Abstract
The corrosion inhibition properties of expired acetazolamide for mild steel in acidic solution were studied by gravimetric and Tafel polarization and Electrochemical Impedance spectroscopy. The inhibition efficiency evaluated by all methods was in good agreement with each other. Results suggest that the inhibition efficiency increases with increase in concentration and decreases with temperature. Inhibitor obeys Langmuir adsorption isotherm and shows inhibition efficiency up to 93% at 313 K for 500 ppm solution. Tafel polarization study suggests that it acts as mixed-type inhibitor. Thermodynamic parameters indicate that the adsorption process is spontaneous. Shifting of frequencies in FTIR spectra and SEM-EDX analysis confirms the adsorption of inhibitor on mild steel surface. Application of expired drug as corrosion inhibitor is useful to reduce environmental pollution and economically beneficial to pharmaceutical industries.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Corrosion is the interaction between a material and its environment leading to degradation in the material properties. Mild steel is an important material which finds wide applications in industry due to its excellent mechanical properties and low cost. Acidic solutions are widely used in several industries for the removal of undesirable oxide films and corrosion products during acid pickling, acid descaling, industrial cleaning and oil-well acidization. Among the various methods to prevent destruction or degradation of metal surface, the corrosion inhibitor is one of the best know methods of corrosion protection and one of the most useful in the industry [1,2,3,4].
The selection of organic inhibitor is mostly based upon the aromaticity, electron density at donor sites, type and number of hetero atoms like N, O and S present in the compounds. Most organic inhibitors are more costly, toxic in nature and hence environmentally unfriendly. Among organic corrosion inhibitors, drugs are found to be safe due to their non-toxicity and environmentally benign nature. Corrosion inhibition properties of drugs have been studied by many researchers for different metal non-toxic and effective inhibitors, and are considered more important and desirable, nowadays, which are also called eco-friendly or green corrosion inhibitors [5,6,7,8,9,10].
For many drugs, though they have retained potency after expiry date, they are not used by the people for medical treatment. Disposal of such expired drugs after the expiry causes public health hazards due to environmental pollution and economic problems due to disposal costs to the pharmaceutical industries. To avoid this, recently many researchers have studied corrosion inhibitive properties for the expired drugs [11,12,13,14,15,16].
Fouda et al. [17] studied corrosion inhibitive properties of the fresh acetazolamide for carbon steel. The aim of the present work is to study the expired acetazolamide drug as corrosion inhibitor for mild steel in acidic solution using gravimetric and electrochemical methods. The name of commercially available acetazolamide is N-(5-Sulfamoyl-1,3,4-thiadiazol-2-yl)acetamide having empirical formula C4H6N4O3S2 with molecular weight 222.24 g mol−1. It is used for the medical treatment of cystinuria, glaucoma, epileptic seizure, idiopathic intracranial hypertension, altitude sickness and periodic paralysis.
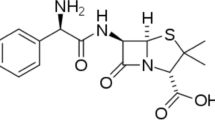
Acetazolamide is a sulfa-based drug and can act as a good corrosion inhibitor due to the presence of four nitrogen, two sulphur and three oxygen atoms in the structure in Fig. 1.
2 Experimental Studies
2.1 Materials
2.1.1 Preparation of the Specimens
Mild steel (MS) coupons of size 3 × 3 × 0.051 cm with small hole of 0.5 cm diameter near the upper edge were prepared and polished with different emery papers ranging from 320 to 1200 grades. Further coupons were rinsed with double-distilled water, degreased in acetone and then dried at room temperature before use.
Electrochemical measurements were carried out on a 6-cm-long coupon with exposed area of 2 cm2 and remaining portion was covered with commercially available lacquers.
Mild steel composition in wt% shown in Table 1 is used in this study which was a ASTM A36 grade confirmed by Spectrochemical test ASTM E-415.
2.1.2 Aggressive Solution
The aggressive solution (2 M HCl) was prepared by dilution of Analytical Grade 35.5% HCl with double-distilled water.
2.1.3 Inhibitor
Acetazolamide with brand name Diamox was purchased from medicine shop and used without further purification after 6 months of the date of expiry. Different concentrations of expired Acetazolamide drug were prepared by diluting appropriate volume of 1000 ppm stock solution.
2.2 Experimental
2.2.1 Gravimetric Study
In this experiment, beakers of 100 ml are labelled from 1 to 6 containing experimental solution with and without inhibitor. Inhibitor concentration of 100–500 ppm is used. Mild steel coupons of predetermined weight were separately immersed in 100-ml beaker containing experimental solution for periods of 1, 3, 6, 9, 15 and 24 h. Experiment was carried out in triplicates and average value of weight loss was reported. Temperature dependence of the inhibition efficiency was studied at temperatures in the range of 303, 313, 323 and 333 K using equitron stirred water bath. All experiments were carried out as described in ASTM standard G-1 procedures.
2.2.2 Electrochemical Study
All electrochemical measurements were carried out using Gamry Instruments Interface 1010T model at 303 K temperatures. Echem Analyst 7.06 Software installed in a laptop was used for fitting data. Three-electrode glass cell assembly with platinum wire as a counter electrode, a saturated calomel electrode (SCE) as a reference electrode and MS specimen (exposed area 2 cm2) were used as working electrode for electrochemical study. To attain a steady-state open circuit potential (OCP) working electrode are immersed in test solution for 30 min before starting each experiment.
The tafel polarization measurement was carried out at the scan rate of 1 mV/s versus the OCP in the potential range ± 150 mV at room temperature. The linear Tafel segments of the anodic and cathodic curves were extrapolated to corrosion potential to obtain the corrosion current densities (Icorr) and corrosion potential (Ecorr). Electrochemical impedance spectroscopy (EIS) measurements were carried out at a frequency range of 20 kHz–0.1 Hz with amplitude of 10 mV.
2.2.3 Surface Analysis Study
2.2.3.1 SEM-EDX Analysis
Mild steel coupons were immersed in 2 M HCl in the absence (blank) and presence of 500 ppm (optimum concentration) of expired acetazolamide solution at 303 K for 6 h. The composition and morphology of the corrosion products formed on the surface were examined by HITACHI High Technologies Corporation (model no.S-4800 Type-II Made in Japan) and also characterized for surface elemental composition using on Bruker AXS Gmbh Germany.
2.2.3.2 Fourier Transform Infrared Spectroscopic Analysis
The surface adhered film was scratched carefully and FTIR spectra were recorded for the inhibitor and that of the corrosion products (in the presence and absence of the inhibitors) using a FTIR (IR Affinity 1), Shimadzu, Japan.
3 Results and Discussion
3.1 Gravimetric Study
Corrosion rate, degree of surface coverage (θ) and inhibition efficiency (% IE) calculated from weight loss using following equations.
The corrosion rate (CR) was computed from the following equation:
, where \(W\) is the average weight loss of MS coupons, a represents the total surface area of one MS coupon, t is the immersion time and D is the density of MS in g cm− 3.
The surface coverage (θ) is calculated as follows:
where CR and inhCR are the corrosion rates of MS in the absence and presence of the inhibitors, respectively.
The inhibition efficiency (% IE) is calculated as follows:
3.1.1 Effect of Inhibitor Concentration and Time of Immersion on Corrosion Inhibition
Inhibition efficiency (% IE) and Corrosion rate (CR) obtained from weight loss measurements are summarized in Table 2. For expired acetazolamide, inhibition efficiency at 313 K temperature observed is higher than that of at temperature 303 K. Corrosion rate decreases with increase in the concentration of inhibitor. After 15 h of immersion time, the inhibition efficiency showed a significant decrease then it slightly increased with the increase in the immersion time. This behaviour is due to desorption/adsorption of inhibitor molecules on the metal surface. Maximum inhibition efficiency was observed for a period of 9 h for 500 ppm inhibitor concentration (Fig. 2a, b.) [18].
3.1.2 Effect of Temperature on Corrosion Inhibition of Expired Acetazolamide
Effect of Temperature was studied in the temperature range 303–343 K for 6 h of immersion time in 2 M HCl and various concentrations of expired acetazolamide. It is observed that the Inhibition efficiency of inhibitor has increased from 303 to 313 K which indicates that adsorbed layer on mild steel coupon is stable up to 313 K after that inhibition efficiency decreases with increase in temperature due to desorption of inhibitor (Fig. 3a, b). The data are summarized in Table 3.
3.2 Electrochemical Study
3.2.1 Tafel Polarization
The polarization study was carried out for mild steel in 2 M HCl solution in the absence and presence of various concentrations (100, 200, 300, 400 and 500 ppm) of expired acetazolamide drug at 303 K shown in Fig. 4. Electrochemical parameters such as tafel constants (βa and βc), corrosion potential (Ecorr), corrosion current density (Icorr) and Inhibition Efficiency (%IE) are obtained by the tafel extrapolation method. The inhibition efficiency (% IE) was calculated using the following Eq. (4) and represented in Table 4.
where I0corr and Icorr are the corrosion current density in the absence and presence of the inhibitor, respectively.
From Table 4, it is concluded that corrosion current density decreases with increased concentration of inhibitor. Inhibition efficiency (% IE) has increased as well as rate of electrochemical reaction is reduced due to the formation of protective layer on the mild steel surface. Mixed mode of inhibition is considered for expired acetazolamide drug as the displacement in Ecorr is less than 85 mV with respect to the corrosion potential of the blank solution. Ecorr value slightly shifts towards negative direction in the presence of inhibitor as compared to the Ecorr value in blank solution, which indicates that inhibitor is with predominant control of cathodic reaction [19].
3.2.2 Electrochemical Impedance Spectroscopy (EIS)
Electrochemical Impedance Spectroscopy (EIS) used to provide information about the kinetics of the electrochemical processes at the mild steel/acid interface. Nyquist plots in the absence and presence of different concentrations of expired acetazolamide inhibitor at 303 K are shown in Fig. 5.
The curves show a similar type of Nyquist plots for carbon steel in the presence of different concentrations of inhibitor. The diameter of the semicircles increases with increasing concentration of inhibitor. This indicates that inhibition efficiency increases with the increase in impedance of mild steel due to the formation of protective layer of inhibitor. Pure double-layer capacitor is replaced by the constant phase element (CPE) to give a more accurate fit.
In a Model (Fig. 6), Ru represents solution resistance, Rp represents the polarization resistance which consists of the charge transfer resistance (Rct), Polarization resistance (Rp) may include the different types of resistances such as Rct, charge transfer resistance; Rd, diffuse layer resistance; Ra, resistance of accumulated species (corrosion products or any existing molecules or ions); Rf, resistance of film.
But to simplify the calculation, they are measured as Rp. [20], Y0 represents CPE coefficient.
Alpha (α) represents CPE exponent (phase shift) which is a measure of surface homogeneity.
The double-layer capacitance (Cdl) was calculated from the following Eq. (6).
where ωmax = 2π fmax (fmax represents the maximum frequency at which the imaginary component of the impedance has a maximum value).
The inhibition efficiency (%IE) of the inhibitor has been found out from the charge transfer resistance values using the following Eq. (7).
where \({Rp}^{inh}\)and \({Rp}^{0}\)are the values of charge transfer resistance in the presence and absence of inhibitor in 2 M HCl, respectively. Results obtained are reported in Table 5.
From Table 5, It is observed that Polarization resistance (Rp) increases, while the double-layer capacitance (Cdl) decreases in the presence of inhibitor compared to the blank solutions.
The increase in Rp values may be due to the gradual replacement of water molecules by the adsorbed inhibitor molecules on the mild steel surface. While the decrease in Cdl results from either decrease in local dielectric constant and/or an increase in the thickness of the double layer, this indicates the adsorption inhibitor molecules at the metal/solution interface [20,21,22].
3.2.3 Adsorption and Thermodynamic Study of the Corrosion Process
Nature of metal–inhibitor interaction can be studied with the help of adsorption isotherms. Data obtained for the degree of surface coverage were used to fit curves for different adsorption isotherms such as Langmuir, Freundlich, El-Awady, Temkin, Flory–Huggins and Frumkin. It is observed that Langmuir adsorption isotherm fits best to the surface coverage data obtained from weight loss measurements. Langmuir model was elucidated in Eq. 8
, where \(C\) is the molar inhibitor concentration, θ is the degree of surface coverage and Kads is the equilibrium constant of the adsorption process [23, 24].
Figure 7 shows the relationship between C/θ and C for the studied temperature range. The results show that the correlation coefficient (R2) and slope are around unity. This suggests that the adsorption of the drug inhibitor on metal surface obeys Langmuir adsorption isotherm.
Free energy of adsorption ΔG0ads was calculated using the following relationship:
where 55.5 is the concentration of water in solution in mol L and R is the universal gas constant. T is the absolute temperature.
Adsorption equilibrium constant (Kads) is given as
From Table 6, the negative value of ΔG0ads implies that the adsorption process is spontaneous. The magnitude of signifies the nature of adsorption, whether it is physisorption or chemisorption [25, 26].
If the value of ΔG0ads is less than − 20 kJ/mol physisorption or less negative concludes physisorption which is due to electrostatic interactions between inhibitor and the charged metal surface while those around − 40 kJ/mol−1 or larger negative are concludes chemisorption due to charge sharing or transferring from organic species to the metal surface to form a coordinate type of metal bond .In this study, ΔG0ads adsorption values are less than − 40 kJ/mol but more than − 20 kJ/mol, indicates that nature of adsorption are both physisorption and chemisorption [27, 28].
3.2.4 Kinetic–Thermodynamic Corrosion Parameters
Mechanism of corrosion inhibition process can be studied with the help of Kinetic–Thermodynamic Corrosion Parameters.
The enthalpy of adsorption (ΔHads) can be calculated from the rearranged Gibbs–Helmholtz equation
The Plot of ΔGads/T versus 1/T gave a straight line with a slope of ΔHads as shown in Fig. 8.
Basic thermodynamic equation is use to determine the entropy of adsorption ΔSads was as follows:
Variation of ΔGads versus T give straight line is useful to deduce value of ΔSads (slope) and ΔHads (intercept).
Van’t Hoff equation is also important to elucidate the values of enthalpy of adsorption (ΔHads) and entropy of adsorption ΔSads as follows:
Plot of ln Kads versus 1/T which gives straight lines with slopes with slope equal to [\(\frac{-{\Delta }\text{Hads}}{\text{R}}\)] and intercept equal to [\(\frac{{\Delta }\text{Sads}}{\text{R}} +\text{ln}\frac{1}{55.5}\)] as shown in Fig. 9.
\(\Delta {\text{H}}_{{{\text{ads}}}} {\text{ }}\) is negative indicates adsorption process is exothermic evinces physisorption, chemisorption, or a mixture of both. The positive value of ΔSads can be attributed to the increase in the solvent entropy. The value of the enthalpy and entropy of adsorption calculated by Van’t Hoff method and Gibbs–Helmholtz relations are in good agreement shown in Table 7 [29,30,31].
The apparent activation energy values (C) for the corrosion process in absence and presence of inhibitor were evaluated from Arrhenius Eq. (13):
where CR1 and CR2 are the corrosion rates at temperatures T1 and T2, respectively.
The heats of adsorption (Qads) also determined as
The calculated values of Ea and Qads are summarized in Table 8. Increase in activation energy (Ea) in the presence of inhibitor as compared to in the absence of inhibitor suggests physical adsorption of drug on mild steel surface. Qads values are negative which indicates that the adsorption of inhibitor on mild steel surface is exothermic [32,33,34].
3.3 Surface Analysis Study
3.3.1 SEM-EDX Analysis
From SEM micrographs (Fig. 10a–c), It is clear that polished mild steel surface is very smooth. In the absence of inhibitor, mild steel is highly damaged and rough due to metal dissolution. Less pits and cracks are found due to the formation of protective film on the mild steel surface in the optimum concentration of inhibitor.
3.3.1.1 Energy Dispersive Spectroscopy
The energy dispersive X-ray analysis (EDX) technique was used to find the element composition of the surface of the mild steel sample from Fig. 11a–c in the presence and absence of inhibitor in 2 M HCl solution after 6 h of immersion is given in Table 9.
EDX spectra were used to determine the elements present on mild steel surface in the presence and absence of the inhibitor solution. From Table 9, we conclude that that Fe peaks are considerably suppressed in the blank solution compared to polished Mild steel surface indicating the free corrosion of MS in 2 M HCl solution.
Also, the percentage of atomic content of Oxygen and Chlorine is low in 500 ppm inhibitor solution as compared to blank solution which indicates that less corrosion occurs due to the formation of protective film of inhibitor. Peaks for Nitrogen (N) and Sulphur (S) are observed in the presence of inhibitors which were absent in the blank solution is evident that protective layer is of expired acetazolamide drug [35, 36].
3.3.2 Fourier Transform Infrared Spectroscopic Analysis
By observing FTIR spectra (Fig. 12) of pure expired acetazolamide drug and scraps of mild steel surface protected by inhibitor, the shift in IR frequencies is summarized in Table 10. The shift in the frequencies of –NH, –C=O and –S=O suggests that there is interaction between the inhibitor and mild steel surface through these groups. Corrosion inhibition occurs though adsorbed layer of expired acetazolamide inhibitor on mild steel surface which supports the result obtained from SEM and EDX.
4 Conclusions
-
1.
Gravimetric studies showed that Expired acetazolamide acts as efficient green inhibitor for mild steel in 2 M HCl. The inhibition efficiency increases with the increase in concentration of inhibitor and found maximum for 500 ppm solution. Efficiency of inhibitor decreases with increasing temperature.
-
2.
Tafel polarization study suggests that Expired acetazolamide acts as mixed inhibitor (Ecorr is < 85 mV) but slightly cathodic in nature
-
3.
Electrochemical impedance spectroscopy (EIS) calculations showed that charge transfer resistance (Rct) increases and double-layer capacitance (Cdl) decreases in the presence of inhibitors, which suggested the adsorption of the inhibitor molecules on the surface of mild steel
-
4.
Expired acetazolamide obeys Langmuir adsorption isotherm in the process of adsorption. Thermodynamic study suggests that the adsorption process is spontaneous (ΔG0ads is −ve), exothermic (Qads is −ve also ΔHads < 0) and follow both physical and chemical adsorption mechanisms.
-
5.
Formation of the protective film of inhibitor on the mild steel was confirmed by surface analysis techniques (SEM-EDXS and FTIR)
-
6.
Results obtained from gravimetric and electrochemical methods were in good agreement as follows:
Gravimetric | Tafel polarization | Electrochemical impedance spectroscopy | |
|---|---|---|---|
% Inhibition efficiency for 500 ppm expired acetazolamide | 85.64 | 93.27 | 96.39 |
References
Doharea P, Chauhana DS, Sorourb AA, Quraishi MA (2017) DFT and experimental studies on the inhibition potentials of expired Tramadol drug on mild steel corrosion in hydrochloric acid. Mater Discov 9:30–41. https://doi.org/10.1016/j.md.2017.11.001
Kamal C, Sethuraman M (2012) Spirulina platensis—A novel green inhibitor for acid corrosion of mild steel. Arab J Chem 5:155–161. https://doi.org/10.1016/j.arabjc.2010.08.006
Sherif ESM, Abbas AT, Gopi D, El-Shamy AM (2014) Corrosion and corrosion inhibition of high strength low alloy steel in 2.0 M sulfuric acid solutions by 3-amino-1,2,3-triazole as a corrosion inhibitor. J Chem. https://doi.org/10.1155/2014/538794
El-Etre AY, Abd El S, Wanees et al (2015) Comparison of the corrosion inhibition by drugs for the corrosion of Nickel in hydrochloric acid. J Am Sci. https://doi.org/10.7537/marsjas110215.03
Ansari KR, Quraishi MA et al (2013) Electrochemical and thermodynamic investigation of diclofenac sodium drug as a potential corrosion inhibitor for mild steel in hydrochloric acid. Int J Electrochem Sci 8:12860–12873
Fouda S, El-Defrawy AM, El-Sherbeni MW (2013) Lornoxicam & tenoxicam drugs as green corrosion inhibitors for carbon steel in 1 M H2SO4 solution. J Electrochem Sci Technol 4(2):47–56. https://doi.org/10.5229/JECST.2013.4.2.47
Naqvi I, Saleemi A, Naveed S (2011) Cefixime: a drug as efficient corrosion inhibitor for mild steel in acidic media. Electrochemical Thermodynamic Studies. Int J Electrochem Sci 6:146–161
Prasanna B, Matad et al (2014) Ketosulfone drug as a green corrosion inhibitor for mild steel in acidic medium. Ind Eng Chem Res 53:8436–8444. https://doi.org/10.1021/ie500232g
Mahmoud N. El-Haddad (2016) Inhibitive action and adsorption behavior of cefotaxime drug at copper/hydrochloric acid interface: electrochemical, surface and quantum chemical studies. RSC Adv 6:57844–57853. https://doi.org/10.1039/C6RA03316D
Kumar SH, Karthikeyan S (2013) Torsemide and furosemide as green inhibitors for the corrosion of mild steel in hydrochloric acid. Ind Eng Chem Res 52(22):7457–7469. https://doi.org/10.1021/ie400815w
Rajeswari V, Devarayan K, Viswanathamurthi P (2017) Expired pharmaceutical compounds as potential inhibitors for cast iron corrosion in acidic medium. Res Chem Intermed 43:3893. https://doi.org/10.1007/s11164-016-2852-9
Gökhan G (2011) Drugs: a review of promising novel corrosion inhibitors. Corros Sci 53:3873–3898. https://doi.org/10.1016/j.corsci.2011.08.006
El-Desoky AM et al (2015) Electrochemical and analytical study of the corrosion inhibitory behavior of expired pharmaceutical compounds for C-steel Corrosion. Int J Electrochem Sci 10:5112–5129
Shukla SK, Singh AK, Ebenso EE (2011) Pharmaceutically active compound as corrosion inhibitor for mild steel in acidic medium. Int J Electrochem Sci 6:4276–4285
Singh P, Chauhan DS, Srivastava K et al (2017) Expired atorvastatin drug as corrosion inhibitor for mild steelin hydrochloric acid solution. Int J Ind Chem 8:363. https://doi.org/10.1007/s40090-017-0120-5
Geethamani P et al (2015) An expired non-toxic drug acts as corrosion inhibitor for mild steel in hydrochloric acid medium. IJCPS 3(1):1442–1448
Fouda AS, Etaiw SH, Wahba A (2015) Effect of acetazolamide drug as corrosion inhibitor for carbon steel in hydrochloric acid solution. Nat Sci 13(9):1–8
Yadav M, Sinha RR, Sarkar TK, Tiwari N (2015) Corrosion inhibition effect of pyrazole derivatives on mild steel in hydrochloric acid solution. J Adhes Sci Technol 29(16):1690–1713. https://doi.org/10.1080/01694243.2015.1040979
Yadav M, Sinha RR, Kumar S, Sarkar TK (2015) Corrosion inhibition effect of spiropyrimidinethiones on mild steel in 15% HCl solution: insight from electrochemical and quantum studies. RSC Adv 5:70832–70848. https://doi.org/10.1039/C5RA14406J
Akbarzadeh E, Ibrahim MNM, Rahim AA (2011) Corrosion inhibition of mild steel in near neutral solution by kraft and soda lignins extracted from oil palm empty fruit bunch. Int J Electrochem Sci 6:5396–5416
Thanapackiam P et al (2016) Electrochemical evaluation of inhibition efficiency of ciprofloxacin on the corrosion of copper in acid media. Mater Chem Phys. https://doi.org/10.1016/j.matchemphys.2016.02.059
Verma C, Quraishi MA, Ebenso EE et al (2018) A green and sustainable approach for mild steel acidic corrosion inhibition using leaves extract: experimental and DFT studies. J Bio Tribo Corros 4:33. https://doi.org/10.1007/s40735-018-0150-3
Saman Zehra M, Mobin JA, Parveen M (2018) Assessment of glycine derivative N-benzylidine-2((2-oxo-2-(10H-phenothiazine-10yl)ethyl)amino) acetohydrazide as inhibitor for mild steel corrosion in 1 M HCl solution: electrochemical and theoretical approach. J Adhes Sci Technol 32(3):317–342. https://doi.org/10.1080/01694243.2017.1354669
Eddy NO, Ebenso EE (2010) Adsorption and quantum chemical studies on cloxacillin and halides for the corrosion of mild steel in acidic medium. Int J Electrochem Sci 5:731–750
Emeka E, Oguzie (2008) Evaluation of the inhibitive effect of some plant extracts on the acid corrosion of mild steel. Corros Sci 50:2993–2998. https://doi.org/10.1016/j.corsci.2008.08.004
Nathiya RS, Perumal S, Murugesan V et al (2018) Expired drugs: environmentally safe inhibitors for aluminium corrosion in 1 M H2SO4. J Bio Tribo Corros 4:4. https://doi.org/10.1007/s40735-017-0120-1
de Souza FS, Spinelli A (2009) Caffeic acid as a green corrosion inhibitor for mild steel, Corros Sci 51:642–649, https://doi.org/10.1016/j.corsci.2008.12.013
Karthik G, Sundaravadivelu M (2016) Studies on the inhibition of mild steel corrosion in hydrochloric acid solution by atenolol drug. Egypt J Pet 25:183–191. https://doi.org/10.1016/j.ejpe.2015.04.003
Praveen BM, Prasanna BM, Hebbar N et al (2018) Experimental and theoretical studies on inhibition effect of the praziquantel on mild steel corrosion in 1 M HCl. J Bio Tribo Corros 4:21. https://doi.org/10.1007/s40735-018-0137-0
Ansari KR, Ramkumar S, Nalini D, Quraishi MA, Slawin AMZ (2016) Studies on adsorption and corrosion inhibitive properties of quinoline derivatives on N80 steel in 15% hydrochloric acid. Cogent Chem 2:1. https://doi.org/10.1080/23312009.2016.1145032
Shukla SK, Singh AK, Ahamad I, Quraishi MA (2009) Streptomycin: a commercially available drug as corrosion inhibitor for mild steel in hydrochloric acid solution. Mater Lett 63:819–822. https://doi.org/10.1016/j.matlet.2009.01.020
Shukla SK, Ebenso EE (2011) Corrosion inhibition, adsorption behavior and thermodynamic properties of streptomycin on mild steel in hydrochloric acid medium. Int J Electrochem Sci 6:3277–3291
Vinutha MR, Venkatesha TV, Nagaraja C (2018) Anticorrosive ability of electrochemically synthesized 2,2′-disulfanediyldianiline for mild steel corrosion: electrochemical and thermodynamic studies. Int J Ind Chem 9:185. https://doi.org/10.1007/s40090-018-0149-0
Ameh PO, Sani UM (2016) Cefuroxime axetil: a commercially available drug as corrosion inhibitor for aluminum in hydrochloric acid solution. Portugaliae Electrochim Acta 34(2):131–141. https://doi.org/10.4152/pea.201602131
Nnaji NJ, Obi-Egbedia NO, Nnabugwu MA (2012) Kinetics and thermodynamics of aluminium corrosion inhibition by anthocleista djalonensis leaf extract in hydrochloric acid solution. Int J Chem Sci 10(1):182–194
Umoren SA (2016) Biomaterials for corrosion protection: evaluation of mustard seed extract as eco-friendly corrosion inhibitor for X60 steel in acid media. J Adhes Sci Technol 30:17,1858–1879. https://doi.org/10.1080/01694243.2016.1168339
Acknowledgements
Mr Lakhan Prakash Chaudhari gratefully acknowledges the research grant provided by the North Maharashtra University Jalgaon under Vice Chancellor Research Motivational Scheme [VCRMS] (Grant No. NMU/11A/VCRMS/Budjet-2015/Science-8/65/2016). Authors are also thankful to Head of the U.I.C.T. department of North Maharashtra University, Jalgaon for SEM-EDX analysis facility.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chaudhari, L.P., Patel, S.N. Corrosion Inhibition Study of Expired Acetazolamide on Mild Steel in Dilute Hydrochloric Acid Solution. J Bio Tribo Corros 5, 20 (2019). https://doi.org/10.1007/s40735-018-0212-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-018-0212-6