Abstract
Ti6Al4V titanium alloy was used as a metallic permanent implant for the internal fixation during surgery of orthopedic and dental implants. But due to poor bioactivity like release of aluminum and vanadium, ions cause allergic problems and weak osseointegration with host environment. Surface modifications of those implants are required to make it biocompatible. The aim of the present research is to modify the surface of Ti6Al4V implant by electrodeposition of cathodic bioactive Calcium Phosphate (CaP) coating. The morphology of the modified surface was characterized by Scanning Electron Microscopy and Atomic Force Microscopy while electrochemical response was measured in simulated body fluid (SBF) at 37 °C by Open Circuit Potential, Cyclic polarization, and Electrochemical Impedance Spectroscopy techniques. In vitro biocompatibility of modified Ti6Al4V surfaces was further evaluated by coculturing them with a human liver carcinoma cell line, HEPG2. The bond strength of the CaP coating is 65 MPa in anodized Ti6Al4V surface. The CaP coating was dominated by hydroxyapatite with flake-like appearance which eventually decreases the Ti6Al4V dissolution rate to 0.206 mpy in SBF and the cell viability analysis further highlighted highest level of biocompatibility of coated titanium alloy which boosted cell growth by 78% competed to other surfaces.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Metallic implants whether prosthetic or dental, should firstly depend on that it must be biocompatible i.e., it must not react with the human tissue and its weight should be light practically same to that of bone and it must have excellent mechanical properties [1]. Ti6Al4V with these similar properties has ever been generally used in assembling of biomedical segments especially hip prostheses, teeth root, and prosthodontics [2]. But beside that Ti6Al4V does show quite poor tribological properties like wear [3].
To solve those issues, different surface modification techniques are attempted to increase its biocompatibility and osseointegration. To improve the osseointegration mechanism, Bruno Ramos Chrcanovic et al. used dual acid etching (DAE). During DAE, the acids react with the surface and produce a barrier oxide film on the titanium surface which improve the osseointegration [4]. Many researchers applied electropolishing for the uniform and smooth modification of titanium and titanium alloy [5]. Moreover, techniques like anodizing with titania nanotubes [6, 7], chemical vapor deposition [8], and physical vapor deposition [9] formed a thin film on the surface of the titanium. Xuanyong Liu et al. discuss the surface modification of titanium and titanium alloy by thermal spraying and ion implantation for biomedical application [10].
Song Park et al. assessed the growth of anodic TiO2 films in glycerol solution containing 1% NH4F and 20% deionized water at a constant voltage of 20 V for 1 h [11]. This process electrolytically increasing the thickness of oxide film on metallic surface enhances the corrosion and wear resistance [12]. Besides formation of oxide layer on the surface of titanium, the surface becomes rough as well due to the pitting action of fluoride. Additionally, that rough surface builds the hydrophilicity action which provides region for cell proliferation and bone formation [13].
Solgel includes the change of a colloidal suspension into a strong film on the surface of the titanium substrate commonly at low or room temperatures [14]. For improving the adhesion, Ming-Fa Hseih et al. blasted the surface of the titanium with alumina particles which causes the roughness and then applied the precursor sol containing calcium nitrate tetrahydrate and triethyl phosphate [15]. Biocompatible calcium phosphate coating techniques to obtain bioactive coatings on Ti6Al4V substrates have been used in the past by the researchers like electrochemical deposition [16], plasma spraying [17], and biomimetic deposition [18].
However, Szu Hao Wang et al. applied pressure of 80 torr on the electrolytic bath of 0.04 M calcium hydro-phosphate solution [16]. Achariya rakngarm et al. deposited the calcium phosphate coating at room temperature in different calcium phosphate solutions [19]. It has also been proved that examination of apatite formation on a material in SBF is useful for predicting the in vivo bone bioactivity of a material [20]. The anodized titania tubes and calcium phosphate coating enhanced osteoblast adhesion and proliferation [21,22,23]. Xiaohua Yu et al. work on correlation made between soluble-treated titanium surface and biomimetic calcium phosphate which increase cell attachment and growth [24].
In this study, the calcium phosphate coating was electrodeposited on different modified surfaces of Ti6Al4V. The developed coating was electrochemically analyzed in simulated body fluid and cell proliferation with HEPG2 cells.
2 Experimental
Ti6Al4V Grade five samples of 20 × 10 mm dimensions were taken out from a sheet of 4 mm thickness. For the smooth grinding of the titanium sample, the sample were cold mounted in polyester resin. Then the mounted samples were grounded with silicon carbide (SiC) papers of grit size 120, 240, 320, 400, 600, 800, 1000, and 1500. The samples were then rinsed with deionized water and degreased with acetone.
Electropolishing was performed on grinded in an electrolyte with bath composition of 80 ml acetic acid and 50 ml perchloric acid solution for 5 min at 20 V DC voltage with stainless steel cathode which was already reported by J. B. Matheiu et al. [5]. The samples were rinsed in water, and dried and dipped in methanol. The electropolished sample was then anodized in a bath containing 98 ml ethylene glycol, 1.8 ml water and 0.8 g ammonium fluoride (NH4F). Graphite rod was used as cathode and 20 V was maintained by DC power supply for 2 h after which, the samples were washed with deionized water and dipped in acetone container for 20 min.
The calcium phosphate (CaP) electrodeposition bath solution was prepared in deionized water with 1.46 M calcium chloride (CaCl2) and 0.87 M sodium dihydrogen phosphate (NaH2PO4). Each solution was stirred on the magnetic stirrer for 1 h, then solutions were cooled to room temperature and mixed together in a 1:1. The solution was further stirred for another 5 min. This method was successfully reported by Achariya Rakngarm et al. [19]. Electrodeposition of CaP was done in a two-electrode setup with graphite rod as anode while anodized titanium as cathode. The voltage of 7 V for 1 min DC power supply yielded the most uniform CaP coating, covering the whole anodized titanium surface.
The surface morphology and elemental analysis of the grinded, polished, anodized as well as CaP-coated sample was studied by Scanning Electron Microscope with Energy Dispersive X-ray analysis. Atomic Force Microscopy technique was used to measure the surface roughness of modified titanium surfaces. The bond strength of CaP coating to the modified surfaces of Ti4Al6V substrate was measured by hydraulic adhesion tester (108 Elcometer, USA). Aluminum dolly was used for bond strength measurement. Firstly, the dolly was stuck to the coating with epoxy adhesive. After curing, at room temperature, the aluminum dolly was pulled by hydraulic adhesion tester and maximum pullout force was recorded which was used to measure bond strength of the CaP coating to the Ti4Al6V substrate.
A three-electrode electrochemical cell was used for electrochemical evaluation with a graphite counter electrode, Ag/AgCl as the reference electrode and surface modified titanium as the working electrode. All the experiments were performed in 0.9% saline solution with pH 7.4 at 37 °C. The open circuit potential (OCP) was measured for 1 h. For cyclic polarization (CP), the sample surface was first polarized from − 0.3 to 1.5 V with forward scan rate of 2.5 mV s and then polarized reversely to − 0.3 V. For Electrochemical Impedance Spectroscopy (EIS), the frequency range was 100 kHz to 10 mHz with AC amplitude of 5 mV.
Human liver carcinoma cell line HEPG2 was used in the current research work. Cells were cultured in Dulbecco’s Modified Eagle Medium with 100 units/ml penicillin, 100 µg/ml streptomycin, and 10% fetal bovine serum. Cells were incubated in a humidified controlled atmosphere at 37 °C, under 5% CO2. Media was routinely changed every third day. To determine the cell attachment and cytotoxicity, HEPG2 cells were cultured in a 6-well plate in the presence of different types of Ti6Al4V surfaces placed in separate wells compared to control without anything. After 24 h, attachment of cells was analyzed under light microscope and number of attached cells were counted randomly in each well. Furthermore, after 72 h, cell cytotoxicity analysis was performed using standard protocol of MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-dipheny). Briefly, 100 μl of MTT solution per well was added and allowed to crystallize at 37 °C for 4 h followed by addition of 1 ml/well of acidified isopropanol as solubilization solution. Solution was thoroughly mixed to properly dissolve formazan crystals and shifted to a reader plate. Absorbance was measured at 570 nm using ELIZA reader. The experiment was repeated thrice for each sample.
3 Results and Discussion
3.1 Surface Analysis
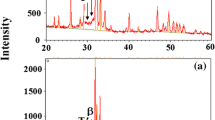
Figure 1 shows the surface morphology of all the samples. The surface of the polished sample is very smooth, uniform, and homogenized as compared to the grinded one because of the usage of perchloric and acetic acid. During electropolishing, gel-like byproduct of yellowish color was observed on the sample which decreases the effect of electrolyte by mass transport reaction on limiting current value due to the reduction of perchlorate ions on the anode. The reaction byproducts remove easily during the electropolishing and some after rinsing with water from the surface easily [25, 26]. After electropolishing, the sample was anodized in electrolyte based on fluoride ions, which formed porous nano-tube oxide film on the surface of the sample. The titania nanotubes were formed on the basis of migration of titanium ions from the pores and to the oxide solution interface while the pitting action of the fluoride ions from the electrolyte gives rise to selective dissolution of the substrate. The nanotubes on the titanium sample served as the adhesion points for the coating due to the increased surface area of nanotubes [27]. Moreover, in the SEM image of CaP coating flake like morphology of the coating is observed. The thickness of the CaP coating was approximately 10 mm with flake like morphology the CaP coating was synthesized in the electrolyte of calcium chloride and sodium dihydrogen phosphate with a pH of 4. The weakly acidic environment favored the fast formation of flakes of calcium phosphate [28]. It was found from the previous research work that morphology of the CaP coatings depended mainly on current densities and agitation of the bath [29]. At 20 mA cm−2, the structure is acicular and with increasing densities, the needles lose their acicularity and become globular [30].
Moreover, the presence of calcium and phosphorous in the coated sample was confirmed by EDX as shown in Fig. 2. The EDX of CaP-coated sample shows a calcium-to-phosphorus ratio of 1.65, which was quite close to the 1.67 ratio of stoichiometric hydroxyapatite coating.
3.2 Atomic Force Microscopy (AFM) and Bond Strength Results
The surface roughness of all surface modified samples was measured by AFM as shown in Fig. 3. The grinded sample shows average roughness of 26.46 nm due to non-uniform surface with an unbalanced ratio of peaks and valleys which can serve as points for localized corrosion [30]. However, the electropolished sample shows low value of roughness 12.11 nm due to uniform dissolution during the electropolishing process. The anodized sample shows maximum roughness of 33.42 nm due to the presence of nanoporous titania layer which is covering the entire surface. The presence of the nanotubular layer which increases the surface area and helps in the adherence of the coating which then in fact further helps in osseointegration [31].
The bond strength or adhesion strength results of CaP coating with the modified surfaces of the Ti6Al4V subtract shows a good adherence of the coating. The bond strength of the CaP depends on the roughness profile of the subtract. The result of the bond strength was in support with the roughness profile results. The bond strength of CaP coating on grinded Ti6Al4V sample is 45 MPa which decreases in the case of polished sample to 22 MPa but in case of anodized sample the value increased to 65 MPa due to high roughness value of 33.42 nm. Thus, the anodized sample shows good adhesion to the CaP coating as compared to other modified samples.
3.3 Electrochemical Testing Results
3.3.1 Open Circuit Potential (OCP)
Figure 4 shows the Open Circuit potential (OCP) of all samples with respect to Ag/AgCl reference electrode. Grinded sample shows a potential of − 104 mVvs Ag/AgCl while electropolished sample shows more active potential of − 331 mVvs Ag/AgCl than all the other samples due to the removal of native oxide barrier layer of titanium during polishing. The result shows that during anodizing the barrier and porous TiO2 formation on the surface of titanium sample, which shift its potential to 208 mVvs Ag/AgCl, shifted towards noble direction which increased its corrosion resistance [28]. However, with electrodeposition of calcium phosphate (CaP) coating it gives ceramic porous surface which resulted in less negative potential of − 183 mVvs Ag/AgCl.
3.3.2 Cyclic Polarization
In Fig. 5, Cyclic Polarization Scans of different surface-modified titanium samples are shown having negative hysteresis loop which indicates that no pitting of the samples in the simulated body fluids (SBF) at 37 °C temperature. Polarization parameters were calculated using tafel fitting in the linear region of activation in cyclic polarization curves. Table 1 shows that corrosion rate of grinded sample is 0.7449 mpy and after polishing, the sample corrosion rate increased to 2.153 mpy which could be due to the removal of oxide film giving active surface to aggressive ions present in the SBF. This behavior is in support to the OCP results which shows that electropolished potential shifted towards more active side. Similarly, anodized and CaP-coated samples show less corrosion rate of 0.2505 and 0.2054 mpy, respectively, as compared to grinded and electropolished.
3.3.3 Electrochemical Impedance Spectroscopy (EIS)
Figure 6 shows Nyquist plot of all samples. It showed that by Anodizing and CaP Coating, the impedance of sample increased which defines that corrosion process becomes slower. By fitting the Equivalent Circuit Models in Nyquist plot, Kinetic parameters like solution resistance (Rs), pore resistance (Rpo), film capacitance (Yo po), double-layer resistance (Rdl), double-layer capacitance (Yo dl), Diffusion resistance (R diff), and Diffusion capacitance (Yo diff) are calculated and reported in Table 2. Figure 7 shows the Equivalent Electrical Circuit Models (EEC) fitted in Nyquist plot. Figure 7a EEC model which was fitted in anodized sample shows three-time constants in Nyquist plot while Fig. 7b EEC model was fitted on grinded, polished, and CaP-coated sample’s Nyquist plot showing two-time constants.

Pore resistance of grinded sample showed highest value, which may be due to the formation of corrosion products on sample. From the Nyquist plot of grinded sample, it can be interpreted that a double was formed on metal–electrolyte interface. Pore resistance of polished sample showed a normal value as compared to grinded sample. Similarly, there was a decrease in pore resistance of anodized and CaP-coated samples which may not be attributed as they corrode earlier. This may be due to chloride ions action on substrate during EIS. Overall, the area of time constants of anodized and CaP-coated samples increased in Nyquist plot resulting in higher impedance values.
3.4 Cell Culturing
Attachment and proliferation of cells in the presence of different Ti–6Al–4V surface modifications are important parameters to understand their significant difference and further clinical use. After 24 h, attachment of cells in the presence of different Ti–6Al–4V surfaces showed that varying number of cells were attached in the presence of different surfaces. Grinded sample showed highest number of attached cells compared to polished, anodized, and CaP-coated specimens Fig. 8a. However, in contrast to cell attachment, cell viability analysis after 72 h showed highest viability in the presence of CaP-coated sample followed by polished and anodized samples, while least number of viable cells were observed in the presence of grinded specimen Fig. 8b. These results indicate that time of 24 h is not enough to study the biocompatibility of titanium surfaces. Cristiane X. Resende et al. [32] have also observed similar results and proposed a time more than 24 h for the biocompatibility analysis of different titanium surfaces. However, differences might be explained by the fact that roughness brought about by the hydrophilicity of bioactive coating in coated surface promoted cell growth and proliferation as shown in Fig. 9. Whereas, polished specimen due to its active surface is promoting the cell growth [33]. The grinded sample had the least viability because although the surface is rough, the pattern of peaks and valleys is not uniform and is highly susceptible to corrosion.
4 Conclusions
Following conclusions are carried out from the research:
-
1.
The electropolishing of Ti6Al4V makes the surface more active forming specific high energy sites for the growth of nano tubes of titania which serve as base for calcium phosphate coating of the coating.
-
2.
Electrochemical results of the coated sample were active due to the hydrophilic CaP coating which promoted interaction with biological environment and stopped dissolution of metal.
-
3.
In vitro biocompatibility tests executed on human hepato-carcinoma cells exhibited that all four different types of Ti–6Al–4V surfaces displayed significant level of biocompatibility. Specially coated and polished specimens with negligible effects on cell viability render them an excellent material for further biomedical application.
References
Oldani C, Dominguez A (2012) Titanium as a biomaterial for implants. In: Fokter SM (ed) Recent advances in arthroplasty, chapter 9. IntechOpen, Vienna
Faria A, Rodrigues R, Claro A, Mattos M, Riberio R (2011) Wear resistance of experimental titanium alloys for dental applications. J Mech Behav Biomed Mater 4:1873–1879
Budiniski KG (1991) Tribological properties of titanium alloys. Wear 151:203–217
Chrcanovic BR, Martins MD (2014) Study of the influence of acid etching treatments on the superficial characteristics of Ti. Mater Res 17:373–380
Mathieu JB, Landolt D (1978) Electropolishing of titanium in perchloric acid—acetic acid solution: II polarization behavior and Stoichiometry. J Electrochem Soc 125(7):1044–1048
Tan AW, Pingguan-Murphy B, Ahmad R, Akbar SA (2012) Review of titania nanotubes: fabrication and cellular reponse. Ceram Int 38:4421–4435
Deen KM, Farooq A, Raza MA, Haider W (2014) Effect of electrolyte composition on TiO2 nanotubular structure formation and its electrochemical evaluation. Electrochim Acta 117:329–335
Martin PM (2010) Handbook of deposition technologies for films and coatings, 3rd edn. Elseveir, Amsterdam
Juhasz JA, Best SM (2011) Surface modification of biomaterials methods analysis and applications: surface modification of biomaterials by calcium phosphate deposition (Chapter 6), Series in Biomaterials. Woodhead Publishing, Sawston, pp 143–169
Liu X, Chu PK, Ding C (2004) Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater Sci Eng R 47:49–121
Park Ii S, Oh HJ, Bae TS (2013) Bioactivity and generation of andozied tubular TiO2 layer of Ti-6Al-4V alloy in glycerol soultion. Thin Solid Films 548:292–298
Brammer KS, Oh S, Cobb CJ, Bjursten LM, van der Heyde H, Jin S (2009) Improved bone-forming functionality on diamter-controlled TiO(2) nanotube surface. Acta Biomater 5:3215–3223
Whangdee P, Sriprsertusk S, Srimaneepong V, Kashima DP (2014) Surface characteristics and hydrophilicity of the as-anodized films formed at high current density on Ti-6Al-4V in different electrolytes. Key Eng Mater 608:274–279
Kim HW, Koh YH, Li LH, Lee S, Kim HE (2004) Hydroxyapatite coating on titanium substrate with titania buffer layer processed by sol gel method. Biomateials 25:2533–2538
Hsieh MF, Perng LH, Chin TS (2002) Hydroxyapatite coating on Ti6Al4V alloy using a sol–gel derived precursor. Mater Chem Phys 74:245–250
Wang SH, Shih WJ, Li WL, Hon MH, Wang MC (2005) Morphology of calcium phosphate coatings deposited on a Ti-6Al-4V substrate by an electrolytic method under 80 Torr. J Eur Ceram Soc 25:3287–3292
Gu YW, Khor KA, Pan D, Cheang P (2004) Activity of plasma sprayed yttria stabilized zirconia reinforced hydroxyapatite/Ti–6Al–4V composite coatings in simulated body fluid. Biomaterials 25:3177–3185
Liang Fanghui, Zhou Lian, Wang Keguang (2003) Apatite formation on porous titanium by alkali and heat-treatment. Surf Coat Technol 165:133–139
Rakngarm Achariya, Mutoh Yoshiharu (2009) Electrochemical deposition of calcium phosphate film on commercially pure titanium and Ti-6Al-4V in two types of electrolyte at room temperature. Mater Sci Eng C 29:275–283
Takadama H, Kokuboand T (2006) How useful is SBF in predicting invivo bone bioactivity? Biomaterials 27:2907–2915
Oh S, Daraio C, Chen LH, Pisanic TR, Finones RR, Jin S (2006) Significantly accelerated osteoblast cell growth on aligned TiO2, nanotubes. J Biomed Mater Res A 78:97–103
Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R (2000) Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials 21:1803–1810
Webster TJ, Ejiofor JU (2004) Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V and CoCrMo. Biomaterials 25:4731–4739
Yu X, Wei M (2013) Cellular performance comparison of biomimetic calcium phosphate coating and alkaline treated titanium surface. Biomed Res Int 2013:9
Han W, Fang F (2019) Fundamental aspects and recent developments in electropolishing. Int J Mach Tools Manuf 139:1
Wilk J (2014) A review of measurements of the mass transfer in minichannels using the limiting current technique. Exp Therm Fluid Sci 57:242
Indira K, Mudali UK, Nishimura T, Rajendran N (2015) A review on TiO2 nanotubes: influence of anodization parameters, formation mechanism, properties, corrosion behavior, and biomedical applications. J Bio Tribo Corros 1(4):28
Eliaz N, Metoki N (2017) Calcium phosphate bioceramics: a review of their history, structure, properties, coating technologies and biomedical applications. Materials 10(4):334
Richard R. et al (2006) Hydroxyapatite with controllable size and morphology, United States Patent 7998219
Koju N, Sikder P, Ren Y, Zhou H, Bhaduri SB (2017) Biomimetic coating technology for orthopedic implants. Curr Opin Chem Eng 15:49–55
Birch MA, Lynn SJ, Nouraei S, Wu Q-B, Ngalim S, Lu W-J, Watchorn C, Yang T-Y, McCaskie AW, Roy S (2012) Effect of electrochemical structuring of Ti6Al4V on osteoblast behaviour in vitro. Biomed Mater 7(3):035016
Resende CX, Lima IR, Gemelli E, Granjeiro JM, Soares GD (2010) Cell adhesion on different titanium coated surfaces. Materia (Rio J) 15(2):386–391
Wua Chunya, Chena Mingjun, Zhengb Ting, Yang Xiaonan (2015) Effect of surface roughness on the initial response of MC3T3-E1 cells cultured on polished titanium alloy. Bio Med Mater Eng 26:155–164
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Farooq, A., Raza, A., Tayyeb, A. et al. Electrochemical and Cell Response of Surface Modified Ti6Al4V for Biomedical Applications. J Bio Tribo Corros 5, 94 (2019). https://doi.org/10.1007/s40735-019-0285-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-019-0285-x