ind Eng Chem Res 53:2851–2859
Rheima AM, Hussain DH, Abdulah HI (2016) Silver nanoparticles: synthesis, characterization and their used a counter electrodes in novel dye sensitizer solar cell. IOSR J Appl Chem 9(10):6–9
Ali AA, Al-Hassani RM, Hussain DH et al (2019) Fabrication of solar cells using novel micro - and nano complexes of triazole Schiff base derivatives. J Southwest Jiaotong Univ 54(6):1–19
Rheima AM, Hussain DH, Almijbilee MMA (2019) Graphene-silver nanocomposite: synthesis, and adsorption study of cibacron blue dye from their aqueous solution. J Southwest Jiaotong Univ 54(6):1–6
Kadhum HA, Salih WM, Rheima AM (2020) Improved PSi/c-Si and Ga/PSi/c-Si nanostructures dependent solar cell efficiency. Appl Phys A 126(10):1–5
Rheima AM, Aboud NA, Jasim BE, Ismail AH, Abbas ZS (2021) Synthesis and structural characterization of ZnTiO3 nanoparticles via modification sol-gel prosses for assessment of their antimicrobial activity. Int J Pharm Res 13(1):342–347
Ismail AH, AL-Bairmani HK, Abbas ZS et al (2020) Synthesis, characterization, spectroscopic and biological studies of Zn (II), Mn (II) and Fe (II) theophylline complexes in nanoscale. Nano Biomed Eng 12(3):253–261
Ismail AH, Al-Bairmani HK, Abbas ZS, Rheima AM (2020) Nano metal-complexes of theophylline derivative: synthesis, characterization, molecular structure studies, and antibacterial activity. IOP Conf Ser: Mater Sci Eng 928(5):052028
Jasim NA, Al-Gashaa FA, Al-Marjani MF et al (2020) ZnO nanoparticles inhibit growth and biofilm formation of vancomycin-resistant S. aureus (VRSA). Biocatal Agric Biotechnol 29:101745
Aboud NA, Alkayat WH, Hussain DH, Rheima AM (2020) A comparative study of ZnO, CuO and a binary mixture of ZnO0.5-CuO0.5 with nano-dye on the efficiency of the dye-sensitized solar cell. J Phys: Conf Ser 1664(1):012094
Rheima A, Anber AA, Shakir A, Salah Hammed A, Hameed S (2020) Novel method to synthesis nickel oxide nanoparticles for antibacterial activity. Iran J Phys Res 20(3):51–55
Pragathiswaran C, Ramadevi P, Karthik Kumar K (2021) Imidazole and Al3+nano material as corrosion inhibitor for mild steel in hydrochloric acid solutions. Mater Today: Proc 37(2):2912–2916
Sowmya shree AS, Somya A, Pradeep Kumar CB, Rao S (2021) Novel nano corrosion inhibitor, integrated zinc titanate nano particles: Synthesis, characterization, thermodynamic and electrochemical studies. Surf Interfaces 22:100812
Al-Uqaily RAH (2015) Corrosion behavior of carbon steel in HCL media and Inhibition by 3-hydroxy-2-methylpyridine. Int J Adv Sci Tech Res 3(5):181–190
Al-Uqaily RAH, Al-Bayaty SA, Hameed S (2019) 2-Amino-6-chlorobenzothiaozole as effective corrosion inhibitor for copper in acidic media. J Int Pharm Res 46(4):342–345
Al-Uqaily RAH, Al-Bayaty SA (2019) Study a corrosion inhibitor of 1-isoquinolinyl phenyl ketone for mild steel in acidic medium as hcl acid. J Phys: Conf Ser. https://doi.org/10.1088/1742-6596/1294/5/052014
Amin MA (2006) Weight loss, polarization, electrochemical impedance spectroscopy, SEM and EDX studies of the corrosion inhibition of copper in aerated NaCl solutions. J Appl Electrochem 36(2):215–226
Patel AS, Panchal VA, Mudaliar GV, Shah NK (2013) Impedance spectroscopic study of corrosion inhibition of Al-pure by organic Schiff base in hydrochloric acid. J Saudi Chem Soc 17(1):53–59
Al-Uqaily RAH (2016) Corrosion inhibition of steel in HCL media using 2- methoxymethyl-benzlamine. J Appl Chem IOSR 8(4):50–55
Hameed S, Awad HA, AL-Uqaily RAH (2020) Boron removal from seawater using adsorption and Ion exchange techniques. Ecol Environ Conserv 26(2):10–17
Al-Bayaty SA, Jubier NJ, Al-Uqaily RAH (2020) Study of thermal decomposition behavior and kinetics of epoxy/polystyrene composites by using TGA and DSC. J Xi’an Univ Archit Technol 12(3):1331–1341
Awad HA, Al-Uqaily RAH, Al-Bayaty SA (2020) Effect of inhibition by “ 2-(2-methoxyphenoxy) benzylamine hydrochloride ”for corrosion of mild Steel in HCl media. J Xidian Univ 14(4):3499–3507
Al-Uqaily RAH, Al-Bayaty SA, Sager AG (2020) Inhibition and adsorption by using“ thiazole-2-carboxylic acid ” as anti-corrosion for copper metal in HCl media. J Southwest Jiaotong Univ. https://doi.org/10.35741/issn.0258-2724.55.2.56
Khadom AA (2015) Kinetics and synergistic effect of iodide ion and naphthylamine for the inhibition of corrosion reaction of mild steel in hydrochloric acid. React Kinet Mech Catal 115(2):463–481
Rathod MR, Rajappa SK, Praveen BM, Bharath DK (2021) Investigation of Dolichandra unguis-cati leaves extract as a corrosion inhibitor for mild steel in acid medium. Curr Res Green Sustain Chem 1(4):100113
Paul A, Joby Thomas K, Raphael VP, Shaju KS (2012) Chelating efficacy and corrosion inhibition capacity of Schiff base derived from 3-formylindole. Orient J Chem 28(3):1501–1507
Kuriakose N, Kakkassery JT, Raphael VP, Shanmughan SK (2014) Electrochemical impedance spectroscopy and potentiodynamic polarization analysis on anticorrosive activity of thiophene-2-carbaldehyde derivative in acid medium. Indian J Mater Sci. https://doi.org/10.1155/2014/124065
Hameed S, Awad HA, AL-Uqaily RAH (2019) Removal of iron and manganese from ground water by different techniques. J Res Lepid 50(4):458–468
Rathod MR, Rajappa SK, Minagalavar RL, Praveen BM, Devendra BK, Kittur AA (2022) Investigation of African mangosteen leaves extract as an environment-friendly inhibitor for low carbon steel in 0.5 M H2SO4. Inorg Chem Commun 140:109488
Rathod MR, Rajappa SK (2022) Corrosion inhibition effect of Cycas revoluta leaves extract on corrosion of soft-cast steel in hydrochloric acid medium. Electrochem Sci Adv 2(2):e2100059
Al-Bayaty SA, Al-Uqaily RAH, Hameed S (2020) Study of thermal degradation kinetics of high density polyethlyene (HDPE) by using TGA technique. In: Ann R (ed) AIP conference proceedings, vol 2290. AIP Publishing LLC, Melville
Dalbin S, Maurin G, Nogueira RP, Persello J, Pommier N (2005) Silica based coating for corrosion protection of electrogalvanized steel. Surf Coat Technol 194(2–3):363–371
Singh Raman RK, Tiwari A (2014) Graphene: the thinnest known coating for corrosion protection. JOM 66:637–642
Chen Y-F et al (2003) The effect of calcination temperature on the crystallinity of TiO2 nanopowders. J Cryst Growth 247(3–4):363–370
Kim JH, Bhattacharjya D, Yu J-S (2014) Synthesis of hollow TiO2@ N-doped carbon with enhanced electrochemical capacitance by an in situ hydrothermal process using hexamethylenetetramine. J Mater Chem A 2(29):11472–11479
Huang C, Young NP, Grant PS (2014) Spray processing of TiO2 nanoparticle/ionomer coatings on carbon nanotube scaffolds for solid-state supercapacitors. J Mater Chem A 2(29):11022–11028
Wang H, Zhang Y, Zhang M, Zhen Q, Wang X, Du X (2016) Gold nanoparticle modified NiTi composite nanosheet coating for efficient and selective solid phase microextraction of polycyclic aromatic hydrocarbons. Anal Methods 8(31):6064–6073
Huang Z, Zhao W (2020) Coupling hybrid of HBN nanosheets and TiO2 to enhance the mechanical and tribological properties of composite coatings. Prog Org Coat 1(148):105881
Chaitra TK, Shetty Mohana KN, Tandon HC (2015) Thermodynamic, electrochemical and quantum chemical evaluation of some triazole Schiff bases as mild steel corrosion inhibitors in acid media. J Mol Liq 211:1026–1038
Bou A, Bisquert J (2021) Impedance spectroscopy dynamics of biological neural elements: from memristors to neurons and synapses. J Phys Chem B 125(35):9934–9949
Mebed AM, De Malsche W, Abd-Elnaiem AM (2021) Fabrication, boron leaching, and electrochemical impedance spectroscopy of nanoporous P-type silicon. SILICON 14:5691–5701
Laschuk NO, Bradley Easton E, Zenkina OV (2021) Reducing the resistance for the use of electrochemical impedance spectroscopy analysis in materials chemistry. RSC Adv 11(45):27925–27936
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest arising from the involvement of other parties either internal or external to the University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rheima, A.M., Khadom, A.A., Kadhim, M.M. et al. Electrochemical Synthesis of Rutile Phase Titanium Dioxide Nanosheets for Corrosion Protection of Mild Steel in Acidic Media. J Bio Tribo Corros 8, 118 (2022). https://doi.org/10.1007/s40735-022-00711-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-022-00711-y