Abstract
Metal and metal oxide nanoparticles (MMO NPs) are excellent antimicrobials, anti-biofilm and possess quorum sense quenching and inhibition property. The biocides used to control biocorrosion are expensive, release unwanted by-products into the environment and in most cases not effective against established biofilm. MMO NPs are ecofriendly, less expensive, and possess excellent antimicrobial, anti-biofilm, and anti-quorum sensing activity and hence are a promising group of inhibitors against biocorrosion. This review highlights the mechanisms of biocorrosion and biofilm formation, microbes involved, and properties of metal and metal oxide NPs that make them suitable as biocorrosion inhibitor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
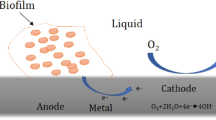
Corrosion is the electrochemical or biological deterioration of metals or metal alloys. Biodegradation activities of bacteria and fungi lead to biodeterioration of materials in which biocorrosion is inclusive. Microbiologically influenced corrosion is a type of corrosion caused by microbes, it is also known as biocorrosion. It is the deteriorating corrosive effect on solid surfaces caused by microbial activities. This can be observed in metals, metal alloys, sewer pipes, concretes, vessels, and tanks, in several piping systems including oil pipelines [145, 146]. Biocorrosion unlike chemical corrosion, which is aerobic, microbiologically influenced corrosion, can be either aerobic or anaerobic. This can be achieved either by direct or indirect microbial actions. Direct mechanism involves direct oxidation of the metal or by utilising at the cathode, a constituent of the electrochemical process (e.g. hydrogen), while indirect mechanism involves the creation of differential aeration cells or secretion of corrosive metabolites. In microbial concentration cells, the colony edge becomes cathodic in the presence of oxygen, while the center of the colony in contact with the metal surface becomes anodic and metal ions are formed there.
Due to the presence of microbes at corrosion sites, some people blame corrosions of unknown causes on microbes. Surfaces of metals and substances are not sterile; so there is need to investigate corrosion causes carefully and thoroughly before attributing them to biocorrosion. Corrosion occurs by the process of oxidation. It is paramount to identify the final electron acceptor in a corrosion process. In aerobic microbial corrosion, oxygen is removed by the aerobic microbes and used as final electron acceptor during metal oxidation. Anaerobic microbial corrosion is a major concern. In the absence of oxygen, the electrons must be accepted by a non-oxygen oxidant like sulphate [63].
Many bacteria have been implicated in corrosion caused by differential aeration cells. Fungi have also been implicated in biocorrosion in fuel tanks. Microorganisms secrete lots of acidic products like ammonia, organic acids, and hydrogen sulphide which can corrode metallic surfaces. In the oil sector, almost 20% of corrosion-related effects in metals can be attributed to biocorrosion [145]. Though several bacterial species are involved in biocorrosion, sulphate reducing bacteria (SRB) are a major concern. Besides SRB, other bacterial groups which include iron oxidizing bacteria (IOB), iron reducing bacteria (IRB), Sulphur oxidizing bacteria (SOB), slime forming bacteria, acid producing bacteria (APB) and some fungi can also cause biocorrosion [102]. SRB are important members of the bacterial population. They are ubiquitous in nature (found in water, soil and animals) and help in sulphur cycle. They are found in sewer pipe, releasing hydrogen sulphide (H2S) through their metabolic activities, which can corrode sewer pipes and cause foul smell in sewers. They can also corrode concrete and metals. They are implicated in pipeline corrosion and in crude oil souring in the oil and gas sector. According to Ali et al. [9], SRB are the major cause of biocorrosion and souring in oil industries. They use sulphate as the final electron acceptor and produce sulphide ions which react with hydrogen to form hydrogen sulphide which is considered a major player in the corrosion process. SRB is a major cause of anaerobic corrosion. They make use of sulphate as a final electron acceptor, but can also use thiosulphate, sulphur, or carbon(IV) oxide. Besides SRB, other microbes also carry out anaerobic biocorrosion-like methanogens and acid producing bacteria (APB).
2 Methods
The databases used in this review for extracting relevant articles are PubMed, Science direct and Google scholar. The search words are ‘metal and metal oxides nanoparticles antimicrobial’, ‘metal oxides nanoparticles antibacterial’, ‘metal oxides nanoparticles antifungal’, ‘metal oxides nanoparticles antibiofilm property’, ‘metal oxides nanoparticles anti-quorum sensing property’, ‘metal oxides nanoparticles on sulphate reducing bacteria’, ‘metal oxides nanoparticles on biocorrosion’, ‘microbial biofilm formation’, ‘biofilm and biocorrosion mechanisms’, ‘biofilm and biocorrosion inhibitors’, ‘silver nanoparticles antimicrobial’, ‘zinc oxide nanoparticles antimicrobial, ‘copper oxide nanoparticles antimicrobial, ‘mechanisms of metal and metal oxide nanoparticles toxicity in microbes’, ‘iron oxide nanoparticle antimicrobial’, ‘tin oxide nanoparticle antimicrobial’, ‘titanium oxide nanoparticle antimicrobial’, ‘zirconium oxide nanoparticle antimicrobial’, ‘aluminium oxide nanoparticle antimicrobial’, ‘nickel nanoparticle oxide antimicrobial’, ‘magnesium oxide nanoparticle antimicrobial’, gold nanoparticle antimicrobial, bismuth nanoparticles antimicrobial, antimony nanoparticles antimicrobial, chromium nanoparticles antimicrobial, silicon nanoparticles antimicrobial, cobalt nanoparticles antimicrobial, cerium oxide nanoparticles antimicrobial, manganese nanoparticles antimicrobial, platinum nanoparticles antimicrobial, palladium nanoparticles antimicrobial. Articles not on metal and metal oxides nanoparticles were excluded. Articles on metal nanocomposite, functionalized metal nanoparticles, conjugated metal nanoparticles, coated metal nanoparticles, nanofibres and nanotubules were all excluded. Publications earlier than 2010 were excluded; metal nanoparticles effects on protozoa and viruses and articles not published using English language were also excluded.
The screening and assessing of relevant articles used in this review is given in Fig. 1. A total of two hundred and eighty-three (283) articles were extracted from three databases stated above. One hundred and thirty-one (131) artilces with irrelevant titles and abstracts were removed, eight (8) duplicate articles removed and one (1) article published in Arabic was removed. The remaining one hundred and forty-three (143) articles were thoroughly read and another twenty-five (25) irrelevant studies were screened out. Lastly, one hundred and eighteen relevant articles were used for this review.
2.1 Microbial Biofilm Formation and Biocorrosion
Several microbial species and groups have been implicated in biocorrosion [102, 129]. Table 1 below shows different microbial groups implicated in biocorrosion, their mechanisms of action, oxygen requirement and corrosive effects. Bacteria can attach to living or non-living surfaces to form microbial communities in the presence of supportive metabolic or biochemical substances including exopolymeric substances and protein. Such microbial community matrix is called biofilm. Presence of moisture or water is a major condition necessary for biofilm formation [174]. It can be formed by single microbial species or a combination of several species, but mainly by a combination of many microbial species. In nature, most bacteria exist in multispecies communities. Biofilms have been studied for many years, both directly in nature or under laboratory conditions. Bacteria like other living things interact with each other and also with their environment. Bacteria by the secretion of extracellular substances can form a matrix and exist in a congregation of interspecies on surfaces interacting and communicating with each other by the release of different kinds of molecules. Bacterial species in biofilms can interact with one another through the secretion of protein and extracellular polymeric substances (EPS) also referred to as exopolymeric substances [86]. Formation of biofilm helps microbes to proliferate and survive harsh condition; it also increases their ability to colonize a surface or persist during infection by the ability to resist several biocides and antibiotics. The production of EPS by microbes is highly responsible for their ability to form biofilm. The EPS contains molecules like lipids, proteins, nucleic acids (extracellular DNA; eDNA) and polysaccharides [112]. Synergistic interactions in multispecies biofilms affect the distribution and multiplication of the species affecting its general function which may lead to a beneficial or detrimental effect and may result to increased pollutant degradation, virulence, antimicrobial resistance, environmental biodeterioration and environmental adaptability [124].
The microbial species in biofilms interact with each other in synergy, contributing in function to achieve the sustenance and survival of the community. Each species in a biofilm perform specific functions which help in maintaining the biofilm. Bacteria in biofilm work in synergy. However, this same synergistic quality is what contributes to resistance and deteriorative effects of biofilms. Horizontal gene transfer may occur in a multispecies biofilm yielding a better specialized bacteria and mutation can increase the symbiotic association and communication among species in a multispecies biofilm leading to a more stable community. A synergistic activity by IOB and SRB has been reported in the corrosion of carbon and low-alloyed steel [119]. The IOB (example Galionella and Leptothrix) attack the metal first forming tubercles. The SRB (example Desulfovibrio) then colonise the tubercles reducing sulphate to sulphide. The sulphides then react with the metals to cause corrosion. Some microbial species implicated in biocorrosion, their isolation sites and the deteriorative effects caused at those sites are shown in Table 2 below. According to Liang et al. [112], identifying the various microbial species in a multispecies biofilm is important because it will help to understand and manipulate the functions of several biofilms. The dynamics in function in a multispecies biofilm is largely due to the interaction among the microbial species. Interaction of species in biofilms is not always a synergistic cooperation; it can also be antagonistic depending on the microorganism involved [32]. This interaction may be competitive or cooperative depending on the conditions and microbial species involved. Despite the beneficial actions of biofilms in cleansing the environment, degrading waste and removing pollutants, they are a serious challenge in the medical, industrial and environmental sector causing several degrees of drug resistant infections, biocorrosion, biofouling and contaminations [57].
To achieve biofilm inhibition in biocorrosion, biofilm need to grow uniformly and cover the surface of the metal with uniform activity, but since this uniformity of biofilm in growth and activity is hard to achieve in nature, biofilm inhibition in biocorrosion is hard to determine. Microorganisms are bio-indicators of environmental contamination and pollution and their capability to form biofilm is a challenge in medical, environmental and oil sector. The microbial biofilms resist antibiotics in medical treatments, biocides in environmental treatment, and cause corrosion of sewer pipes and oil pipelines including souring of crude oil [124]. Most antibiotic resistant, environmental resistant and microbial influenced corrosion are caused by biofilm forming microbes.
Biocorrosion involves the material surface, abiotic corrosion products, microbial cells and their corrosive metabolites. Biocorrosion is higher with mixed cultures than pure cultures due to synergistic activities. Enzymes have been reported to facilitate biocorrosion by accelerating cathodic reaction [114]. Biocorrosion cannot be attributed to a single mechanism or single bacterial species. Several mechanisms have been propounded for biocorrosion which includes the formation of oxygen gradient, generating corrosive metabolites, increasing the resistance to mass transport at the metal surface, removal of protective films from the metal surface during detachment, and altering the redox conditions at the boundary between bulk solution and the metal [114]. The presence of oxygen gradient in biofilm has been widely demonstrated and it is believed that anaerobic microbes can be found beneath or within biofilms in aerated surfaces. SRB are believed to exist in such environment due to the report that they possess oxygen resistant proteins.
2.2 Biocorrosion Preventive Measures
Information on the general bacterial species and their mechanism of attack can aid control biocorrosion. Tables 1, 2, and Fig. 2 listed the microbial groups, some microbial species and the mechanisms of biocorrosion attack. Biocorrosion does not only rely on environmental conditions and type of bacteria, but can also depend on the type of metal [114]. Bacillus sp. behaved differently when colonizing different metals showing that biocorrosion do not only depend on environmental factors and type of microbe but also on the type of metal [114]. According to Lin and Ballim [114], a better biocorrosion management can be achieved by utilizing proactive measures which include using suitable construction materials to prevent corrosion, applying protective film/coatings like paints, plastics, tar and metal plating on metal surfaces, controlling biofilm formation and cathodic protection using sacrificial metals at the anode. The mould (Hormonconis resinae) caused corrosion of aluminium in aircraft tanks by degrading kerosene and releasing acidic/corrosive metabolites [147]. It was prevented by the use of high chromium paints until it stopped due to environmental concern from chromium. Copper, copper alloys, stainless steel mainly austenitic stainless steel and titanium are more resistant to corrosion though biofouling and biocorrosion has been observed over time. Chromium in stainless steel aids its resistance and titanium is believed to be the least metal susceptible to biocorrosion. Like chromium in stainless steel, noble metals can be used to prevent biocorrosion by incorporating them in metal alloys of biocorrosion susceptible metals.
Besides the use of nanoparticles for biocorrosion control, there are reports of the use of biocides and phytochemicals like ozone, sodium azide, chlorine, glutaraldehyde, diketopiperazines, tetra-kis-hydroxymethylphosphonium sulphate (THPS), hydrogen peroxide with silver and extract from Korean green tea [4, 9, 33, 79, 119, 187]. BIOCOMPETITIVE exclusion by the use of nitrate reducing bacteria (NRB) to competitively eliminate SRB has also been described [142]. The use of specific bacteriophage to scavenge the SRB has also been postulated [161].
Biocide can be used to prevent the development of biofilm. Current research focus is on the development of cheap and non-toxic inhibitors of biocorrosion. The use of biocides to inhibit enzyme production like catalase, superoxide dismutase and hydrogenase can also prevent biocorrosion accelerated or facilitated by enzyme production. The use of biocides in preventing biocorrosion is challenging due its environmental concerns and research is shifted to its replacement with green biocides that are environmentally friendly. Without attachment or adhesion to substratum or surfaces to form biofilm, biocorrosion will not be effective. Measures to prevent microbial adsorption on metal surface will be a proactive step to curb biocorrosion.
2.3 Nanoparticles Prospects in Biocorrosion Inhibition
A review by Rasheed et al. [145] showed the effectiveness of nanomaterial coatings on metal surfaces in biocorrosison inhibition. Figure 2 shows the antimicrobial mechanisms of metal and metal oxide NPs that make them prospective agents for biocorrosion control. They can also act as biocide in aqueous solution thereby inhibiting microbial growth and hence preventing biofilm formation and biocorrosion. Nanoparticles are ecofriendly, small in size, cost efficient, have large surface area and high number of particles, bioavailable, easily delivered to target cells, can cross cell barrier and can permeate and immobilize in biofilms [150]. These qualities account for MMO NPs toxicity on microorganisms. Nanoparticles attach to bacteria EPS in biofilms and become immobilized and entrapped within the microbial biofilm [150]. This makes it firmly attached providing more exposure time and then it slowly permeates and distributes around the entire microbial community or matrix. Metal NPs from metals in period four in the periodic table prevented biofouling in freshwater to a degree of 125% [35]. Biocides are majorly utilized in biocorrosion control measures, but these biocides are expensive, not environmentally friendly and release unwanted by-products after use in disinfection thereby presenting environmental health challenges. Biosynthesized metal and metal oxides NPs are ecofriendly and unwanted by-products are not generated. Some metal and metal oxides NPs possess antimicrobial, anti-biofilm and quorum sense inhibition potential. MMO NPs, based on their properties, are the best alternatives to conventional biocides used in biocorrosion control.
2.4 Quorum Sense Inhibition by MMO NPs
The microorganisms secrete signalling molecules that are diffusible which mediates mutualistic growth and cooperation among species. Microorganisms in biofilms interact with each other through quorum sensing by the secretion and recognition of some micro molecules by both Gram-positive (GP) and Gram-negative (GN) bacteria. Biocides, metal alloys and NPs can prevent and block this interaction thereby controlling the formation of biofilm. In quorum sensing, bacteria use extracellular chemicals (auto-inducers) to communicate and monitor population size. It helps bacteria to coordinate their activity in a biofilm. It is found in both GP and GN bacterial species. Scientists have identified three profound types of auto-inducers; auto-inducing peptides (GP bacteria), acyl homoserine lactones (GN bacteria) and Al-2s molecules in both bacteria groups [72]. Quorum sense inhibition is a major factor in bacteria growth and virulence control. Agents capable of inhibiting Quorum sensing are important antimicrobial agents with broad-spectrum activity. The unique features of nanoparticles and their reported antimicrobial action make them potential, suitable and prospect quorum sense quenchers and inhibitors. Quorum sense ability is responsible for biofilm formation and virulence factors.
To mitigate biocorrosion, it is paramount to prevent biofilm formation than trying to control an already formed biofilm. It is best to be proactive rather than reactive. Microorganisms in biofilms communicate majorly by quorum sensing. The physical–chemical activities in biofilms take place in the EPS [174]. It performs several functions including facilitating genetic transfer or molecular switches in biofilms. Controlling EPS will control biofilm formation and controlling biofilm will control biocorrosion. According to Lars and Douglas [108] the surface properties of materials affects biofilm formation. According to them, the physical and chemical properties regulate cell attachment, physiology, biofilm formation and community development. Therefore, manipulating the properties of material surfaces to inhibit biofilm formation will also help to prevent and control biocorrosion.
Several engineered MMO NPs (Ag, ZnO, Fe, TiO2, SiO2, Fe2O3) have different impacts on bacterial growth and bacterial biofilm at low level concentration depending composition, size and the NPs aggregation [111]. Nanoparticles of Ag, Au, TiO2, ZnO, SiO2 and chitosan can act as quorum sensing jammers in biofilms [72]. Nanoparticles act as biofilm and efflux pump inhibitor thereby assisting antibiotics in their bacteriocidal actions [65]. QS depends on bacterial population (density) and anything that affects bacterial proliferation will affect quorum sensing [111]. AgCl–TiO2 NPs exhibited anti-quorum sensing action preventing the interaction of microbes in biofilm [132]. From [140], Ag NPs inhibited quorum sensing in Pseudomonas aeruginosa (at 30 μg/mL), Serratia marcescens (at 20 μg/mL) and Chromobacterium violaceum (at 10 μg/mL).
The effect of Ag NPs, ZnO NPs and TiO2 NPs on quorum sensing in Chromobacterium violaceum (a model organism for quorum sensing study) was evaluated by [60]. According to their report, ZnO NPs affect signal response and perception while Ag NPs and TiO2 NPs affect biosynthesis of QS autoinducer molecules. The production of violacein (a naturally occurring bis-indole pigment with violet to purple colour) by some bacterial species including Chromobacterium violaceum is a behaviour that is regulated by quorum sensing activity therefore inhibiting violacein expression is synonymous with quorum sense inhibition. [121] reported anti-quorum sensing property of NiO NPs through violacein degradation. Ag NPs showed antibacterial property and inhibited violacein production and swarming motility [18, 160]. QS in certain bacteria (example Pseudomonas aeruginosa) directs virulence factors expression, production of certain metabolites and resistance to oxidative stress. [152] demonstrated that Au NPs inhibited biofilm formation and pyocyanin (a toxic, virulence quorum sense dependent metabolite) synthesis in Pseudomonas aeruginosa. Au NPs down regulated quorum sensing related genes in Aeromonas hydrophila [52]. [151] reported the Quorum sense inhibition activity of ZnO NPs on Pseudomonas aeruginosa. ZnO NPs and Ag NPs can inhibit biofilm and QS in Pseudomonas aeruginosa [8, 56, 157, 163]. Anju and Saraja [12] also reported quorum sensing inhibition activity of Ag NPs. [168] reported anti-biofilm and anti-QS property of Ag NPs. Ag NPs showed antibacterial and anti-QS action against Klebsiella pneumoniae [17].
2.5 Antimicrobial and Anti-Biofilm Actions of MMO NPs
MMO NPs are environmentally friendly and have antimicrobial potentials. In biocorrosion and biofouling (biodeterioration) control, nanoparticles and nanomaterials are regarded as the next alternative biocides with regards to conventional biocides. MMO NPs have been shown to possess antimicrobial activity against certain microbes including SRB. MMO NPs utilization is a promising approach with increasing reports of their antimicrobial activity. They can be used to coat surfaces or included in aqueous solutions as biocides. Metal oxide (MO) NPs have been reported to possess antimicrobial property. Nanoparticles and metal alloys inhibit biofilm formation [48]; Bakkiyarj and Pandian [26, 93].
2.6 Some Metals in Period Three
2.6.1 Magnesium Oxide Nanoparticles (MgO NPs)
Magnesium oxide (MgO) NPs were found effective for biofilm formation inhibition and the detachment or removal of already formed biofilm [73]. The effective MIC values for these activities ranged from 125 to 500 mg/mL. MgO NPs were found to be more effective in membrane disruption against GN bacteria. It reduced significantly the biomass of 48–120 h old biofilm, prevented attachment of cells to plastic and caused cellular protein leakage. MgO NPs showed effective antimicrobial property from a study by [172]. MgO NPs showed good anti-biofilm and antifungal activity against Fusarium oxysporum [2]. It affected the hyphal appearance of the fungus and biofilm formation. At a concentration of 15 μg/mL, it totally inhibited fungal mycelia while at 1.92 μg/mL, it suppressed biofilm formation. From a study by El-Sayyad et al. [47], MgO NPs proved an effective antimicrobial agent against Candida albicans, Enterococcus faecalis, and Klebsiella pneumonia. [34] used MgO NPs to preserve a eighteenth century paper. They reported that MgO NPs have antibacterial potency against GP and GN bacteria. They reported the toxicity mechanisms to be through ROS generation and cell membrane damage finally resulting in cell death. From [138], the NPs of MgO showed potent antimicrobial action on human pathogens. He et al. [74] reported MgO NPs mechanism of action against food borne pathogens to be through oxidative stress and membrane damage. MgO NPs showed antibacterial and anti-biofilm effect at MIC of 500 μg/mL and MBC of 1000 μg/mL [133].
2.6.2 Aluminium Oxide Nanoparticles (Al2O3 NPs)
Al2O3 NPs were shown to possess anti-biofilm and antibacterial properties against multidrug resistant (MDR) Acinetobacter baumannii [131]. The MIC and MBC for the NPs were 125 mg/mL and 1000 mg/mL. Bacteriocidal action was through disruption of cell membrane accompanied by cellular content leakage. Inhibition of biofilm formation was up to 70.2% and bacterial surface attachment ability was reduced to 51.9%. EPS synthesis and biomass of already formed biofilm were also reduced. Aluminium oxide NPs were effective against MDR Pseudomonas aeruginosa [13]. The MIC was between 1600 to 3200 μg/mL; complete inhibition of bacterial growth was observed at concentration above 2000 μg/mL. Al2O3 NPs of concentrations 0.25 mg/L, 0.5 mg/L, and 1.0 mg/L were used to determine the toxicity of the NPs on GN (Pseudomonas aeroginosa) and GP (Bacillus altitudinis) bacteria [29]. The NPs were observed to release metal ions (Al3+) into the medium at 13 μg/L, 17 μg/L, and 20 μg/L for 0.25 mg/L, 0.5 mg/L, and 1.0 mg/L, respectively. Release of metal ion was not concentration dependent hence difference in toxicity could not be related to concentration dependent metal ion leaching. Pseudomonas aeruginosa was more susceptible to the Al2O3 NPs than Bacillus altitudinis. Toxicity is through ROS generation and cell membrane damage (lipopolysaccharide leakage) with an estimated DNA damage and these were dose dependent. Al2O3 NPs were found to attach to cell membrane and internalized in cells. A dose dependent increase in the production of EPS and formation of biofilm was observed in the bacteria as a defensive response to NPs exposure.
2.6.3 Silicon and Silicon Oxide Nanoparticles (Si and SiO2 NPs)
Si NPs were shown to possess antibacterial property at concentration above 1.5 μg/mL [176]. According to [166], Si NPs showed antibacterial effect against both GN and GP bacteria. They suggested ROS (singlet oxygen) on Si NPs surface (which can cause oxidative stress, damage to bacterial cell wall and possibly death) may be the mechanism of antimicrobial action of Si NPs. [99] examined the antifungal property of CuO NPs, ZnO NPs, MgO NPs and SiO2 NPs against Candida albicans and obtained the minimum fungicidal concentration (MFC) and MIC of the four metal oxide NPs. The MIC of MgO NPs and SiO2 NPs were above 3200 μg/mL. The MIC and MFC of ZnO NPs were 200 μg/mL and 400 μg/mL, while the MIC and MFC for CuO NPs was 400 μg/mL. ZnO NPs and CuO NPs showed antifungal activity against the tested fungus but the effect was lower when compared to Amphotericin B (an antifungal drug) which had MIC and MFC at 0.5 μg/mL and 2.0 μg/mL. [96] reported SiO2 NPs and ZnO NPs to show antibacterial property against several GP and GN bacteria isolated from human infections but reported ZnO NPs to show better effect than SiO2 NPs.
2.7 Some Metals in Period Four
2.7.1 Zinc Oxide Nanoparticles (ZnO NPs)
Ezealisiji and Noundou [49] examined the antibacterial effect of ZnO NPs on selected GN bacterial species (Pseudomonas aeruginosa, Escherichia coli, Proteus mirabilis, Salmonella enteritidis and Klebsiella pneumonia). They found the MIC of the ZnO NPs to vary depending on the bacteria involved. The MIC of ZnO NPs recorded against the bacteria are 0.26 µg/mL, 2.01 µg/mL, 0.14 µg/mL, 6.42 µg/mL, and 0.18 µg/mL for Pseudomonas aeruginosa, Escherichia coli, Proteus mirabilis, Salmonella enteritidis and Klebsiella pneumonia, respectively. [78] identified ZnO NPs as antimicrobial agent at 50 µg per well against Bacillus subtilis and Escherichia coli. At 85 µg/mL, 100% bacteriocidal action was observed. [85] found ZnO NPs to be antifungal against phyto-fungal pathogens. ZnO NPs have antibacterial and antimicrobial property as evidenced in literature [30, 91, 97]. ZnO NPs showed positive antibacterial and anti-biofilm property against Proteus vulgaris, Streptococcus mutans, Vibrio parahaemolyticus and Lysinibacillus fusiformis [183]. ZnO NPs showed antibacterial and anti-biofilm effect to Bacillus subtilis [77]. Bacillus subtilis biofilm was also inhibited by ZnO NPs [20]. There was a reduction in viable bacteria in fluvial system and an increase in ROS generation when exposed to a concentration of 100 mg/L ZnO NPs and 7.85 mg/L Zn2+, respectively [186]. Algal biofilm was observed to drastically reduce when exposed to 100 mg/L of ZnO NPs and 7.85 mg/L of Zn2+, respectively [186]. From [95], ZnO NPs inhibited biofilm formation by Candida tropicalis at 5.2 mg/mL for fluconazole-sensitive strain and 5.42 mg/mL for fluconazole-resistant strain. The EPS adsorption level of ZnO NPs and CuO NPs was evaluated by [184] and CuO NPs were better adsorbed by EPS than ZnO NPs. They also stated that the smaller NPs had higher EPS adsorption (the smaller the NPs the better and higher the EPS adsorption).
In a study by [137] using Pseudomonas putida, low levels of ZnO NPs (0.5 mg/L to 30 mg/L) stimulated bacterial growth, EPS formation, quorum sensing, antibiotic resistance gene expression and biofilm formation while at higher concentrations greater than 30 mg/L there was biofilm inhibition. At a concentration of 250 mg/L of ZnO NPs, there was biofilm inhibition and toxicity which was observed in the down regulation of genes responsible for biofilm formation and the upregulation of genes responsible for antioxidant production. ZnO NPs inhibited the biofilm formation by a facultative anaerobic bacterium and Streptococcus sp. [1]. ZnO NPs and zinc ions inhibited biofilm and virulence in Pseudomonas aeruginosa [109]. ZnO NPs showed bacteriocidal property (Bacillus licheniformis, Escherichia coli, Proteus vulgaris, and Bacillus pumilis) and great anti-biofilm property under light and dark conditions [80]. ZnO NPs inhibited biofilm formation by MR Staphylococcus aureus at 8–10 μg/mL concentration [181]. Subsequently, Jayabalan et al. [89] showed biofilm inhibition of ZnO NPs at 10–50 μg/mL on Bacillus cereus and Enterococcus faecalis. ZnO NPs showed anti-biofilm activity on Vancomycin Resistant (VR) Staphylococcus aureus at MIC of 625 μg/mL [88]. ZnO NPs at 100 μg/mL exhibited antibacterial effect against Klebsiella pneumonia, Salmonella typhimurium, Candida albicans, Pseudomonas aeruginosa, Acinetobacter baumannii, Escherichia coli, Enterococcus faecalis, Salmonella enteritidis, and Staphylococcus aureus [134]. It also showed profound inhibition effect on Pseudomonas aeruginosa and Staphylococcus aureus. ZnO NPs (at 100 mg/mL) affected bacterial proliferation and biofilm in Pseudomonas aeruginosa [45]. ZnO NPs were effective against eight groups of microbes affecting building materials suggesting ZnO NPs as a good coating for building materials against biodeterioration [46].
2.7.2 Copper and Copper Oxide Nanoparticles (Cu and CuO NPs)
Altikatoglu et al. [10] tested CuO NPs potency against bacteria (antibacterial potency) and found it to be effective against Staphylococcus aureus and Escherichia coli. [58] reported antibacterial properties of CuO NPs. [25] concentrated on the toxicity of MO NPs on microorganisms (Bacillus subtilis, Escherichia coli, and Streptococcus aureus). They tested CuO NPs, NiO NPs (nickel oxide NPs), Sb2O3 NPs (Antimony oxide NPs), and ZnO NPs against colony forming units (CFU) counts of bacteria and used dose–response analysis. They found CuO NPs most toxic to the microbes and others in the order Sb2O3 < NiO < ZnO. Copper oxide nanoparticles (CuO NPs) inhibited Escherichia coli, Bacillus subtilis, and Pseudomonas aeruginosa [22, 25, 39]. Cu NPs showed effective anti-biofilm property against GN bacteria. The Cu NPs exhibited excellent anti-biofilm activity against Salmonella typhi, Pseudomonas aeruginosa, Shigella flexneri, and Escherichia coli [156].
CuO NPs and Fe2O3 NPs showed antibacterial activity against GN and GP bacteria [3]. They were active against MRSA and Escherichia coli with CuO NPs being more effective with anti-biofilm property in a dose dependent manner. The MIC for the NPs against MRSA was 30 μg/mL and 40 μg/mL for CuO NPs and Fe2O3 NPs, while the MIC for Escherichia coli was 35 μg/mL and 45 μg/mL for CuO NPs and Fe2O3 NPs, respectively. Cu NPs inhibited biofilm formation in Pseudomonas aeruginosa and Listeria monocytogenes [59]. The Cu NPs MIC for Pseudomonas aeruginosa was 32 mg/L and 16 mg/L for Listeria monocytogenes. It was observed that Cu NPs reduced biofilms formation and microbial colonization on surfaces coated with Cu NPs. Cu NPs affect bacterial growth and biofilm synthesis through attachment to microbial cell wall and disrupting growth and functional activities [139]. The MIC and MBC of the Cu NPs for Pseudomonas aeruginosa and Staphylococus aureus were 50 μg/mL and 100 μg/mL, respectively, while the MIC and MBC for Escherichia coli were 25 μg/mL and 50 μg/mL, respectively.
2.7.3 Iron Oxide Nanoparticles (Fe2O3 NPs; Fe3O4 NPs)
According to Jagathesan and Rijiv [84] iron oxide nanoparticles showed good inhibition against some selected bacteria species (Staphylococcus aureus and Pseudomonas fluorescens) at 100 μg/mL. Fe2O3 NPs (0.01 mg/mL, 0.05 mg/mL, 0.1 mg/mL and 0.15 mg/mL) reduced bacterial surface adhesion and biofilm formation which was surface type and dose dependent [175]. The NPs performed best at high concentration (0.15 mg/mL) on polymer brush coating than other surfaces. At 0.15 mg/mL concentration of Fe2O3 NPs, highest bacterial toxicity and biofilm inhibition was observed which was higher for Staphylococcus aureus (Gram positive) when compared to the GN bacteria (Pseudomonas aeruginosa and Escherichia coli). From Gabrielyan et al. [53], Fe3O4 NPs showed antibacterial effect against GP (Enterococcus hirae) and Gram-negative (Escherichia coli) bacteria in a dose-dependent approach. Fe3O4 NPs was effective against Escherichia coli and Staphylococcus aureus [98]. Fe3O4 NPs was effective against antibiotic resistant Escherichia coli [54]. Fe3O4 NPs has been shown to inhibit biocorrosion of iron by iron corroding bacterium (Halanaerobium sp.) at doses ranging from 0.1 to 100 mg/L [40]. They revealed that the NPs adsorbed and deformed the bacterial cells. They reported a genotoxic effect of the NPs with DNA damage in a dose dependent manner. Fe3O4 NPs reduced sulphide production rate of the iron corroding bacterium with exposure concentration of 100 mg/L.
2.7.4 Nickel and Nickel Oxide Nanoparticles (Ni and NiO NPs)
Nickel oxide nanoparticles antimicrobial activity was reported by Din et al. [44]. According to Khan et al. [100] nickel (Ni NPs) with average size of 41.23 nm showed an IC50 of 73.37 μg/mL for total oral bacteria, NiO NPs with net size of 35.67 nm showed IC50 of 197.18 μg/mL and Ni ions exhibited the best bacteriocidal effect against oral bacteria at IC50 of 70 μg/mL. At 200 μg/mL of NiCl2, Ni NPs and NiO NPs, respectively, against oral biofilm; NiCl2 caused 40% biofilm inhibition and Ni NPs caused 30% biofilm inhibition, while NiO NPs resulted in 7% biofilm inhibition (NiCl2 > Ni NPs > NiO NPs). [149] showed NiO NPs to have antibacterial and anti-biofilm effect against Pseudomonas auroginosa, Escherichia coli, MR and MS (methicillin sensitive) Staphylococcus aureus. NiO NPs eradicated biofilm of Pseudomonas auroginosa at 60 μg/mL concentration [121]. Vahedi et al. [177] studied NiO NPs effect on biofilm formation by Staphylococcus epidermidis. NiO NPs significantly reduced biofilm formation at 0.05 mg/mL, 0.1 mg/mL, and 1.0 mg/mL with a non-significant reduction observed at 0.01 mg/mL NiO NPs concentration.
2.7.5 Titanium Oxide Nanoparticles (TiO2 NPs)
Titanium oxide NPs antibacterial effects have been reported by [162]. [36] reported that titanium dioxide NPs showed antimicrobial potency. TiO2 NPs caused over 60% inhibition in periphytic biofilm [76]. TiO2 NPs exhibited anti-biofilm activity against Pseudomonas auroginosa [144]. The MIC of TiO2 NPs against Pseudomonas aeroginosa PAO1 was determined to be 31.25 μg/mL and this value exhibited profound inhibitory activity on biofilm formation of Pseudomonas aeruginosa. Using in silico analysis, [14] examined the interaction between TiO2 NPs and Pseudomonas aeruginosa PAO1 and found that the NPs bind to the KatA protein of the bacteria. KatA is responsible for hydrogen peroxide resistance (catalase) and virulence. TiO2 NPs showed anti-biofilm activity by generating H2O2 at NPs biofilm interface to suppress biofilm formation in aquatic system [42]. TiO2 NPs slowed the biofilm synthesis and growth rate of Shewanella oneidensis [123].
2.7.6 Manganese and Manganese Oxide Nanoparticles (Mn; MnO2; Mn3O4 NPs)
Manganese (Mn) NPs showed antimicrobial activity against selected microbial species [90]. MnO2 (manganese (iv) oxide) NPs were shown to possess antifungal and antibacterial property [68, 69]. [117] stated that MnO2 NPs possess antibacterial property but are less effective when compared to Ag NPs. Mn3O4 (manganese (ii,iii) oxide) NPs antibacterial activity was tested against two human pathogens (Staphylococcus aureus and Escherichia coli) and it inhibited the growth and multiplication of the microorganisms [23]. The NPs were better against GN bacteria than GP bacteria.
2.7.7 Cobalt and Cobalt Oxide Nanoparticles (Co and Co3O4 NPs)
Co NPs were reported by [92] to possess antimicrobial property. Co3O4 NPs showed good and considerable antibacterial effect on certain pathogenic microbes [135]. Co NPs showed effective antibacterial action on pathogenic Pseudomonas aeruginosa and Escherichia coli [104]. [188] reported the antibacterial property of Co NPs and further stated that they are more effective against GN bacteria than they are against Gram-positive bacteria. Co NPs at 35 μg/mL caused complete lysis of the cell of Escherichia coli bacterium [154]. The Co3O4 NPs antibacterial activity were tested against GP (Bacillus subtillus and Bacillus lichenifermia) and GN (Escherichia coli and Klebseilla pneumonia) bacteria and it was found effective [67]. From Arsalan et al. [15], Co3O4 NPs showed significant and promising antibacterial activity against certain pathogenic bacteria species (Pseudomonas aeruginosa, Escherichia coli, and Staphylococcus aureus). Cobalt oxide NPs was reported by [127] to have antibacterial effect against Escherichia coli, and Staphylococcus aureus.
2.8 Some Metals in Period Five
2.8.1 Silver Nanoparticles (Ag NPs)
Ezealisiji et al. [50, 51] showed that Ag NPs have antibacterial effect against GN and GP bacteria. [51] studied the effect of Ag NPs (average particle size 22 nm; synthesized using plant extract) on Klebsiella pneumonia, Escherichia coli, Bacillus subtilis, Staphylococcus aureus, and Pseudomonas aeruginosa and found the Ag NPs to show inhibitory activity against the tested bacterial species at 5 µg/mL. Secondly, [50] examined the antibacterial property of Ag NPs (average particle size 69 nm synthesized using bacterial extract) using selected pathogenic bacteria (Klebsiella pneumonia, Escherichia coli, Bacillus subtilis, Staphylococcus aureus, and Pseudomonas aeruginosa) and found it to be effective (dose dependent) against all the bacterial species tested at a concentration between 0.05 mg/mL and 0.10 mg/mL. [110] showed silver oxide NPs to be antimicrobial. There was a report of a dose dependent inhibitory effect of Ag NPs on Candida albicans [107]. Ag NPs inhibited drug resistant Pseudomonas aeruginosa [13]. Ag NPs are effective against a range of GN bacteria. It also inhibited several foodborne pathogens [116]. The CFU, disc diffusion, MIC, and MBC was used to examine its effect on Salmonella enteritidis, Salmonella typhimurium, Escherichai coli, and Klebsiella pneumonia. Ag NPs showed a wide antimicrobial growth inhibition against GP and GN bacteria [167]. AgNPs is antimicrobial against multidrug resistant Pseudomonas aeruginosa [113]. Ag NPs showed antibacterial activity at MIC of 3 µg/mL against Staphylococcus aureus and 5 µg/mL against Escherichia coli [61]. MR coagulase negative Staphylococcus aureus was sensitive to 50 µg/mL of Ag NPs [143].
Ag NPs showed antibacterial and anti-biofilm effect on MR Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa [180]. Ag NPs was also shown to exhibit anti-biofilm effect on multi-drug-resistant Staphylococcus aureus [128]. Ag NPs enhanced the quorum sensing inhibition in Pseudomonas aeruginosa [115]. Ag NPs eradicated biofilm of Staphylococcus aureus and Escherichia coli and the eradication was concentration dependent. At 15 µg/mL of Ag NPs, Staphylococcus aureus and Escherichia coli biofilms were eradicated to 89% and 75%, respectively [61]. Ag NPs at 55 µg/mL resulted in 91% biofilm inhibition in the same MR coagulase negative Staphylococcus aureus bacterium [143]. A 50 mg/L Ag NPs affected biofilm level, syntrophy relationship and microbial diversity in a microbial electrolysis cell at the anode [189]. The microbes increased EPS production and quorum sensing gene expression (as a defense mechanism) to combat the toxic effect of the Ag NPs. The species of Bacteroides, Synergistaceae vadin CA02, and Dysgonomonas were sensitive to the Ag NPs and the population of Geobacter reduced allowing Enterobacteriaceae to dominate. From [103], Ag NPs exhibited antibacterial and anti-biofilm effect on a range of GP (Bacillus subtilis, Enterococcus durans, Enterococcus faecalis, Enterococcus faecium, Listeria innocua, Listeria monocytogenes, Staphylococcus aureus, Staphylococcus epidermis) and GN bacteria (Enterobacter aerogenes, Escherichia coli CFAI, Kleb-siella pneumonia, Pseudomonas aeruginosa, Salmonella enteridis,Salmonella infantis, Salmonella kentucky, Salmonella typhimurium).
Ag NPs inhibited growth and biofilm of Bacillus subtilis and Pseudomonas aeruginosai [118]. According to [140], Ag NPs inhibited biofilm and virulence in Pseudomonas aeruginosa PAO1, Serratia marcescens and Chromobacterium violaceum. The MIC of AgNPs against the bacteria tested was reported to be 30 μg/mL, 20 μg/mL, and 10 μg/mL, respectively. The Ag NPs also showed bacteriocidal activity against MRSA, Bacillus subtilis, Salmonella typhi and Pseudomonas aeruginosa. Ag NPs showed antibacterial activity and anti-biofilm effect against Klebsiella pneumonia [143]. The bacteriocidal effect was observed at 50 μg/mL, while 88% biofilm reduction was observed at 100 μg/mL of Ag NPs. Ag NPs of size 8.3 nm prevented biofilm formation by Serratia proteamaculans 94, Pseudomonas aeruginosa PAO1, and Escherichia coli AB1157 at 10–20 μg/mL, 10 μg/mL, and 4–5 μg/mL, respectively [141]. Ag NPs prevented the expression of virulence, synthesis of EPS, formation of biofilm and swarming in Pseudomonas aeruginosa [7]. Ag NPs showed MIC of 6.25 μg/mL against Pseudomonas aeruginosa and MIC of 5 μg/mL against Escherichia coli with MBC of 12.5 μg/mL and 25 μg/mL against the two bacteria, respectively [164]. No inhibitory effect of Ag NPs was observed on Staphylococcus epidermidis at ≥ 50 μg/mL concentration. Ag NPs hindered the proliferation and biofilm formation of Escherichia coli, Serratia liquefaciens, Salmonella sp., and Aeromonas hydrophila [182].
2.8.2 Palladium Nanoparticles (Pd NPs)
Palladium nanoparticles were effective against B. subtilis and P. aeruginosa at 200 μg/well [120]. Pd NPs were promising as antibacterial agents against GP and GN bacteria [159]. It was more effective against E. coli and P. aeruginosa than it was against Staphylococcus aureus and Bacillus subtilis. Pd NPs showed good inhibitory quality against S. aureus and Escherichia coli [171]. By [24], Pd NPs were good against Escherichia coli and Staphylococcus aureus. Biosynthesized Palladium (Pd) NPs and Platinum (Pt) NPs were good against the tested microbial strains at 400 μg/mL [19]. The Pt NPs were more effective than the Pd NPs and their effect was higher against bacterial strains than it was against fungal strains. [136] tested the antifungal potential of Pd NPs against Colletotrichum gloeosporioides and Fusarium oxysporum and found it to have antifungal property. Pd NPs exhibited a broad-spectrum antibacterial effect against GP (Staphylococcus auerus; Streptococcuspyrogens; Bacillus subtilis) and GN bacteria with a greater effect against Gram-positive (Entrococcus aerogenes; Klebsiella pneumoniae; Proteus vulgaris) bacteria [126].
2.8.3 Zirconium Oxide (Zirconia) Nanoparticles (ZrO2 NPs)
In an article published by [106], zirconium oxide (zirconia) NPs where shown to have antibacterial activity against GP bacterium (Bacillus subtilis) and GN bacteria (Salmonella typhi and Escherichia coli). ZrO2 NPs affected the adhesive ability of Candida albicans to attach to surfaces hence it can prevent biofilm formation [55]. Zirconia NPs and Zr(IV) complexes were tested for antimicrobial activity. ZrO2 NPs was effective against E. coli, while the Zr(IV) were effective against E. coli, S. aureus and fungi [87]. They speculated that shape of NPs may be responsible for their cytotoxic effects stating that NPs of same surface area but different shape will exhibit different antimicrobial effect. Zirconia NPs proved to have antimicrobial property by inhibiting bacterial and fungal species [62]. The NPs profoundly inhibited the growth of Escherichia coli, Staphylococcus aureus, Candida albicans and Aspergillus niger.
2.8.4 Tin Oxide Nanoparticles (SnO2 NPs)
SnO2 NPs showed bacteriocidal property against Escherichia coli [179]. In a study by [11], SnO2 were synthesized, characterized, and used against S. aureus, and Escherichia coli. It was observed that the NPs have antibacterial effect which was more profound against E. coli (Gram negative) than it was against S. aureus (Gram positive). From a study by [70], SnO2 NPs were good against elected microbes and the result showed that SnO2 NPs are more effective against GN bacteria than GP and fungi as shown in the order (Gram negative > Gram positive > fungi). SnO2 NPs were tested for antibacterial property using GP bacterium (Micrococcus luteus) and GN bacterium (Escherichia coli) and it showed excellent antibacterial property against the bacteria [173].
2.9 Some Metals in Period Six
2.9.1 Gold Nanoparticles (Au NPs)
Gold nanoparticles (Au NPs) showed antibacterial activity on Pseudomonas putida [153]. Au NPs showed antibacterial effect on Staphylococcus aureus at MIC of 25 µg/mL [148]. Higher concentration of Au NPs and iron oxide NPs (0.05 mg/mL, 0.10 mg/mL, and 0.15 mg/mL) reduced biofilm of Staphylococcus aureus and Pseudomonas aeruginosa [155]. Gold nanoparticles (Au NPs) distorted matured biofilm which was observed using fluorescent and light microscope [148]. Au NPs showed both bacteriocidal and anti-biofilm effect against Pseudomonas fluorescens [66]. Au NPs showed effective bacteriocidal action against several bacterial pathogens [5]. It inhibited biofilm formation in Pseudomonas aeruginosa by 80%. Au NPs acted against Pseudomonas aeruginosa with antibacterial, anti-biofilm and anti-virulence activity [101]. The MIC was 512 μg/mL with antibacterial activity. At sub-MIC level, Au NPs inhibited biofilm formation and eradicated established mature biofilm with no effect on bacterial growth. The minimum biofilm inhibition concentration (MBIC) including the minimum biofilm eradication concentration (MBEC) was recorded as 128 μg/mL. At this sub-MIC concentration, Au NPs was observed to inhibit virulence factors (swimming, swarming and twitching) of Pseudomonas aeruginosa. Au NPs was good against Escherichia coli and Bacillus subtilis [169]. [165] showed the antibacterial effect of Au NPs on Mycobacterium smegmatis (which is a model bacterium for Mycobacterium tuberculosis). From the report of [27], Au NPs showed a wide range of antimicrobial effects against several microbes (Candida albicans, Stretococcus pyogenes, Aspergillus fumigatus, Escherichia coli, Trichoderm viride, Staphylococcus aureus, Lecanicillium lecanii and Klebsiella pneumonia). Au NPs caused bacterial cell damage in Escherichia coli and Salmonella typhi [43].
2.9.2 Cerium Oxide Nanoparticles (CeO2 NPs)
The sorption of ceria NPs in biofilms was studied using biofilm of Pseudomonas fluorescens and Mycobacterium smegmatis. The study showed that NPs adsorption takes place majorly on the cell wall and spores of bacterial cells [94]. After exposure, cells in the biofilm move away from the bulk solution of NPs as a self-protection measure and some outer layer of the biofilm was detached thereby releasing CeO2 NPs in this portion of the biofilm back into the bulk solution. From a study by Mohamed et al. [125] CeO2 NPs were reported to have antibacterial property. Cerium oxide NPs affected the outer membrane of GN bacteria (Klebsiella pneumonia, Escherichia coli) and increased the effectiveness of antibiotics [28]. A study by [16] showed that GP bacteria are sensitive to CeO2 NPs than GN bacteria. CeO2 NPs antibacterial activity was evaluated using Staphylococcus aureus and Escherichia coli by Surendra and Roopan [170] and it proved to be more effective against Escherichia coli than Staphylococcus aureus. CeO2 NPs exhibit profound bacteriocidal activity against GN bacteria (Escherichia coli and Pseudomonas aeruginosa) than GP bacteria (Staphylococcus saprophyticus and Bacillus cereus) [130].
2.9.3 Bismuth and Bismuth Oxide Nanoparticles (Bi and Bi2O3 NPs)
Bismuth oxide NPs (Bi2O3 NPs) showed antifungal and anti-biofilm effect against Candida albicans better than many antifungal agents like nystatin, terbinafine and chlorhexidine [75]. Bi NPs showed antimicrobial and anti-biofilm effect when tested against Staphylococcus aureus and Candida albicans [178]. Bi2O3 NPs showed 16% antibacterial activity against MRSA strain with MIC of 1500 ppm [38]. This result is lower when compared to what is obtainable when using conventional antibiotics like Ciprofloxacin. Though, increasing the concentration of the NPs, resulted in increased activity and reduction in bacterial proliferation. [31] studied the effect of Bi NPs and Ag NPs on eight bacterial species linked with subgingival biofilm including three pathogenic species (Pseudomonas aeruginosa, Escherichia coli and Staphylococus aueus). The Bi NPs showed MIC against the subgingival bacteria between 66 to 133 μg/mL and MIC of 267 μg/mL for the pathogenic bacteria, while the Ag NPs showed MIC against the sungingival bacteria between 16 to 32 μg/mL and between 32 to 65 μg/mL for the three pathogenic bacteria. Bi NPs showed MIC between 0.625 μg/mL to 20 μg/mL and MBC between 1.25 μg/mL to 40 μg/mL against Entrococcus faecalis [21].
2.9.4 Platinum Nanoparticles (Pt NPs)
Pt NPs exhibited antibacterial activity against bacterial species which are related to the dental system (Porphyromonas gingivalis, Streptococcus mutans and Enterococcus faecalis) [81, 82]. From [71], Pt NPs inhibited biofilm formation in Staphylococcus mutans, an oral bacterium. By [6], Pt NPs inhibited bacterial multiplication through membrane integrity dirsuption and generation of ROS. Pt NPs showed antimicrobial activity in a study by Gupta and Chundawat [64]. The MIC of Pt NPs against Escherichia coli was 62.5 μg/mL, 100 μg/mL for Staphylococcus aureus, Klebsiella pneumoniae, and Pseudomonas aeruginosa, while it was 300 μg/mL for Aspergillus niger. The NPs of Pt, Ag, Cu, and Au were tested against microbial species isolated form mastitis in cattle to determine their antimicrobial activity [185]. The microbes isolated are Candida krusei, Escherichia coli, Candida albicans, Staphylococcus aureus, and Staphylococccus uberis. After the study, Ag NPs was better, followed by Cu NPs and a little inhibitory activity by Au NPs. It was reported that Pt NPs has no significant inhibitory effect on the tested pathogens. Pt NPs were effective against dental/oral related bacterial species (Enterococcus faecalis, Streptococcus mutans, Porphyromonas gingivalis) by suppressing their proliferation [81]. The Pt NPs were also found to decompose and eliminate LPS; which an important component of Gram-negative bacteria cell wall. [158] reported broad-spectrum activity of Pt NPs against bacteria. The Pt NPs were effective against coagulase negative Streptococcus sp., Staphylococcus aureus, Proteus vulgaris, Escherichia coli, while it showed a poor antibacterial activity against Pseudomonas aeruginosa and Klesiella pneumoniae.
2.9.5 MMO NPs Effect on SRB
Sulphate reducing bacteria (SRB) is the major cause of biocorrosion and crude oil souring in the oil sector including biocorrosion of pipes and metals outside oil field [119]. They colonize surfaces and cause corrosion in sewer pipes, concretes, buildings, metals, oil pipelines and in automobile tanks. The actions of SRB and other biocorrosion inducing microbes cause great maintenance and operational challenges and ways of mitigating, inhibiting, controlling and preventing these effects are of serious research interest. Their deteriorative activities lead to huge economic loss. Most corrosion caused by SRB in biofilm are resistant to antibiotics, biocides and several antimicrobial agents but with the qualities of NPs they are a promising approach to tackling these challenges. MMO NPs have been shown to possess antimicrobial repertoire and SRB inhibition potential. Inhibiting SRB is synonymous with inhibiting biocorrosion because they are the major microbial group involved in biocorrosion.
NPs of silver, chitosan, copper, zinc, iron, and titanium have been recorded to inhibit sulphate reducing bacteria (SRB) and biocorrosion [37, 105, 122, 145]. The mechanisms include release of reactive oxygen species (ROS), release of toxic metal ions, by destruction of cell membrane, preventing adhesion to surfaces and hence inhibiting biofilm formation, damage to mitochondria, disturbing electron transport chain, inhibiting protein and DNA synthesis, damage to nucleus and causing cell death [37, 145]. Iron nanoparticles (Fe NPs), CuO NPs and TiO NPs inhibited the growth and proliferation of SRB [37, 105, 122]. The SRB affected are Desulfotomaculum nigrificans, Desulfovibrio vulgaris, and Desulfovibrio desulfuricans. ZnO/chitosan nanocomposite inhibited SRB biofilm at 250 μg/mL on carbon steel with 74% SRB inhibition efficiency indicating the nanocomposite as a good biocorrosion inhibitor [146]. Ag NPs was bacteriocidal to Desulfovibio sp. and inhibited biocorrosion at MIC of 2.88 mg/L [83]. The corrosion inhibition was temperature dependent with 76.2% inhibition at 303 K and 61.8% inhibition at 333 K. CuO NPs at concentration above 50 mg/L inhibited metabolic activities in Desulfovibrio vulgaris lowering sulphate reduction rate and cell growth [37]. Mechanism of action of the CuO NPs was attributed to ROS generation and down regulation of genes needed for respiration and electron transfer. TiO2 NPs SRB inhibition potential was tested against two SRB species (Desulphovibrio desulfuricans and Desulfotomaculum nigrificans) and a consortium of both bacteria [122]. The growth rate and sulphate reduction rate of the bacteria were reduced at 1.0 μg/mL of the NPs, while lower concentration below 1.0 μg/mL produced no significant effect on bacterial proliferation and sulphate reduction. Biosynthesized CuO NPs inhibited the growth of Desulfovibrio marinisediminis with MIC ≥ 100 μg/mL [190]. Au NPs showed bacteriocidal property against SRB (Desulfovibrio sp.) with MIC value of 200 mg/mL [41]. The sulphate reduction activity and proliferation of the bacterium were reduced by 7% and 12%, respectively. Genotoxic effect of Au NPs on SRB was shown by Comet assay and this toxic effect was responsible for the inhibition of sulphide reduction and bacterial growth.
3 Conclusion
MMO NPs are promisingly effective in preventing biocorrosion (including SRB related biocorrosion) based on their awesome antimicrobial properties. They were found effective against antibiotic resistant strains of bacteria and antifungal resistant strains of fungi. Some of the noble metals (Ag, Pd, Au and Pt) are promising as biocorrosion inhibitors due to their excellent antimicrobial, anti-quorum sensing and anti-biofilm qualities. The metals in period four have good antimicrobial quality, second when compared with the noble metals. The utilization of NPs of noble metals and some period four metals is a promising approach to handling and preventing the challenges posed by biocorrosion. The MMO NPs show broad-spectrum effect on bacteria and fungi, but were observed to be more effective against GN bacteria than GP bacteria. This may be due to the presence of lipopolysaccharide (LPS) as major part of GN bacterial outer membrane as opposed to the high peptidoglycan in GP bacterial cell wall. The MMO NPs were reported to disrupt LPS hence they can damage the cell wall of Gram-negative bacteria faster than that of Gram-positive bacteria and cause cell death through leakage of cell components. Conclusively, MMO NPs based on their properties are the best alternatives to conventional biocides used in biocorrosion prevention and control.
Data Availability
Data sources are PubMed, Google scholar and Science direct.
References
Abdulkareem EH, Memarzadeh K, Allaker RP, Huang J, Pratten J, Spratt D (2015) Anti-biofilm activity of zinc oxide and hydroxyapatite nanoparticles as dental implant coating materials. J Dent 43:1462–1469. https://doi.org/10.1016/j.jdent.2015.10.010
Abdel-Aziz MM, Emam TM, Elsherbiny EA (2020) Bioactivity of magnesium oxide nanoparticles synthesized from cell filtrate of endobacterium Burkholderia rinojensis against Fusarium oxysporum. Mater Sci Eng, C 109:110617. https://doi.org/10.1016/j.msec.2019.110617
Agarwala M, Choudhury B, Yadav RNS (2014) Comparative study of antibiofilm activity of copper oxide and iron oxide nanoparticles against multidrug resistant biofilm forming uropathogens. Indian J Microbiol 54(3):365–368. https://doi.org/10.1007/s12088-014-0462-z
Agwa OK, Iyalla D, Abu GO (2017) Inhibition of biocorrosion of steel coupon by sulphate reducing bacteria and iron oxidizing bacteria using Aloe Vera (Aloe barbadensis) extracts. J Appl Sci Environ Manag 21(5):833–838
Ahiwale SS, Bankar AV, Tagunde S, Kapadnis BP (2017) A bacteriophage mediated gold nanoparticles synthesis and their anti-biofilm activity. Indian J Microbiol 57(2):188–194. https://doi.org/10.1007/s12088-017-0640-x
Ahmed KBA, Raman T, Anbazhagan V (2016) Platinum nanoparticles inhibit bacteriaproliferation and rescue zebrafish from bacterialinfection. RSC Adv 2016(6):44415–44424. https://doi.org/10.1039/c6ra03732a
Akther T, Khan MS, Hemalatha S (2020) Biosynthesis of silver nanoparticles via fungal cell filtrate and their antiquorumsensing against Pseudomonas aeruginosa. J Environ Chem Eng 8:104365. https://doi.org/10.1016/j.jece.2020.104365
Al-Shabib NA, Husain FM, Ahmed F, Khan RA, Ahmad I, Alsharaeh E, Khan MS, Hussain A, Rehman MT, Yusuf M, Hassan I, Khan JM, Ashraf GM, Alsalme A, Al-Ajmi MF, Tarasov VV, Aliev G (2016) Biogenic synthesis of Zinc oxide nanostructures from Nigella sativa seed: prospective role as food packaging material inhibiting broad-spectrum quorum sensing and biofilm. Sci Rep 6:36761. https://doi.org/10.1038/srep36761
Ali AS, Gires U, Fathul KS, Asmat A (2016) Inhibition of the planktonic and sessile growth and biocorrosion of Desulfovibrio sp Solution which is isolated from crude oil fluid by the ethyl acetate extraction from marine Alcaligenes faecalis for carbon steel protection. Austral J Basic Appl Sci 10(18):233–243
Altikatoglu M, Attar A, Erci F, Cristache CM, Isildak I (2017) Green synthesis of copper oxide nanoparticles using Ocimum basilicum extract and their antibacterial activity. Fresenius Environ Bull 26(12A):7832–7837
Amininezhad SM, Rezvani A, Amouheidari M, Amininejad SM, Rakhshani S (2015) The antibacterial activity of SnO2 nanoparticles against Escherichia coli and Staphylococcus aureus. Zahedan J Res Med Sci. 17(9): https://doi.org/10.17795/zjrms-1053
Anju S, Sarada J (2016) Quorum sensing inhibiting activity of silver nanoparticles synthesized by Bacillus isolate. Int J Pharm Biol Sci. 6(1):47–53
Ansari MA, Khan HM, Khan AA, Cameotra SS, Saquib Q, Musarrat J (2014) Gum arabic capped-silver nanoparticles inhibit biofilm formation by multi-drug resistant strains of Pseudomonas aeruginosa. J Basic Microbiol. https://doi.org/10.1002/jobm.201300748
Anupama R, Lulu S, Madhusmita R, Vino S, Mukherjee A, Babu S (2019) Insights into the interaction of key biofilm proteins in Pseudomonas aeruginosa PAO1 with TiO2nanoparticle: an in silico analysis. J Theor Biol 462:12–25. https://doi.org/10.1016/j.jtbi.2018.10.057
Arsalan N, Kashi EH, Hasan A, Doost ME, Rasti B, Paray BA, Nakhjiri MZ, Sari S, Sharifi M, Shahpasand K, Akhtari K, Haghighat S, Falahati M (2020) Exploring the interaction of cobalt oxide nanoparticles with albumin, leukemia cancer cells and pathogenic bacteria by multispectroscopic, docking, cellular and antibacterial approaches. Int J Nanomed 15:4607–4623. https://doi.org/10.2147/IJN.S257711
Arumugam A, Karthikeyan C, Hameed ASH, Gopinath K, Gowri S, Karthika V (2015) Synthesis of cerium oxide nanoparticles using Gloriosa superba L. leaf extract and their structural, optical and antibacterial properties. Mater Sci Eng, C 49:408–415. https://doi.org/10.1016/j.msec.2015.01.042
Arunkumar M, Mahesh N, Balakumar S, Sivakumar R, Priyadharshni S (2013) Antiquorum sensing and antibacterial activity of silver nanoparticlessynthesized by mutant Klebsiella pneumoniae MTCC 3354. Asian J Chem 25(17):9961–9964. https://doi.org/10.14233/ajchem.2013.15754
Arunkumar M, Suhashini K, Mahesh N, Ravikumar R (2014) Quorum quenching and antibacterial activity of silver nanoparticles synthesized from Sargassum polyphyllum. Bangladesh J Pharmacol 9:54–59
Arya A, Gupta K, Chundawat TS (2020) In vitro antimicrobial and antioxidant activity ofbiogenically synthesized palladium and platinum nanoparticles using Botryococcus braunii. Turk J Pharm Sci 17(3):299–306. https://doi.org/10.4274/tjps.galenos.2019.94103
Awasthi A, Sharma P, Jangir L, Kamakshi et al (2020) Dose dependent enhanced antibacterial effects and reduced biofilm activity against Bacillus subtilis in presence of ZnO nanoparticles. Mater Sci Eng, C 113: https://doi.org/10.1016/j.msec.2020.111021
Azad A, Rostamifar S, Modaresi F, Bazrafkan A, Rezaie (2020) Assessment of the antibacterial effects of bismuth nanoparticles against Enterococcus faecalis. Biomed Res Int. https://doi.org/10.1155/2020/5465439
Azam A, Ahmed AS, Oves M, Khan M, Memic A (2012) Size-dependent antimicrobial properties of CuO nanoparticles against Gram-positive and-negative bacterial strains. Int J Nanomed 7:3527–3535
Azhir E, Etefagh R, Shahtahmasebi N, Mashreghi M, Pordeli P (2015) Preparation, characterization and antibacterial activity of manganese oxide nanoparticles. Phys Chem Res 3(3):197–204. https://doi.org/10.22036/pcr.2015.9329
Azizi S, Shahri M, Rahman HS, Rahim RA, Rasedee A, Mohamad R (2017) Green synthesis palladium nanoparticles mediated by white tea (Camellia sinensis) extract with antioxidant, antibacterial, and antiproliferative activities toward the human leukemia (MOLT-4) cell line. Int J Nanomed 12:8841–8853
Baek YW, An YJ (2011) Microbial toxicity of metal oxide nanoparticles (CuO, NiO, ZnO, and Sb2O3) to Escherichia coli, Bacillus subtilis, and Streptococcus aureus. Sci Total Environ 409:1603–1608
Bakkiyaraj D, Pandian SK (2014) Biofilm inhibition by nanparticles. In: Rumbaugh K, Ahmad I (eds) Antibiofilm agents. Springer series on Biofilms. Springer, Berlin
Balasubramanian S, Kala SMJ, Pushparaj TL (2020) Biogenic synthesis of gold nanoparticles using Jasminum auriculatum leaf extract and their catalytic, antimicrobial and anticancer activities. J Drug Deliv Sci Technol 57:101620. https://doi.org/10.1016/j.jddst.2020.101620
Bellio P, Luzi C, Mancini A, Cracchiolo S, Passacantando M, Pietro LD, Perilli M, Amicosante G, Santucci S, Celenza G (2018) Cerium oxide nanoparticles as potential antibiotic adjuvant. Effects of CeO2 nanoparticles on bacterial outer membrane permeability. BBA - Biomembranes 1860:2428–2435. https://doi.org/10.1016/j.bbamem.2018.07.002
Bhuvaneshwari M, Bairoliya S, Parashar A, Chandrasekaran N, Mukherjee A (2016) Differential toxicity of Al2O3 particles on Gram-positive and Gram-negative sediment bacterial isolates from freshwater. Environ Sci Pollut Res 23:12095–12106. https://doi.org/10.1007/s11356-016-6407-9
Bhuyan T, Mishra K, Khanuja M, Prasad R, Varma A (2015) Biosynthesis of zinc oxide nanoparticles from Azadirachta indica for antibacterial and photocatalytic applications. Mater Sci Semicond Process 32:55–61
Campos V, Almaguer-Flores A, Velasco-Aria D, Díaz D, Rodil SE (2018) Bismuth and silver nanoparticles as antimicrobial agent over subgingival bacterial and nosocomial strains. J Mater Sci Eng A 8(7–8):142–146. https://doi.org/10.17265/2161-6213/2018.7-8.002
Carey DN, Knut D, Kevin RF (2016) Spatial structure, cooperation and competition in biofilm. Nat Rev Microbiol 14:589–600
Carvalho MP, Wolf-Rainer A (2012) Antimicrobial and biofilm inhibiting diketopiperazines. Curr Med Chem 19(21):3564–3577
Castillo IF, Matteis LD, Marquina C, Guillén EG, Fuente JM, Mitchell SG (2019) Protection of 18th century paper using antimicrobial nano-magnesium oxide. Int Biodeter Biodegrad 141:79–86. https://doi.org/10.1016/j.ibiod.2018.04.004
Chapman J, Weir E, Regan F (2010) Period four metal nanoparticles on the inhibition of biofouling. Colloids Surf B 78:208–216. https://doi.org/10.1016/j.colsurfb.2010.03.002
Chatterjee A, Nishanthini D, Sandhiya N, Abraham J (2016) Biosynthesis of titanium dioxide nanoparticles using Vigna radiata. Asian J Pharm Clin Res 9(4):85–88
Chen Z, Gao S-H, Jin M, Sun S, Lu J, Yang P, Bond PL, Yuan Z, Guo J (2019) Physiological and transcriptomic analyses reveal CuO nanoparticle inhibition of anabolic and catabolic activities of sulfate-reducing bacterium. Environ Int 125:65–74. https://doi.org/10.1016/j.envint.2019.01.058
Dalvand LF, Hosseini F, Dehaghi SM, Torbati ES (2018) Inhibitory effect of bismuth oxide nanoparticles produced by Bacillus licheniformis on methicillin- resistant Staphylococcus aureus strains (MRSA). Iranian J Biotech 16(4): https://doi.org/10.21859/ijb.2102
Das D, Nath BC, Phukon P, Dolui SK (2013) Synthesis and evaluation of antioxidantand antibacterial behavior of CuO nanoparticles. Colloids Surf B 101:430–433
Das KR, Kerkar S, Meena Y, Mishra S (2017) Effects of iron nanoparticles on iron-corroding bacteria. Biotech 7:385. https://doi.org/10.1007/s13205-017-1018-9
Das KR, Tiwari AK, Kerkar S (2020) Psychrotolerant Antarctic bacteria biosynthesize gold nanoparticles activeagainst sulphate reducing bacteria. Prep Biochem Biotechnol 50(5):438–444. https://doi.org/10.1080/10826068.2019.1706559
Dhandapani P, Maruthamuthu S, Rajagopal G (2012) Bio-mediated synthesis of TiO2 nanoparticles and its photocatalytic effecton aquatic biofilm. J Photochem Photobiol, B 110:43–49. https://doi.org/10.1016/j.jphotobiol.2012.03.003
Dhas TS, Sowmiya P, Kumar VG, Ravi M, Suthindhiran K, Borgio JF, Narendrakumar G, Kumar VR, Karthick V, Kumar CMV (2020) Antimicrobial effect of Sargassum plagiophyllum mediated gold nanoparticles on Escherichia coli and Salmonella typhi. Biocatal Agric Biotechnol 26:101627. https://doi.org/10.1016/j.bcab.2020.101627
Din MI, Nabi AG, Rani A, Aihetasham A, Mukhtar M (2018) Single step green synthesis of stable nickel and nickel oxide nanoparticles from Calotropis gigantean: catalytic and antimicrobial potentials. Environ Nanotechnol Monit Manag 9:29–36
Dwivedi S, Wahab R, Khan F, Mishra YK, Musarrat J, Al-Khedhair AA (2014) Reactive oxygen species mediated bacterial biofilm inhibition via zinc oxide nanoparticles and their statistical determination. PLoS ONE 9(11): https://doi.org/10.1371/journal.pone.0111289
Dyshlyuk L, Babich O, Ivanova S, Vasilchenco N, Atuchin V, Korolkov I, Russakov D, Prosekov A (2020) Antimicrobial potential of ZnO, TiO2 and SiO2 nanoparticles in protecting building materials from biodegradation. Int Biodeterior Biodegrad. https://doi.org/10.1016/j.ibiod.2019.104821
El-Sayyad GS, Mosallam FM, El-Batal MI (2018) One-pot green synthesis of magnesium oxide nanoparticles using Penicillium chrysogenum melanin pigment and gamma rays withantimicrobial activity against multidrug-resistant microbes. Adv Powder Technol 29:2616–2625. https://doi.org/10.1016/j.apt.2018.07.009
Erlin Z, Lei Y, Jianwei X, Haiyan C (2010) Microstructure, mechanical properties and biocorrosion properties of Mg–Si (-Ca, Zn) alloy for biomedical application. Acta Biomater 6(5):1756–1762
Ezealisiji KM, Noundou XS (2020) Green synthesis of zinc oxide nanoparticles and their antibiotic-potentiation activities of mucin against pathogenic bacteria. Res J Nanosci Nanotechnol 10(1):9–14. https://doi.org/10.3923/rjnn.2020.9.14
Ezealisiji KM, Noundou XS, Ukwueze SE, Atuzie W (2018) Biosynthesis, characterization and antimicrobial activity of silver nanoparticles using cell free lysate of Bacillus subtilis: a biotechnology approach. Am J Nanosci Nanotechnol Res 6:18–27
Ezealisiji KM, Noundou XS, Ukwueze SE (2017) Green synthesis and characterization of monodispersed silver nanoparticles using root bark aquous extract of Annona muricata Linn and their antimicrobial activity. Appl Nanosci 7:905–911. https://doi.org/10.1007/s13204-017-0632-5
Fernando SID, Cruz KSJ (2019) Ethnobotanical biosynthesis of gold nanoparticles and itsdownregulation of Quorum Sensing-linked AhyR gene in Aeromonas hydrophila. SN Appl Sci 2:570. https://doi.org/10.1007/s42452-020-2368-1
Gabrielyan L, Hovhannisyan A, Gevorgyan V, Ananyan M, Trchounian A (2019) Antibacterial effects of iron oxide (Fe3O4) nanoparticles: distinguishingconcentration- dependent effects with different bacterial cells growthand membrane-associated mechanisms. Appl Microbiol Biotechnol 103:2773–2782. https://doi.org/10.1007/s00253-019-09653-x
Gabrielyan L, Hakobyan L, Hovhannisyan A, Trchounian A (2019) Effects of iron oxide (Fe3O4) nanoparticles on Escherichia coli antibiotic-resistant strains. J Appl Microbiol 126:1108–1116. https://doi.org/10.1111/jam.14214
Gad MM, Al-Thobity AM, Shahin SY, Alsaqer BT, Ali AA (2017) Inhibitory effect of zirconium oxide nanoparticles on Candida albicans adhesion to repaired polymethyl methacrylate denture bases and interim removable prostheses: a new approach for denture stomatitis prevention. Int J Nanomed 12:5409–5419. https://doi.org/10.2147/IJN.S142857
Garcıa-Lara B, Saucedo-Mora MA, Roldan-Sanchez JA, Perez-Eretza B, Ramasamy M, Lee J, Coria-Jimenez R, Tapia M, Varela-Guerrero V, Garcıa-Contreras R (2015) Inhibition of quorum-sensing-dependent virulence factorsand biofilm formation of clinical and environmental Pseudomonas aeruginosa strains by ZnO nanoparticles. Lett Appl Microbiol 61:299–305. https://doi.org/10.1111/lam.12456
Giovanna B, Giuseppantoni M, Semih E (2015) Antimicrobial peptides and their interaction with biofilm of medically relevant bacteria. Biochem Biophys Acta 1858:1044–1060
Ghareib M, Abdallah W, Tahon MA, Tallima A (2019) Biosynthesis of copper oxide nanoparticles using the preformed biomass of Aspergillus fumigatus and their antibacterial and photocatalytic activities. Digest J Nanomater Biostruct 14(2):291–303
Ghasemian E, Naghoni A, Rahvar H, Kialha M, Tabaraie B (2015) Evaluating the effect of copper nanoparticles in inhibiting Pseudomonas aeruginosa and Listeria monocytogenes biofilm formation, Jundishapur. J Microbiol 8(5):e17430. https://doi.org/10.5812/jjm.8(5)2015.17430
Gómez-Gómez B, Arregui L, Serrano S, Santos A, Pérez-Corona T, Madrid Y (2019) Unravelling mechanisms of bacterial quorum sensing disruption bymetal-based nanoparticles. Sci Total Environ 696: https://doi.org/10.1016/j.scitotenv.2019.133869
Goswami SR, Sahareen T, Singh M, Kumar S (2015) Role of biogenic silver nanoparticles in disruption of cell–cell adhesion in Staphylococcus aureus and Escherichia coli biofilm. J Ind Eng Chem 26:73–80. https://doi.org/10.1016/j.jiec.2014.11.017
Gowri S, Gandhi RR, Sundrarajan M (2014) Structural, optical, antibacterial and antifungal propertiesof zirconia nanoparticles by biobased protocol. J Mater Sci Technol 30(8):782–790. https://doi.org/10.1016/j.jmst.2014.03.002
Gu T (2012) New understanding of biocorrosion mechanisms and their classifications. J Microb Biochem Technol 4(4):3–6
Gupta K, Chundawat TS (2019) Bio-inspired synthesis of platinum nanoparticles from fungus Fusarium oxysporum: its characteristics, potential antimicrobial, antioxidant and photocatalytic activities. Mater Res Express 6:10506. https://doi.org/10.1088/2053-1591/ab4219
Gupta D, Singh A, Khan AU (2017) Nanoparticles as efflux pump and biofilm inhibitor to rejuvenate bactericidal effect of conventional antibiotics. Nanoscale Res Lett 12:454. https://doi.org/10.1186/s11671-017-2222-6
Habimana O, Zanoni M, Vitale S, O’Neill T, Scholz D, Xu B, Casey E (2018) One particle, two targets: a combined action of functionalized gold nanoparticles, against Pseudomonas fluorescens biofilms. J Colloid Interface Sci 526:419–428. https://doi.org/10.1016/j.jcis.2018.05.014
Hafeez M, Shaheen R, Akram B, Abdin Haq et al (2020) Green synthesis of cobalt oxide nanoparticles for potential biological applications. Mater Res Express 7: https://doi.org/10.1088/2053-1591/ab70dd
Haneefa MM, Jayandran M, Balasubramanian V (2017) Green synthesis characterization and antimicrobial activity evaluation of manganese oxide nanoparticles and comparative studies with salicylalchitosan functionalized nanoform. Asian J Pharm 11(1):65–74
Haneefa MM, Jayandran M, Balasubramanian V (2017) Evaluation of antimicrobial activity of green-synthesized manganese oxide nanoparticles and comparative studies with curcuminaniline functionalized. Asian J Pharm Clin Res 10(3):347–352. https://doi.org/10.22159/ajpcr.2017.v10i3.16246
Haq S, Rehman W, Waseem M, Shah A, Khan AR, Rehman MU, Ahmad P, Khan B, Ali G (2020) Green synthesis and characterization of tin dioxide nanoparticles for photocatalytic and antimicrobial studies. Mater Res Express 7: https://doi.org/10.1088/2053-1591/ab6fa1
Hashimoto M, Yanagiuchi H, Kitagawa H, Honda Y (2017) Inhibitory effect of platinum nanoparticles on biofilm formation of oral bacteria. Nano Biomed 9(2):77–82
Hayat S, Muzammil S, Aslam B, Siddigue MH, Saqalein M, Nisar MA (2019) Quorum quenching: role of nanoparticles as signal jammers in Gram-negative bacteria. Future Microbiol 14(1):61–72
Hayat S, Muzammil S, Rasool MH, Nisar Z, Hussain SZ, Sabri AN, Jamil S (2018) In vitro anti-biofilm and anti-adhesion effects ofmagnesium oxide nanoparticles against antibiotic resistantbacteria. Microbiol Immunol 62:211–220. https://doi.org/10.1111/1348-0421.12580
He Y, Ingudam S, Reed S, Gehring A, Strobaugh TP Jr, Irwin P (2016) Study on the mechanism of antibacterial action of magnesium oxide nanoparticles against foodborne pathogens. J Nanobiotechnol 14:54. https://doi.org/10.1186/s12951-016-0202-0
Hernandez-Delgadillo R, Velasco-Arias D, Martinez-Sanmiguel JJ, Diaz D, Zumeta- Dube I, Arevalo-Niño K, Cabral-Romero C (2013) Bismuth oxide aqueous colloidal nanoparticles inhibit Candida albicans growth and biofilm formation. Int J Nanomed 8:1645–1652
Hou J, Li T, Miao L, You G, Xu Y, Liu S (2019) Effects of titanium dioxide nanoparticles on algal and bacterialcommunities in periphytic biofilms. Environ Pollut 251:407–414. https://doi.org/10.1016/j.envpol.2019.04.136
Hsueh Y-H, Ke W-J, Hsieh C-T, Lin K-S, Tzou D-Y, Chiang C-L (2015) ZnO nanoparticles affect Bacillus subtilis cell growth and biofilm formation. PLoS ONE 10(6): https://doi.org/10.1371/journal.pone.0128457
Hussain A, Oves M, Alajmi MF, Hussain I, Amir S, Ahmed J, Rehman MT, El- Seedi HR, Ali I (2019) Biogenesis of ZnO nanoparticles using Pandanus odorifer leaf extract: anticancer and antimicrobial activities. R Soc Chem Adv 9:15357. https://doi.org/10.1039/cra01659g
Immanuel OM, Abu GO, Stanley HO (2016) Inhibition of biogenic sulphide production and biocorrosion of carbon steel by sulphate reducing bacteria using Ocimum gratissimum essential oil. J Adv Biol Biotechnol 10(2):1–12
Ishwarya R, Vaseeharan B, Kalyani S, Banumathi B, Govindarajan M, Alharbi NS, Kadaikunnan S, Al-anbr MN, Khaled JM, Benelli G (2018) Facile green synthesis of zinc oxide nanoparticles using Ulva lactuca seaweed extract and evaluation of their photocatalytic, antibiofilm and insecticidal activity. J Photochem Photobiol, B 178:249–258. https://doi.org/10.1016/j.jphotobiol.2017.11.006
Itohiya H, Matsushima Y, Shirakawa S, Kajiyama S, Yashima A, Nagano T, Gomi K (2019) Organic resolution function and effects of platinum nanoparticles on bacteria and organic matter. PLoS ONE 14(9): https://doi.org/10.1371/journal.pone.0222634
Itohiya H, Matsushima Y, Shirakawa S, Kajiyama S, Yashima A, Nagano T, Gomi K (2019) Organic resolution function and effects of platinum nanoparticles on bacteria and organic matter. PLoS ONE 14(9):e0222634. https://doi.org/10.1371/journal.pone.0222634
Ituen E, Ekemini E, Yuanhua L, Singh A (2020) Green synthesis of Citrus reticulata peels extract silver nanoparticlesand characterization of structural, biocide and anticorrosion properties. J Mol Struct 1207: https://doi.org/10.1016/j.molstruc.2020.127819
Jagathesan G, Rijiv P (2018) Biosynthesis and characterization of iron oxide nanoparticles using Eichhornia crassipes leaf extract and assessing their antibacterial activity. Biocatal Agric Biotechnol 13:90–94
Jamdagni P, Khatri P, Rana JS (2018) Green synthesis of zinc oxide nanoparticles using flower extract of Nyctanthes arbor-tristis and their antifungal activity. J King Saud Univ Sci 30:168–175
James AG, Steve M (2012) Interaction in bacterial biofilm development: a structural perspective. Curr Protein Pept Sci 13(8):739–755
Jangra SL, Stalin K, Dilbaghi N, Kumar S, Tawale J, Singh SP, Pasricha R (2012) Antimicrobial activity of zirconia (ZrO2) nanoparticles and zirconium complexes. J Nanosci Nanotechnol 12:7105–7112
Jasim NA, Al-Gashaa FA, Al-Marjani MF, Al-Rahal AH, Abid HA, Al-Kadhmi NA, Jakaria M, Rheima AM (2020) ZnO nanoparticles inhibit growth and biofilm formation of vancomycin-resistant S. aureus (VRSA). Biocatal Agric Biotechnol 29:101745. https://doi.org/10.1016/j.bcab.2020.101745
Jayabalan J, Mani G, Krishnan N, Pernabas J, Devadoss JM, Jang HT (2019) Green biogenic synthesis of zinc oxide nanoparticles using Pseudomonas putida culture and it’s In vitro antibacterial and anti-biofilm activity. Biocatal Agric Biotechnol 21: https://doi.org/10.1016/j.bcab.2019.101327
Jayandran M, Haneefa MM, Balasubramanian V (2015) Green synthesis and characterization of Manganese nanoparticles using natural plant extracts and its evaluation of antimicrobial activity. J Appl Pharm Sci 5(12):105–110. https://doi.org/10.7324/JAPS.2015.501218
Jayaseelan C, Rahuman AA, Kirthi AV, Marimuthu S, Santhoshkumar T, Bagavan A, Gaura K, Karthik L, Rao KVB (2012) Novel microbial route to synthesize ZnO nanoparticles using Aeromonas hydrophila and the activity against pathogenic bacteria and fungi. Spectrochimica Acta part A 90:78–84. https://doi.org/10:1016/j.saa.2012.01.006
Jeevigunta NLL, Navva VR (2017) Purification and antimicrobial activity of cobalt nano particles from Neurospora crassa. Res J Biotechnol 12(10):60–69
Jin-Hyung L, Jintae L (2014) ZnO nanoparticles inhibit Pseudomonas aeruginosa biofilm formation and virulence factor production. Microbiol Res 169(12):888–896
Jing H, Mezgebe B, Hassan AA, Sahle-Demessie E, Sorial GA, Bennett-Stamper C (2014) Experimental and modeling studies of sorption of ceria nanoparticle on microbial biofilms. Biores Technol 161:109–117. https://doi.org/10.1016/j.biortech.2014.03.015
Jothiprakasam V, Sambantham M, Chinnathambi S, Vijayaboopathi S (2017) Candida tropicalis biofilm inhibition by ZnO nanoparticles and EDTA. Arch Oral Biol 73:21–24. https://doi.org/10.1016/j.archoralbio.2016.09.003
Kadhum SA (2017) The effect of two types of nano-particles (ZnO & SiO2) on different types of bacterial growth. Biomed Pharmacol J 10(4):1701–1708. https://doi.org/10.13005/bpj/1282
Kalpana VN, Kataru BAS, Sravani N, Vigneshwari T, Panneerselvam A, Rajeswari VD (2018) Biosynthesis of zinc oxide nanoparticles using culture filtrates of Aspergillus niger: antimicrobial textiles and dye degradation studies. OpenNano 3:48–55
Kanagasubbulakshmi S, Kadirvelu K (2017) Green synthesis of iron oxide nanoparticles using Lagenaria siceraria and evaluation of its antimicrobial activity. Defence Life Sci J 2(4):422–427. https://doi.org/10.14429/dlsj.2.12277
Karimiyan A, Najafzadeh H, Ghorbanpour M, Hekmati-Moghaddam SH (2015) Antifungal effect of magnesium oxide, zinc oxide, silicon oxide and copper oxide nanoparticles against Candida albicans. Zahedan J Res Med Sci. 17(10): https://doi.org/10.17795/zjrms-2179
Khan ST, Ahamed M, Alhadlaq HA, Musarrat J, Al-Khedhairy A (2013) Comparative effectiveness of NiCl2, Ni- and NiO-NPs in controlling oral bacterial growth and biofilm formation on oral surfaces. Arch Oral Biol 58:1804–1811. https://doi.org/10.1016/j.archoralbio.2013.09.011
Khan F, Manivasagan P, Lee J-W, Pham DTN, Oh J, Kim Y-M (2019) Fucoidan-stabilized gold nanoparticle-mediated biofilm inhibition, attenuation of virulence and motility properties in Pseudomonas aeruginosa PAO1. Marine Drugs 17:208. https://doi.org/10.3390/md17040208
Kip N, Veen JAV (2015) The dual role of microbes in corrosion. ISME J 9:542–551. https://doi.org/10.1038/ismej.2014.169
Korkmaz N, Ceylan Y, Karada A, Bülbül AS, Aftab MN, Saygıli S, Sen F (2020) Biogenic silver nanoparticles synthesized from Rhododendronponticum and their antibacterial, antibiofilm and cytotoxic activities. J Pharm Biomed Anal 179: https://doi.org/10.1016/j.jpba.2019.112993
Kuchekar SR, Dhage PM, Aher HR, Han SH (2018) Green synthesis of cobalt nanoparticles, its characterization and antimicrobial activities. Int J Chem Phys Sci 7:190–198
Kumar N, Omoregie EO, Rose J, Masion A, Lloyd JR, Diels L, Bastiens L (2014) Inhibition of sulphate reducing bacteria in aquifer sediment by iron nanoparticles. Water Res 51:64–72
Kumaresan M, Anand KV, Govindaraju K, Tamilselvan S, Kumar VG (2018) Seaweed Sargassum wightii mediated preparation of zirconia (ZrO2) nanoparticles and their antibacterial activity against Gram positive and Gram negative bacteria. Microb Pathog 124:311–315. https://doi.org/10.1016/j.micpath.2018.08.060
Lara HH, Romero-Urbina DG, Pierce C, Lopez-Ribot JL, Arellano-Jimenez MJ, Jose-Yacaman (2015) Effect of silver nanoparticles on Candida albicans biofilms: an ultrastructural study. J Nanobiotechnol 13:91. https://doi.org/10.1186/s12951-015-0147-8
Lars DR, Douglas BW (2011) Physicochemical regulation of biofilm formation. MRS Bull 36(5):347–355
Lee J-H, Kim Y-G, Cho MH, Lee J (2014) ZnO nanoparticles inhibit Pseudomonas aeruginosa biofilm formation and virulence factor production. Microbiol Res 169:888–896. https://doi.org/10.1016/j.micres.2014.05.005
Li R, Chen Z, Ren N, Wang Y, Wang Y, Yu F (2019) Biosynthesis of silver oxide nanoparticles and their photocatalytic and antimicrobial activity evaluation for wound healing applications in nursing care. J Photochem Photobiol, B 199: https://doi.org/10.1016/j.jphotobiol.2019.111593
Li N, Wang L, Yan H, Wang M, Shen D, Yin J, Shentu J (2018) Effects of low- level engineered nanoparticles on the quorumsensing of Pseudomonas aeruginosa PAO1. Environ Sci Pollut Res 25:7049–7058. https://doi.org/10.1007/s11356-017-0947-5
Liang Y, Yang L, Hong W, Niels B, Soren M, Zhi-jun S (2011) Current understanding of multispecies biofilm. Int J Oral Sci 3:74–81
Lioa S, Zhang Y, Pan X, Zhu F, Jiang C, Liu Q, Cheng Z, Dai G, Wu G, Wang L, Chen L (2019) Antibacterial activity and mechanism of silver nanoparticles against multidrug resistant Pseudomonas aeruginosa. Int J Nanomed 14:1469–1487
Lin J, Ballim R (2012) Biocorrosion control: current strategies and promising alternatives. Afr J Biotech 11(91):15736–15747
Liu L, Li J-H, Zi S-H, Liu F-R, Deng C, Ao X, Zhang P (2019) AgNP combined with quorum sensing inhibitor increased the antibiofilm effect on Pseudomonas aeruginosa. Appl Microbiol Biotechnol 103:6195–6204. https://doi.org/10.1007/s00253-019-09905-w
Loo YY, Rukayadi Y, Nor-Khaizura M-A-R, Kuan CH, Chieng BW, Nishibuchi M, Radu S (2018) In vitro antimicrobial activity of green synthesized silver nanoparticles against elected Gram negative foodborne pathogens. Front Microbiol 9:1555. https://doi.org/10.3389/fmicb.2018.01555
Mahlangeni NT, Magura J, Moodley R, Baijnath H, Chenia H (2020) Biogenic synthesis, antioxidant and antimicrobial activity of silver and manganese dioxide nanoparticles using Cussonia zuluensis Strey. Chem Pap 74:4253–4265. https://doi.org/10.1007/s11696-020-01244-9
Majumdar M, Khan SA, Biswas SC, Roy DN et al (2020) In vitro and in silico investigation of anti-biofilm activity of Citrus macroptera fruit extract mediated silver nanoparticles. J Mol Liq 302: https://doi.org/10.1016/j.molliq.2020.112586
Manafi Z, Hashemi M, Abdollahi H, Gregory JO (2013) Biocorrosion of water pipeline by sulphate reducing bacteria in a mining environment. Afr J Biotech 12(46):6504–6516. https://doi.org/10.5897/AJB11.3250
Manikandan V, Velmurugan P, Park J-H, Lovanh N, Seo S-K, Jayanthi P, Park Y-J, Cho M, Oh B-T (2016) Synthesis andantimicrobialactivityofpalladium nanoparticlesfrom Prunus_yedoensis leaf extract. Mater Lett 185:335–338. https://doi.org/10.1016/j.matlet.2016.08.120
Maruthupandy M, Rajivgandhi GN, Quero F, Li W-J (2020) Anti-quorum sensing and anti-biofilm activity of nickel oxide nanoparticles against Pseudomonas aeruginosa. J Environ Chem Eng 8: https://doi.org/10.1016/j.jece.2020.104533
Mathur A, Bhuvaneshwari M, Babu S, Chandrasekaran N, Mukherjee A (2017) The effect of TiO2 nanoparticles on sulphate reducing bacteria and their consortium under anaerobic conditions. J Environ Chem Eng 5:3741–3748
Maurer-Jones MA, Gunsolus IL, Meyer BM, Christenson CJ, Haynes CL (2013) Impact of TiO2 nanoparticles on growth, biofilm formation, and flavin secretion in Shewanella oneidensis. Anal Chem 85(12):5810–5818. https://doi.org/10.1021/ac400486u
Mette B, Dawei R, Thomas B, Soren JS (2014) Interactions in multispecies biofilm: do they actually matter? Trends Microbiol 22(2):84–91
Mohamed HEA, Afridi S, Khalil AT, Ali M, Zohra T, Akhtar R, Ikram A, Shinwari ZK, Maaza M (2020) Promising antiviral, antimicrobial and therapeutic properties of green nanoceria. Nanomedicine (Lond.) 15(5):467–488
Mohana S, Sumathi S (2020) Multi-functional biological effects of palladium nanoparticles synthesized using Agaricus bisporus. J Clust Sci 31:391–400. https://doi.org/10.1007/s10876-019-01652-2
Moradpoor H, Safaei M, Rezaei F, Golshah A, Jamshidy L, Hatam R, Abdullah RS (2019) Optimisation of cobalt oxide nanoparticles synthesis as bactericidal agents. Macedonian J Med Sci 7(17):2757–2762. https://doi.org/10.3889/oamjms.2019.747
Moulavi P, Noorbazargan H, Dolatabadi A, Foroohimanjili F, Tavakoli Z, Mirzazadeh S, Hashem M, Ashrafi F (2019) Antibiofilm effect of green engineered silver nanoparticles fabricated from Artemisia scoporia extract on the expression of icaA and icaR genes against multi drug resistant Staphylococcus aureus. J Basic Microbiol 59:701–712
Moura MC, Pontual EV, Paiva PMG, Coelho LCBB (2013) An outline to corrosive bacteria. In: Méndez-Vilas A (ed) Microbial pathogens and strategies for combating them: science, technology and education. Formatex, Badajoz, pp 11–22
Muthuvel A, Jothibas M, Mohana V, Manoharan C (2020) Green synthesis of cerium oxide nanoparticles using Calotropis procera flower extract and their photocatalytic degradation and antibacterial activity. Inorg Chem Commun 119: https://doi.org/10.1016/j.inoche.2020.108086
Muzammil S, Khurshid M, Nawaz I, Siddique MH, Zubair M, Nisar MA, Imran M, Hayat S (2020) Aluminium oxide nanoparticles inhibit EPS production, adhesion and biofilm formation by multidrug resistant Acinetobacter baumannii. Biofouling 36(4):492–504. https://doi.org/10.1080/08927014.2020.1776856
Naik K, Kowshik M (2014) Anti-quorum sensing activity of AgCl-TiO2 nanoparticles with potential use as active food packaging material. J Appl Microbiol 117:972–983. https://doi.org/10.1111/jam.12589
Noori AJ, Kareem FA (2019) The effect of magnesium oxide nanoparticles on the antibacterial and antibiofilm properties of glass-ionomer cement. Heliyon 5: https://doi.org/10.1016/j.heliyon.2019.e02568
Obeizi Z, Benbouzid H, Ouchenane S, Yılmaz D, Culha M, Bououdina M (2020) Biosynthesis of zinc oxide nanoparticles from essential oil of Eucalyptus globulus with antimicrobial and anti-biofilm activities. Mater Today Commun 25:101553. https://doi.org/10.1016/j.mtcomm.2020.101553
Omran BA, Nassar HN, Younis SA, El-Salamony RA, Fatthallah NA, Hamdy A, El-Shatoury EH, El-Gendy NSh (2019) Novel mycosynthesis of cobalt oxide nanoparticles using Aspergillus brasiliensis ATCC 16404—optimization, characterization and antimicrobial activity. J Appl Microbiol 128:438–457. https://doi.org/10.1111/jam.14498
Osonga FJ, Kalra S, Miller RM, Isika D, Sadik OA (2020) Synthesis, c haracterization and antifungal activities of eco-friendly palladium nanoparticles. RSC Adv 10:5894–5904. https://doi.org/10.1039/c9ra07800b
Ouyang K, Mortimer M, Holden PA, Cai P, Wu Y, Gao C, Huang Q (2020) Towards a better understanding of Pseudomonas putida biofilm formation in the presence of ZnO nanoparticles (NPs): role of NP concentration. Environ Int 137: https://doi.org/10.1016/j.envint.2020.105485
Pugazhendhi A, Prabhu R, Muruganantham K, Shanmuganathan R, Natarajan S (2019) Anticancer, antimicrobial and photocatalytic activities of green synthesized magnesium oxide nanoparticles (MgONPs) using aqueous extract of Sargassum wightii. J Photochem Photobiol, B 190:86–97. https://doi.org/10.1016/j.jphotobiol.2018.11.014
Punniyakotti P, Panneerselvam P, Perumal D, Aruliah R, Angaiah S (2020) Anti- bacterial and anti-biofilm properties of green synthesized coppernanoparticles from Cardiospermum halicacabum leaf extract. Bioprocess Biosyst Eng 43:1649–1657. https://doi.org/10.1007/s00449-020-02357-x
Qais FA, Shafiq A, Ahmad I, Husain FM, Khan RA, Hassan I (2020) Green synthesis of silver nanoparticles using Carum copticum: assessment of its quorum sensing and biofilm inhibitory potential against gram negative bacterial pathogens. Microb Pathog 144: https://doi.org/10.1016/j.micpath.2020.104172
Radzig MA, Nadtochenko VA, Koksharova OA, Kiwi J, Lipasova VA, Khmel IA (2013) Antibacterial effects of silver nanoparticles on gram-negative bacteria: influence on the growth and biofilms formation, mechanisms of action. Colloids Surf B 102:300–306. https://doi.org/10.1016/j.colsurfb.2012.07.039
Rahul B, Shaily MB, Brajendra M, David LO (2010) Microbiologically influenced corrosion and its mitigation. Mater Sci Res India 7(2):407–412
Rajivgandhi GN, Ramachandran G, Maruthupandy M, Manoharan N, Alharbi NS, Kadaikunnan S, Khaled JM, Almanaa TN, Li W-J (2020) Anti-oxidant, anti-bacterial and anti-biofilm activity of biosynthesized silver nanoparticles using Gracilaria corticata against biofilm producing K. pneumoniae. Colloids Surf A 600: https://doi.org/10.1016/j.colsurfa.2020.124830
Rajkumari J, Magdalane CM, Siddhardha B, Madhaven J, Ramalingam G, Al-Dhabi NA, Arasu MV, Ghilan AKM, Duraipandiayan V, Kaviyarasu K (2019) Synthesis of titanium oxide nanoparticles using Aloe barbadensis mill and evaluation of its antibiofilm potential against Pseudomonas aeruginosa PAO1. J Photochem Photobiol, B 201: https://doi.org/10.1016/j.jphotobiol.2019.111667
Rasheed PA, Jabbar KA, Mackey HR, Mahmoud KA (2019) Recent advancements in nanomaterials as coatings and biocides for the inhibition of sulphate reducing bacteria induced corrosion. Curr Opin Chem Eng 25:35–42
Rasheed PA, Jabbar KA, Rasool K, Pandey RP, Sliem MH, Helal M, Samara A, Abdullah AM, Mahmoud KA (2019) Controlling the biocorrosion of sulfate-reducing bacteria (SRB) on carbon steel using ZnO/chitosan nanocomposite as an eco- friendly biocide. Corros Sci 148:397–406. https://doi.org/10.1016/j.corsci.2018.12.028
Renxing L, Deniz FA, Egemen A, Vincentt B, Jan S, Joseph MS (2016) Marine environments. Environ Sci Technol 50(9):4844–4853
Salam FD, Vinita MN, Puja P, Prakash S, Yuvakkumar R, Kumar P (2020) Anti-bacterial and anti-biofilm efficacies of bioinspired gold nanoparticles. Mater Lett 261: https://doi.org/10.1016/j.matlet.2019.126998
Saleem S, Ahmed B, Khan MS, Al-Shaeri M, Musarrat J (2017) Inhibition of growth and biofilm formation of clinical bacterial isolates by NiO nanoparticles synthesized from Eucalyptus globulus plants. Microb Pathog 111:375–387. https://doi.org/10.1016/j.micpath.2017.09.019
Saleh NB, Chambers B, Aich N, Plazas-Tuttle J, Phung-Ngoc HN, Kirisits MJ (2015) Mechanisticles on learned from studies of planktonic bacteria with metallic nanomaterials: implications for interactions between nanomaterials andbiofilm bacteria. Front Microbiol 6:677. https://doi.org/10.3389/fmicb.2015.00677
Saleh MM, Sadeq RA, Abdel Latif HK, Abbas HA, Askoura M (2019) Zinc oxide nanoparticles inhibits quorum sensing and virulence in Pseudomonas aeruginosa. Afri Health Sci 19(2):2043–2055. https://doi.org/10.4314/ahs.v19i2.28
Samanta S, Singh BR, Adholeya A (2017) Intracellular synthesis of gold nanoparticles using an ectomycorrhizal strain EM-1083 of laccaria fraternal and its nanoanti-quorum sensing potential against Pseudomonas aeruginosa. Indian J Microbiol 57(4):448–460. https://doi.org/10.1007/s12088-017-0662-4
Samanta A, Podder S, Kumarasamy M, Ghosh CK, Lahiri D, Roy P, Bhattacharjee G et al (2019) Au nanoparticle-decorated aragonite microdumbbells for enhanced antibacterial and anticancer activities. Mater Sci Eng, C 103:109734. https://doi.org/10.1016/j.msec.2019.05.019
Satpathy G, Manikandan E (2019) Cobalt nanoparticle as the antibacterial tool in vitro. Int J Eng Adv Technol 8(6):3684–3687. https://doi.org/10.35940/ijeat.F9374.088619
Sathyanarayanan MB, Balachandranath R, Srinivasulu YG, Kannaiyan SK, Subbiahdoss G (2013) The effect of gold and iron-oxide nanoparticles on biofilm-forming pathogens. ISRN Microbiol. https://doi.org/10.1155/2013/272086
Sengan M, Subramaniyan SB, Prakash SA, Kamlekar R, Veerappan A (2019) Effective elimination of biofilm formed with waterborne pathogens using copper nanoparticles. Microb Pathog 127:341–346. https://doi.org/10.1016/j.micpath.2018.12.025
Shah S, Gaikwad S, Nagar S, Kulshrestha S, Vaidya V, Nawani N, Pawar S (2019) Biofilm inhibition and anti-quorum sensing activity of phytosynthesized silver nanoparticles against the nosocomial pathogenPseudomonas aeruginosa. Biofouling 35(1):34–49. https://doi.org/10.1080/08927014.2018.1563686
Sharma KD (2017) Antibacterial activity of biogenic platinum nanoparticles: an in vitro study. Int J Curr Microbiol Appl Sci 6(2):801–808
Sharmila G, Fathima MF, Haries S, Geetha S, Kumar NM, Muthukumaran C (2017) Green synthesis, characterization and antibacterial efficacy of palladium nanoparticles synthesized using Filicium decipiens leaf extract. J Mol Struct 1138:35–40. https://doi.org/10.1016/j.molstruc.2017.02.097
Sheikh S, Tale V (2017) Green synthesis of silver nanoparticles: its effect on quorum sensing inhibition of urinary tract infection pathogens. Asian J Pharm Clin Res 10(5):302–305. https://doi.org/10.22159/ajpcr.2017.v10i5.16949
Sillankorva S, Azeredo J (2014) Bacteriophage attack as an anti-biofilm strategy. In: Donelli G (ed) Microbial biofilms. Methods in molecular biology (methods and protocols). Humana Press, New York
Singh P (2016) Biosynthesis of titanium dioxide nanoparticles and their antibacterial property. Int J Chem Mol Eng 10(2):275–278
Singh BR, Singh BN, Singh A, Khan W et al (2015) Mycofabricated biosilver nanoparticles interrupt Pseudomonas aeruginosa quorum sensing systems. Sci Rep 5:13719. https://doi.org/10.1038/srep13719
Singh P, Pandit S, Garnæs J, Tunjic S, Mokkapati VRSS, Sultan A, Thygesen A, Mackevica A, Mateiu RV, Daugaard AE, Baun A, Mijakovic I (2018) Green synthesis of gold and silver nanoparticles from Cannabis sativa (industrial hemp) and their capacity for biofilm inhibition. Int J Nanomed 13:3571–3591
Singh RK, Behera SS, Singh KR, Mishra S, Panigrahi B, Sahoo TR, Parhi PK, Mandal D (2020) Biosynthesized gold nanoparticles as photocatalysts for selective degradation of cationic dye and their antimicrobial activity. J Photochem Photobiol, A 400: https://doi.org/10.1016/j.jphotochem.2020.112704
Smirnov NA, Kudryashov SI, Nastulyavichus AA, Rudenko AA, Saraeva IN, Tolordava ER, Gonchukov SA, Romanova YM, Ionin AA, Zayarny DA (2018) Antibacterial properties of silicon nanoparticles. Laser Phys Lett 15:105602. https://doi.org/10.1088/1612-202X/aad853
Soliman H, Elsayed A, Dyaa A (2018) Antimicrobial activity of silver nanoparticles biosynthesized by Rhodotorula sp. Strain ATL72. Egypt J Basic Appl Sci 5:228–233
Srinivasan R, Vigneshwari L, Rajavel T, Durgadevi R, Kannappan A, Balamurugan K, Devi KP, Ravi AV (2018) Biogenic synthesis of silver nanoparticles using Piper betle aqueous extract and evaluation of its anti-quorum sensing and antibiofilm potential against uropathogens with cytotoxic effects: an in vitro and in vivo approach. Environ Sci Pollut Res 25:10538–10554. https://doi.org/10.1007/s11356-017-1049-0
Sunderam V, Thiyagarajan D, Lawrence AV, Mohammed SSS, Selvaraj A (2019) In-vitro antimicrobial and anticancer properties of green synthesizedgold nanoparticles using Anacardium occidentale leaves extract. Saudi J Biol Sci 26:455–459. https://doi.org/10.1016/j.sjbs.2018.12.001
Surendra TV, Roopan SM (2016) Photocatalytic and antibacterial properties of phytosynthesized CeO2 NPs using Moringa oleifera peel extract. J Photochem Photobiol, B 161:122–128. https://doi.org/10.1016/j.jphotobiol.2016.05.019
Surendra TV, Roopan SM, Arasu MV, Al-Dhabi NA, Rayalu GM (2016) RSM optimized Moringa oleifera peel extract for green synthesis of M. oleifera capped palladium nanoparticles with antibacterial and hemolytic property. J Photochem Photobiol, B 162:550–557. https://doi.org/10.1016/j.jphotobiol.2016.07.032
Suresh J, Pradheesh G, Alexramani V, Sundrarajan M, Hong SI (2018) Green synthesis and characterization of hexagonal shaped MgO nanoparticles using insulin plant (Costus pictus D. Don) leave extract andits antimicrobial as well as anticancer activity. Adv Powder Technol 29:1685–1694. https://doi.org/10.1016/j.apt.2018.04.003
Thoker BA, Bhat AA, Wani AK, Kaloo MA, Shergojri GA (2020) Preparation and characterization of SnO2 nanoparticles for antibacterial properties. Nanomaterial Chem Technol 2(1):1–5. https://doi.org/10.33805/2690-2575.109
Thomas KW, Seok H, Qun M (2011) Engineering biofilm formation and dispersal. Trends Biotechnol 29(2):87–94
Thukkaram M, Sitaram S, Kannaiyan SK, Subbiahdoss G (2014) Antibacterial efficacy of iron-oxide nanoparticles against biofilms on different biomaterial surfaces. Int J Biomater. https://doi.org/10.1155/2014/716080
Tiwari A, Sherpa YL, Pathak AP, Singh LS, Gupta A, Tripathi A (2019) One- pot green synthesis of highly luminescent silicon nanoparticles using Citrus limon (L.) and their applications in luminescent cell imaging and antimicrobial efficacy. Mater Today Commun 19:62–67. https://doi.org/10.1016/j.mtcomm.2018.12.005
Vahedi M, Hosseini-Jazani N, Yousefi S, Ghahremani M (2017) Evaluation of anti- bacterial effects of nickel nanoparticles on biofilmproduction by Staphylococcus epidermidis. Iran J Microbiol 9(3):160–168
Vazquez-Munoz R, Arellano-Jimenez MJ, Lopez-Ribot J (2020) Bismuth nanoparticles obtained by a facilesynthesis method exhibit antimicrobialactivity against Staphylococcus aureus andCandida albicans. BMC Biomed Eng 2:11. https://doi.org/10.1186/s42490-020-00044-2
Vidhu VD, Philip D (2015) Biogenic synthesis of SnO2 nanoparticles: evaluation of antibacterial and antioxidant activities. Spectrochim Acta Part A Mol Biomol Spectrosc 134:372–379. https://doi.org/10.1016/j.saa.2014.06.131
Vijayakumar S, Malaikozhundan B, Saravanakumar K, Duran-Lara EF, Wang M-H, Vaseeharan K (2019) Garlic clove extract assisted silver nanoparticle – antibacterial, antibiofilm, antihelminthic, anti-inflammatory, anticancer and ecotoxicity assessment. J Photochem Photobiol, B 198:111558. https://doi.org/10.1016/j.jphotobiol.2019.111558
Vijayakumar S, Vinoj G, Malaikozhundan B, Shanthi S, Vaseeharan B (2015) Plectranthus amboinicus leaf extract mediated synthesis of zinc oxide nanoparticles and its control of methicillin resistant Staphylococcus aureus biofilm and blood sucking mosquito larvae. Spectrochim Acta Part A Mol Biomol Spectrosc 137:886–891. https://doi.org/10.1016/j.saa.2014.08.064
Vijayan SR, Santhiyagu P, Singamuthu M, Ahila NK, Jayaraman R, Ethiraj K (2014) Synthesis and characterization of silver and goldnanoparticles using aqueous extract of seaweed, Turbinaria conoides, and their antimicrofouling activity. Sci World J. https://doi.org/10.1155/2014/938272
Vinotha V, Iswarya A, Thaya R, Govindarajan M, Alharbi Ns, Kadaikunnan S, Khaled Jm, Al-Anbr Mn, Vaseeharan B (2019) Synthesis of zno nanoparticles using insulin-rich leaf extract: anti-diabetic, antibiofilm and anti-oxidant properties. J Photochem Photobiol, B 197:11541. https://doi.org/10.1016/J.Jphotobiol.2019.111541
Wei L, Ding J, Xue M, Qin K, Wang S, Xin M, Jiang J, Zhao Q (2019) Adsorption mechanism of ZnO and CuO nanoparticles on two typicalsludge EPS: effect of nanoparticle diameter and fractional EPS polarity on binding. Chemosphere 214:210–219. https://doi.org/10.1016/j.chemosphere.2018.09.093
Wernicki A, Puchalski A, Urban-chmiel R, Dec M, Stęgierska D, Dudzic A, Wójcik A (2014) Antimicrobial properties of gold, silver, copper andplatinum nanoparticles against selected microorganismsisolated from cases of mastitis in cattle. Med Weter 70(9):564–567
Xu Y, Wang C, Hou J, Dai S, Wang P, Miao L, Lv B, Yang Y, You G (2016) Effects of ZnO nanoparticles and Zn2+ on fluvial biofilms and the related toxicity mechanisms. Sci Total Environ 544:230–237. https://doi.org/10.1016/j.scitotenv.2015.11.130
Yun-Seok C, Jay JO, Kye-Heon O (2010) Antimicrobial activity and biofilm formation inhibition of green tea polyphenols on human teeth. Biotechnol Bioprocess Eng 15:359–364
Zaib M, Shahzadi T, Muzammal I, Farooq U (2020) Catharanthus roseus extract mediated synthesis of cobalt nanoparticles: evaluation of antioxidant, antibacterial, hemolytic and catalytic activities. Inorg Nano-metal Chem 50(11):1171–1180. https://doi.org/10.1080/24701556.2020.1737819
Zakaria BS, Dhar BR (2020) Changes in syntrophic microbial communities, EPS matrix, and gene expression patterns in biofilm anode in response to silver nanoparticles exposure. Sci Total Environ 734: https://doi.org/10.1016/j.scitotenv.2020.139395
Zarasvand KA, Rai VR (2016) Inhibition of a sulfate reducing bacterium, Desulfovibrio marinisediminis GSR3, by biosynthesized copper oxideNanoparticles. Biotech 6:84. https://doi.org/10.1007/s13205-016-0403-0
Funding
No funding.
Author information
Authors and Affiliations
Contributions
CE conducted the search, extracted the data and drafted manuscript. ANE and KME conducted the search and reviewed the draft manuscript. OEO reviewed the draft manuscripts and certified final manuscript. This is a concise article on biocorrosion challenges and the prospects of MMO NPs as promising biocorrosion inhibitors.
Corresponding author
Ethics declarations
Conflict of interest
This review was carried out without any commercial or financial relationships among the authors that could lead to any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ejileugha, C., Ezealisiji, K.M., Ezejiofor, A.N. et al. Microbiologically Influenced Corrosion: Uncovering Mechanisms and Discovering Inhibitor—Metal and Metal Oxide Nanoparticles as Promising Biocorrosion Inhibitors. J Bio Tribo Corros 7, 109 (2021). https://doi.org/10.1007/s40735-021-00545-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-021-00545-0