Abstract
A sequential anaerobic-aerobic batch reactor was used to treat 3,6-dichloro-2-methoxybenzoic acid (dicamba) during a long operation period of 340 days in the presence of disodium anthraquinone-2,6-disulphonate (AQS) as redox mediator. The sludge activity was evaluated for different dosages of dicamba over constant hydraulic retention time (HRT), neutral pH (6.5–7.5) and at ambient reactor temperature. Effects of increased dicamba concentration, solids retention time (SRT) and oxidation reduction potential (ORP) on the biodegradation of dicamba was monitored and compared with control reactor containing no dicamba. Results revealed that long operation period, long SRT and ORP were playing important role in the breakdown of dicamba to its transformation products and subsequent removal in the system. The system was capable of degrading the compound completely during long operation period, long SRT and at low ORP in the presence of AQS. Reducing condition in the anaerobic reactor significantly contributed to the treatment process through demethylation, dehalogenation and dechlorination reactions in the presence of different reducing bacteria. The results of GC-HRMS identified the anaerobic transformation products of dicamba as oleic acid (C18H34O2), 9-Octadecenoic acid (Z), 2-hydroxy-1-(hydroxymethyl)ethyl ester (C21H40O4), trans-13-Ocatadecenoic acid (C18H34O2) compounds which were then oxidised in the aerobic reactor.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Dicamba (3,6-dichloro-2-methoxybenzoic acid) is one of the halogenated aromatic herbicide commonly used to remove and control broad leaf weeds in various crop fields. This herbicide is mainly used to control post emergence of broad leaf type of weeds in the crop field (González et al. 2006). The application of dicamba is not limited to agricultural field but also used to eradicate weeds in railway embankments, drainages and gardens. Due to its high water solubility (4500 mg/L), half-life period (28.3 day) and other physico-chemical properties the herbicide may weakly be adsorbed on to soil (Hamilton and Crossley 2004). Dicamba exists in water as anions, which makes it weakly adsorbed (Ghoshdastidar and Tong 2013) and it is often detected more in surface than ground-water. Dicamba has been detected at concentrations of up to 97.4 mg/L in sugar cane crop land runoff water (Sangami and Manu 2017a). Dicamba can cause various health effects on aquatic life, animals and also on human (Shin et al. 2011). Therefore, the US EPA has recommended 200 μg/L as standard limit for drinking water (Hamilton et al. 2003). Dicamba has molecular weight of 221 g/mol and water solubility of 4500 mg/L at 25 °C (Sangami and Manu 2017a). Dicamba is weakly adsorbed on to soil with pKa of 1.87, and it is highly water soluble (William and Armbrust 2002) and highly mobile in soil (Comfort et al. 1992). Several treatment methods including physical, chemical and biological have been used to treat it in wastewater, but they have limitations like formation of excess sludge, incomplete mineralization, low capacity and high operating costs (Mondal et al. 2010). Therefore, biological treatment methods are considered as efficient, eco-friendly and economical alternatives (Ge et al. 2017).
The halogenated aromatic compounds can be treated using biological methods as they lack in formation of toxic intermediates (Kuppusamy et al. 2017). There are studies reported to treat herbicides including dicamba in membrane bio-reactors (MBR) and aerobic biofilm reactors (Milligan and Häggblom 1999; Ghoshdastidar and Tong 2013), and specific microbial cultures (González-Cuna et al. 2016). Use of specific microbial consortia creates a competition between the species and becomes unrealistic in wastewater treatment plants (Khan et al. 2011b). It has been found that the long sludge retention in the bioreactor would positively contribute to the efficiency of the system (Navaratna et al. 2012) and sequential batch reactors (SBR) provide the flexibility of handling long SRT as the sludge will be retained in the reactor. SBR works on the simple principle of fill, react, settle and draw, has advantages like low sludge production, easy operation, and is economically driven which makes SBR self-sustainable (Chin et al. 2005). Despite some issues, like formation of recalcitrant substances for complex chemicals, the aerobic SBR has been widely used to treat various types of organic chemicals including 2,4,6-trichlorophenol by modifying an existing SBR (Khorsandi et al. 2018). SBR has also been used as a pre-treatment process to reduce the shock load (Yeruva et al. 2015). The reductive conditions in anaerobic reactor support the biotransformation of halogenated compounds (Field et al. 1995). Several researchers have reported use of anaerobic sequential batch reactor (ASBR) in the treatment of different pollutants including refractory organics by reducing bacteria through dehalogenation, dechlorination reactions (Suflita et al. 1982; Taraban et al. 1993; Weinberg and Teodosiu 2012). Therefore, it was assumed that the refractory halogenated aromatic compounds, which cause recalcitrance, can be efficiently treated in ASBR followed by aerobic SBR system. There are investigations on sequential anaerobic-aerobic treatment of various environmental pollutants including azo dyes removal (Manu and Chaudhari 2002), and textile wastewater treatment (Abiri et al. 2017) but no such study has been found on dicamba treatment.
A redox mediator, like anthraquinone-2,6-disulphonate (AQS), can accelerate the reaction by lowering the activation energy of a reaction. Redox mediators (electron shuttles) are organic molecules which can be either reduced or oxidised reversibly (Van der Zee and Cervante 2009). Redox mediators are capable of transferring electrons over wide variety of organic and inorganic compounds. Reduction of redox mediators can be promoted through chemical reactions of anaerobic environments in the presence of reductants like sulphides, cysteine (Curtis and Reinhard 1994). The reduction of redox mediator can be linked to anaerobic oxidation of organic matter by microorganisms. It has been reported that some of the electron withdrawing compounds accept the electron from reduced redox mediators; such re-oxidation has been observed with azo dyes (Rau et al. 2002) and some polyhalogenated compounds (Kappler and Haderlein 2003). In the presence of redox mediator, the polychlorinated pollutant removal presented six-fold reduction rates (Cervantes et al. 2004). Enhanced removal efficiency of nitroaromatic pollutants like aniline was observed for AQS amended reactions (Tratnyek et al. 2001). Several redox mediated treatment processes have received ample attention for the treatment of different types of pollutants and the scope of this work is limited to briefly discuss the impact of dicamba removal in the absence and presence of different levels of AQS.
The literature on use of sequential anaerobic-aerobic batch reactor and use of disodium anthraquinone-2,6-disulphonate (AQS) as redox mediator to treat dicamba in water is limited and more insight is required on this treatment method. Therefore, this study was conducted to investigate treatment efficiency of dicamba using sequential anaerobic-aerobic batch reactor in the presence of AQS.
2 Materials and Methods
2.1 Chemicals and Synthetic Water Preparation
Analytical grade dicamba and AQS were purchased from Sigma-Aldrich (India), starch and sodium bicarbonate from Hi-media, HPLC grade methanol and ultra-pure water were purchased from Merck, India. The stock dicamba solution was prepared by dissolving 250 mg in 1 L of tap water and stock feed solution was prepared by dissolving 10 g/L of starch and 20 g/L of NaHCO3 in 1 L tap water. The trace metal solution was prepared using COCl2.6H2O:1.613, FeSO4:8.39, MgSO4.7H2O:5, H3BO3:0.1, ZnCl2:0.0473, CuSO4.5H2O:0.0782, NiSO4.62O:1.698, (NH4)6MO7O24.4H2O:0.54, CaCl2:7.776, MnCl2. 4H2O:7.863 in g/L as per the protocols (Manu and Chaudhari 2002), which can serve as nutrients for the microbes.
2.2 Reactor Design and Biomass Acclimatization
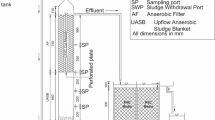
The anaerobic reactor was made using 2.5 L capacity glass bottle with working volume of 2 L, 12 cm in diameter, 20 cm in height and provision for gas collection was made (Fig. 1). The aerobic reactor was made using 2.5 L plastic beaker, with 2 L working volume, 14 cm in diameter, 18 cm in height and fitted with an air diffuser (Fig. 1). The reactors were designed to operate manually to avoid operational issues and involve only manual feeding and decanting. Fresh synthetic wastewater of 1 L was fed daily to the anaerobic reactor, and the reactor was completely air tightened to collect and quantify the gas produced. The aerobic reactor was also designed to work manually, and was fed with 0.5 L effluent from anaerobic reactor.
Seed sludge for the anaerobic reactor was collected from the outlet of a UASB reactor in a municipal treatment plant, and the aerobic sludge was collected from the aeration tank of an STP located on the Institute campus. The sludge was cleaned with water, sieved using 250 μm and characterised for mixed volatile suspended solids (MLVSS) before inoculation. Then, anaerobic and aerobic reactors were inoculated with 9000 mg/L and 2500 mg/L of MLVSS using anaerobic sludge and aerobic sludge, respectively. The activated anaerobic and aerobic sludge was cultured in the respective reactors using synthetic water containing chemical oxygen demand (COD) of 2100 ± 50 mg/L using 2 g/L of stock starch solution. A trace metal solution was supplied to provide nutrient requirement for the microbes. pH was maintained at 7 ± 0.5 using stock NaHCO3 solution (4 g/L). The anaerobic and aerobic reactors hydraulic retention time (HRT) was maintained at 48 h with volumetric exchange ratio of 50% (Khan et al. 2011b). The ambient liquid temperatures recorded in the anaerobic reactor was in the range of 28 ± 0.3 to 31 ± 0.2 °C and in the aerobic reactor was 28 ± 0.3 °C. The sequence of operation was carried out on daily basis as follow: (i) feeding – 0.5 h; (ii) reaction – 22 h; (iii) settling – 1 h; (iv) decanting and idle – 0.5 h.
After achieving consistent biological activity for influent COD concentration of 900 ± 50 mg/L the influent COD concentration was raised to 2100 ± 50 mg/L. Then, the influent COD was maintained in the range of 2100 ± 50 mg/L using 2 g/L of starch to both anaerobic reactors throughout the study period. The steady-state condition for the anaerobic reactor was achieved in 48 days with less than 5% variation in COD removal efficiency (82%) and for the aerobic reactor was in 15 days with less than 3% variation during 3–5 consecutive days. Then, one set of anaerobic-aerobic reactors was used to treat dicamba, and one set was used as control without dicamba and AQS. The dicamba and its TPs were measured both in the liquid and sludge of the reactor using HPLC and GC-HRMS analysis. Influent concentration of dicamba was increased from 10 to 60 mg/L and the influence of dicamba on COD reduction, sludge toxicity and biogas production over different operation periods and SRT was evaluated, and the results were compared with the control.
2.3 Analytical Methods
The transformation products (TPs) have been detected using a gas chromatograph with high resolution mass spectrometer (GC-HRMS, GC – Agilent 7890 and MS – Jeol (AccuTOF GCV)). The GC-HRMS method had column type: column length 60 m; carrier gas Helium; active phase RTX-1; column diameter 0.22 mm; phase thickness 0.25 μm; data type linear RI; program type Ramp; start temperature 60 °C; end temperature 230 °C; heat rate 10 K/min; end time 35 min.
Dicamba was measured using high performance liquid chromatography (HPLC, Agilent Technologies, 1260) equipped with diode array detector at 274 nm wavelength, C18 column with reverse phase and 100 × 4.6 mm, 3.5 μm pore size, flow rate of 0.8 mL/min, and mobile phase ratio of 40:60 methanol and water. The retention time obtained for dicamba was 1.292 min. The maximum wavelength (λmax = 274 nm) was measured using UV-VIS spectrophotometer (AU – 2701, Systronics). Sludge analysis was carried out according to procedure mentioned (Weaver et al. 2004) to find dicamba adsorption. The known quantity of sludge was resuspended with 20 mL methanol (100%) and allowed to mix using a shaker (at 150 rpm) for 24 h; then, centrifuged at 6000 rpm and filtered using 0.2 μm filter paper.
COD (closed reflux method), alkalinity, MLSS and MLVSS were measured as per the standard methods (APHA 2016). All the samples were filtered before analysis using 0.2 μm membrane filter. ORP, pH and temperature were measured using ORP and pH meter (edge, Hanna Instruments). DO meter (HI 9741, Hanna Instruments) was used to measure dissolved oxygen in the aerobic reactor.
2.4 Effect of Influent Dicamba Concentration, SRT and ORP on the Reactor Performance
Dicamba acted as a toxic inhibitor on microbial community even at low concentrations, and appeared as persistent over 112 days even at low concentrations of 3.5 mg/L (Ghoshdastidar and Tong 2013). Dicamba concentration of 19.7 mg/L was treated up to 77% using aerobic packed bed reactor over 150 days operation. Another study reported complete mineralization of dicamba to CO2 and water in an anaerobic reducing condition (Milligan and Häggblom 1999). Hence, an initial dicamba concentration of 10 mg/L was selected and after observing the system performance the concentration was raised up to 60 mg/L successively during the operation period (Table 1). The impact of dicamba introduction on anaerobic and aerobic biomass was monitored through dicamba and COD removal.
Solids retention time is an important parameter which greatly influences the reactor performance. The daily sludge wasting from the reactor effluent was less than 6000 mg/L. The reactor biomass was collected for analysis on specific days to avoid the loss of biomass. The initial MLSS concentration was 80,000 mg/L, and the MLSS measured during every specific day was in the range of 19,200–23,100 mg/L. The SRT of the SBR can be calculated the Eq. (1) and the maximum SRT found was >200 days during stabilization period in all the reactors.
where, SRT is the solids retention time (days); Vt is the total reactor volume (L); X is the MLSS in the reactor (mg/L); Tc is the total operating cycle (h); Vw is the volume of MLSS wasted (L); and Xw is the MLSS wasted (mg/L).
Oxidation reduction potential (ORP) is an important parameter which indicates the reaction mechanism in the reactor. Reducing condition in the anaerobic reactor indicates a negative ORP value. An efficient biological reactor should have optimal ORP of −320 mV (Van der Zee and Cervante 2009). ORP can be maintained in the reactor using a redox mediator like AQS, which is considered as a powerful redox mediator (Rau et al. 2002). The impact of addition of different concentrations of AQS (5–20 mg/L) on anaerobic dicamba removal was studied by monitoring the ORP in the reactor.
3 Results and Discussion
3.1 Acclimatization and Treatment of Dicamba
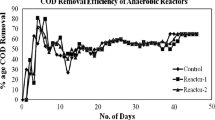
The anaerobic reactors were stabilized using starch as carbon at 24 h HRT with COD removal efficiency of 83% in 49 days, and aerobic reactors using anaerobic effluent as feed with COD removal of 86% in 15 days. Yeruva et al. (2015) have reported that aerobic SBR and anoxic SBR have shown 95% and 92% COD reduction, respectively (out of 3000 mg/L). The reactors were fed with 1 g/L of starch for 20 days, later acclimatised to 2 g/L, and then, 10 mg/L of dicamba was introduced. Anaerobic SBR acclimation can take more time than that of aerobic reactor (Speece 1996). Whereas the aerobic SBR was acclimated in 15 days, this may vary for different compounds from hours to several days up to 25 days (Khorsandi et al. 2018). The influent feed to the anaerobic reactor had COD of 2100 ± 50 mg/L, and pH of 7.5–8.3, and to the aerobic reactor COD of 360–1050 mg/L and pH of 6.3–7.6.
The treatment process was carried out in four stages with different influent dicamba concentrations from 10 to 60 mg/L, AQS dosage (5 to 20 mg/L) and overall reactor operation period of 340 days. In stage I, operation conditions were continued with introduction of 10 mg/L dicamba and the performance of the reactor is shown in Fig. 2. The toxicity of dicamba was found to inhibit anaerobic sludge during the first 27 days of introduction, which was indicated by reduced MLVSS in the reactor, and lower COD removal and biogas production compared to the control. The COD in the effluent of acclimated reactor was 900–1300 mg/L, whereas the control reactor COD was at 300–460 mg/L. High effluent COD may indicate incomplete degradation of dicamba (González-Cuna et al. 2016). Initial dicamba removal between 52nd – 75th day was due to the accumulation of compounds through adsorption on the reactor sludge; this was confirmed after characterising the sludge according to Weaver et al. (2004). Adsorption on the sludge was in the range of 5–8 mg/g MLVSS, indicating that significant amount of dicamba was adsorbed on the sludge. Adsorption of dicamba was reduced with continuous operation, which may be due to high water solubility of 4500 mg/L of the compound (William and Armbrust 2002), and other characteristics, which make it weakly adsorbed on soil (pKa of 1.87), and highly mobile in soil (Comfort et al. 1992). Effluent from the dicamba acclimated anaerobic reactor contained biotransformation products, which may be due to the inability of anaerobic bacteria to completely biodegrade the compound under anaerobic condition. The dicamba TPs have been degraded by the aerobic bacteria in the aerobic reactor indicated by disappearance of intensity peak at retention time of 1.292 min in HPLC (Fig. 3). The HPLC report obtained for the aerobic effluent indicates that the transformation products of dicamba are mineralised. Further, decline in COD concentration and stable biogas production after 65 days indicate that the anaerobic sludge was restored slowly with a consistent biological activity in the reactor, which may be an indication of acclimatization to 10 mg/L of dicamba. Acclimation to 20 mg/L of 2,4-D took more than 80 days to aerobic reactor and inhibitory effects of herbicide was avoided in the presence of glucose (Chin et al. 2005). Therefore, the treatment was continued with the same influent dicamba concentration and the anaerobic reactor reported 65% of dicamba removal, whereas the aerobic reactor removed up to 95% of dicamba. The anaerobic reactor produces dehalogenated and dechlorinated compounds like 3,6-dichlorosalicylate and 6-chlorosalicylate of dicamba which may be difficult to degrade in anaerobic condition (Milligan and Häggblom 1999), whereas in this study formation of long chain fatty acids have been detected from the GC-HRMS analysis.
It was found that at low ORP in the anaerobic reactor, reductive reactions like demethylation and dechlorination occur, which lead to the breakup of methyl, chlorine and halogen group. Therefore, the ORP value was reduced from −250 to −300 ± 10 mV using 5 mg/L AQS, which increased the anaerobic treatment efficiency by 5–12%. It was observed that further increase in AQS to 10 mg/L showed increased dicamba removal efficiency but there was a raise in TPs concentrations with high effluent COD when compared to control. Treatment of anaerobic effluent in the aerobic reactor was found to be maximum for degradation of dicamba and COD by 65% and 67%, respectively. Apparently the appearance of sludge granulation on day 40 was an indication of active growth of aerobic bacteria by utilizing the anaerobic TPs as their nutrient sources. The formation of sludge granules may be due to the reactor operating conditions which was promoted by the dense microbial consortia of different bacterial species, which support the degradation of dicamba; and similar observations have been reported (Dutta and Sarkar 2015). The initial dicamba concentration was raised to 20 mg/L in the acclimatised bioreactor and continued in stage II.
The initial dicamba concentration was increased to 20 mg/L after the consistent removal of 10 mg/L. Toxic effect of dicamba appeared to be negligible when compared to the previous stage as the bacteria in the reactor have been acquainted to the compound over the 113 days of operation. After increase in the influent dicamba concentration, there was raise in dicamba TP and COD in the effluent which may be attributed to increased toxicity load inhibited in the anaerobic sludge; high COD in the effluent is an indication of incomplete dicamba degradation (González-Cuna et al. 2016). Reduced biogas production and reduced MLVSS in the reactor was an indication of sludge toxicity. During this time period from 140 to 160 days, it should also be noted that the acclimatised bacteria were able to degrade the compound partially, which indicates presence of insufficient dominant bacteria to degrade dicamba. Increase in AQS supported the dicamba removal along with reduced effluent COD, which may indicate the development of dicamba degrading bacteria favoured by the redox mediator. MLVSS concentration was increased from 8550 mg/L to 9300 mg/L after 160 days. ORP during this period was observed to be around −300 mV with existing influent AQS concentration of 10 mg/L. It has been reported that the addition of quinones like AQS activated the ability of unacclimated biomass to degraded azo dyes (Rau et al. 2002). At ORP of −270 to −320 mV, anaerobic reactor performance was reported to be stable for dicamba removal, and incomplete degradation in anaerobic reactor followed the previous degradation pattern (Fig. 2). It can be observed that the aerobic reactor performance was exceptionally better than before with removal efficiency of TPs of 92% and COD of 97% (Fig. 4). Hence, the aerobic effluent reported negligible TPs with effluent COD of 45–110 mg/L. In contrast, the aerobic treatment was able to remove >99% (3.5 mg/L) dicamba over 112 days of treatment (Ghoshdastidar and Tong 2013). The influent dicamba concentration was raised to 2 times (40 mg/L) and the treatment was conducted in stage III.
Performance of the anaerobic reactor for the treatment of 40 mg/L dicamba was conducted during 166–243 days of operation in acclimated biomass (Fig. 2) and aerobic reactor performance (Fig. 4). The reduction of anaerobic biological activity was indicated by high effluent dicamba concentration, high COD, reduced MLVSS (<6500 mg/L) and biogas production. The reduced sludge activity in anaerobic reactor compared to previous stage and the control reactor may be due to sudden toxicity, but it was not observed in the aerobic reactor. The operation was preceded using 10 mg/L of AQS till 45 days (between 166 and 211 days), ORP remained at −270 to −320 mV and the treatment efficiencies for dicamba and COD were 74% and 77%, respectively. AQS was increased to 15 mg/L (from day 243) and ORP was reduced slightly (−5 mV), and ORP was almost negligible sometimes. It was assumed that long SRT (>90 days) would have supported the degradation indicated by consistent reactor performance after 40 days of introduction of 40 mg/L of dicamba. Development of specific degradation pathway by the microorganisms might have lead to the increased dicamba removal over long operation period (Koh et al. 2008). Consequently, the aerobic reactor showed better performance than the anaerobic reactor as usually by degrading the anaerobic TP of dicamba (~80%) with COD removal efficiency of 90% till the day 77. The study was continued with increase in influent dicamba concentration to 60 mg/L from day 244 onwards and the results are discussed in stage IV.
Toxicity was slightly inhibited on anaerobes indicated by poor performance and reduced MLVSS concentration below 7000 mg/L till 300 days of operation and continued further as the MLVSS concentration of 2000–10,000 mg/L can support the anaerobic treatment process (Speece 1996). The continued treatment process regenerated the MLVSS concentration over 20 days. It was observed from the graph that at average ORP values around −310 mV the anaerobic reactor showed >70% reduction in dicamba and produced some biotransformation products with COD removal efficiency of 81%. The dicamba removal efficiency of Fenton’s treatment process reported 85% of 86.1 mg/L of dicamba with COD removal of 83% (Sangami and Manu 2017b). HPLC analysis reports obtained for the effluent and influent samples during the study can suggest the formation of TPs in anaerobic reactor and mineralization in aerobic reactor (Supplementary material 1). Further, the TPs of dicamba in the anaerobic effluent was determined using the GC-HRMS analysis and some of the major compounds detected are oleic acid (C18H34O2), 9-Octadecenoic acid (Z), 2-hydroxy-1-(hydroxymethyl) ethyl ester (C21H40O4), trans-13-Ocatadecenoic acid (C18H34O2). These products have tendency to get oxidised when they are treated in aerobic reactor. Aerobic reactor was able to remove dicamba TPs up to 85% with COD removal of 92%. The long chain fatty acids removed by losing 2 carbon atoms by β-oxidation pathway produces acetyl-CoA, which can be further oxidised to CO2 through tricarboxylic acid cycle (Ratledge 1992). Formation of different types of fatty acids and other TPs during the anaerobic treatment of dicamba can be used by aerobic bacteria as nutrient and supported sludge granulation. Degradation of fluroaromatics compounds (type of herbicides) by aerobic bacteria in the presence of oxygenase enzyme has supported our findings (Murphy et al. 2009). The granules formation in the dicamba treating reactor compared with the control reactor sludge is shown in Fig. 5. Granules are cultivated to treat xenobiotic compounds in aerobic SBR as the bacteria uses the compound as their sole carbon source (Khan 2011a). After 300 days of operation with influent AQS (15 mg/L), the concentration was raised to 20 mg/L. This dosage was able to maintain the existing ORP (−310 mV) but it was expected to reduce below −320 mV. The low ambient reactor temperature (28.4–29.5 °C) might not have supported the redox reactions.
3.2 Effect of Influent Dicamba Concentration on Anaerobic Sludge Toxicity
The anaerobic reactor was found to be under the toxic risk on introduction of dicamba which can be evaluated based on the reduction in MLVSS concentration and biogas production. The averaged MLVSS concentration and biogas production was compared with respect to the influent dicamba concentration. It can be observed that the inoculated 9000 mg/L sludge concentration was raised up to 11,000 mg/L initially in both reactors (Table 1). The excess sludge was wasted in both reactors to maintain MLVSS of 9000 mg/L. After 48 days of total stabilization period, 10 mg/L of dicamba was introduced to one of the anaerobic reactors. At the beginning, it appeared that there was slight toxicity which reduced MLVSS concentration (7000 mg/L), whereas in the control it was 9300 mg/L. The reduced sludge activity was an indication of toxicity induced by the transformation products of dicamba on bacteria than the dicamba itself (Ghoshdastidar and Tong 2013; Kuppusamy et al. 2017). MLVSS was restored in the dicamba treating reactor with continued operation on day 85. On the 113th day MLVSS in the control was 9800 mg/L but in the dicamba containing reactor it was 9400 mg/L, and then, influent dicamba concentration was raised to 20 mg/L and the toxicity inhibition followed similar pattern as before and was restored on continued treatment due to the adoptability of bacteria to the TP of dicamba. In spite of the toxicity, the biogas production was found to be consistently higher than that in the control reactor. This may be another evidence for well adopted dominant anaerobic bacteria (Fig. 6b). Release of electron donors from the dicamba under reducing conditions might have been utilized by the anaerobic bacteria and thus high biogas was produced during the treatment. This was also supported by lower ORP values (−310 mV) than the ORP (<−250 mV) in the control reactor. Redox mediators have enhanced the biodegradation of chloroaromatic pollutants like dyes by electron shuttling (Dos Santos et al. 2005), and nitroaromatic pollutants like aniline (Tratnyek et al. 2001). Dicamba containing methyl, chloride, and hydroxyl components after undergoing demethylation, dechlorination and dehalogenation reactions produces CH4, H2S and CO2 gases. CO2 further utilized as carbon source by some of the anaerobic methanogenic bacteria produces methane (Suflita et al. 1982). Therefore, the biogas production in the anaerobic dicamba treating reactor is greater than the control reactor. Further the concentration was doubled by 2 times (40 mg/L) which appeared as toxic load (shock load) on the sludge (Weinberg and Teodosiu 2012) and reduced the MLVSS concentration (̴ 6400 mg/L). The sludge activity was improved over 40 days with regeneration of improved MLVSS concentration and biogas production. The biogas production during stage II (i.e., for 40 mg/L) has varied from 535 to 670 mL/d, lower being on the day 5 and day 31 (after raise of 40 mg/L) due to possible toxicity and it is supported by low COD removal efficiency (45–51%). Further increase in dicamba concentration of 60 mg/L reduced MLVSS to 7000 mg/L initially and recovered over 40 days of operation which indicate the consistent bacterial performance. Increase in biogas production over a long operation period after the raise in influent dicamba concentration compared to the control indicates the utilization of influent dicamba by the activated biomass. Though there was shock load effects observed during initial stages of dicamba introduction, it was recovered after certain time of operation due to the adaptation and activation of inactive biomass of the anaerobic reactor. A gradual adaptation and development of anaerobic biomass (MLVSS) over long operation period has been reported during the treatment of phenoxy acetic acid herbicide (Chin et al. 2005). Another significant observation during this study is that the temperature variation, biogas production was comparatively more at high temperature ranges in the anaerobic dicamba reactor (30–31.2 °C) than the control reactor (29.5–30 °C). It can be observed that different influent dicamba concentration was removed gradually over time; dicamba (in the form of TP) remaining in the effluent after each anaerobic and anaerobic-aerobic treatment can be observed in Fig. 7.
3.3 Effect of Solids Retention Time on the Reactor Performance
MLSS and MLVSS detected in the effluent was considerably less (i.e., MLSS: 800–6000 mg/L and MLVSS: 600–2800 mg/L). The maximum and minimum SRT calculated was 200 days and 26 days during the stabilization period, and dicamba treatment period respectively. In stage I, after addition of 10 mg/L of dicamba the MLVSS in the reactor was reduced to 7200 mg/L (quantification of MLVSS was done after and before the raise in influent dicamba to avoid biomass loss). The loss of MLSS and MLVSS found in the effluent during daily decanting was considered to be very small, of the order of 2200–2600 mg/L and 1800–2000 mg/L, respectively, and the corresponding SRT was 60–70 days. Khan et al. (2011b) have reported SRT of 20 days for 5% loss of biomass. With increase in operation period, the sludge quality was improved, as indicated by the lower appearance of sludge in the effluent (<1200 mg/L). The SRT calculated during the long operation period (89–113 days) was >150 days. The long operation period supported the growth of slow and inactive biomass to get adapted to the toxic condition, and hence, the increased reactor performance was observed.
It can be seen that in all the successive stages (II to IV) similar finding are observed. During the first 20 days of each stage with increased dicamba concentration there was always lower SRT (<55 days) compared to the second phase of that stage. Increase in influent dicamba concentration to 20 mg/L followed the same pattern as observed in the previous stage, whereas further raise to 40 mg/L has appeared as shock loading with reduced sludge activity. The shock load impacts are gradually overcome due to adaptation of bacteria over long operation period (Chin et al. 2005) and the SRT found was 28–35 days during the first 10 days. MLVSS concentration dropped to <6500 mg/L during stage III and recovered over 76 days. Further raise in dicamba concentration to 60 mg/L had low impact on the biomass compared to previous stage. Long SRT in a biological reactor enable growth of slowly growing micro-organisms which have further enhanced the removal of EDCs (Koh et al. 2008).
3.4 Effect of Redox Mediator (AQS) on the Treatment Process
ORP is an important parameter which has greatly influenced the dicamba treatment in the anaerobic reactor. AQS produces free radicals which enhance the redox reaction in reductive environments by oxidising various types of organic and inorganic compounds (Van der Zee and Cervante 2009), by transferring the electrons from electron donors (starch) to electron acceptor (dicamba) (Da Silva et al. 2012). ORP in the anaerobic reactors during the stabilization period was in the ranges of −220 to −270 mV at the ambient temperature ranges of 28 to 30 °C and ORP was dependent on temperature as observed in the experiment. The variation of ORP was linked to redox reactions between the various substrates and hence degradation of dicamba at different ORP and biogas production has been compared with control (Fig. 6a–b). Introduction of 10 mg/L dicamba after the stabilization has activated redox reaction under reducing condition indicated by reduced ORP (−260 to −285 mV). Decrease in the ORP was observed with addition of 5 mg/L AQS solution at similar ambient temperature ranges, which increased the degradation efficiency of dicamba (Fig. 2). In the second stage, with 20 mg/L of influent dicamba concentration, ORP remained the same around −270 to −290 mV, which was sufficient enough to enhance the anaerobic degradation, where the control ORP ranged from −220 to −270 mV. The raise in AQS by 10 mg/L has improved the redox reactions with further reduction of ORP (−10 ± (−4) mV) at the ambient temperature. Da Silva et al. (2012) have reported increased dye removal with addition of AQS which enhanced the colour removal by mediating the redox reactions in the acidogenic and anaerobic reactors. Then, the dicamba concentration was doubled to 40 mg/L keeping all the other dosages constant. At this stage the ORP was found to be −310 ± (−12) mV, which indicates that there were active substrates (dicamba) available for the redox reaction of the anaerobes; this is a clear indication of compound being transformed to its residuals (TPs). As the effluent water contained high residuals concentration and COD up to 750 to 1200 mg/L, it was believed that there are available substrates which can be degraded in anaerobic reactor and hence the AQS was increased to 15 mg/L. Though there was a reduction in ORP (around −12 mV), the reduction in the residual compounds took place only after certain days of operation from herbicide introduction and also after the introduction of AQS. The acclimatization of bacteria over 20–45 days after raise in dicamba had indicated the reduced risk of dicamba for shock loads and contributed to biodegradation. Dicamba concentration was raised to 60 mg/L and the ORP remained the same, maybe due to the inability of bacteria to undergo redox reactions at existing AQS of 15 mg/L. Even after raise in AQS to 20 mg/L, no significant change in ORP was observed but the removal took place around 70%. It was observed that attainment of saturation kinetics during the decolouration studies (Field and Brady 2003) may be due to reduced ambient temperature in the reactor (28–29.2 °C) influencing the redox reactions. ORP remained around −270 to −300 mV but the effluent contained significant amounts of non-degraded compound which contributed to high COD values. The AQS addition to anaerobic reactor had no influence on the sludge activity of the aerobic reactor. The high rate of herbicide removal in the aerobic reactor was due to low influent load (COD <400 mg/L), and presence of readily oxidising long chain fatty acids.
4 Conclusions
Treatment of dicamba in a sequential anaerobic-aerobic batch reactor was conducted. Biotransformation of dicamba in the anaerobic reactor, followed by the mineralization of transformation products (TPs), was achieved. Treatment in the anaerobic reactor was studied using varying concentrations of AQS as redox mediator which influenced the anaerobic transformation to certain extent. Anaerobic transformation products were identified as long chain fatty acids using GC-HRMS analysis and the post-treatment in aerobic reactor supported the complete mineralization. Significant outcomes of this study are:
-
Toxicity on anaerobic biomass was reduced over long operation period with increased SRT contributed to dicamba removal.
-
Increased SRT over long operation period supported the efficacy of the system.
-
Addition of AQS was significant in the enhancement of redox reactions under reductive conditions and AQS (>20 mg/L) may be insignificant at low temperatures.
-
COD remaining in the anaerobic effluent indicated the presence of biodegradable organic matter in the form of TPs.
-
Individual reactor treatment efficiency is considered as inefficient.
-
Aerobic treatment is essential for the maximum reduction of TPs and COD.
References
Abiri F, Fallah N, Bonakdarpour B (2017) Sequential anaerobic–aerobic biological treatment of colored wastewaters: case study of a textile dyeing factory wastewater. Water Sci Technol 75(6):1261–1269. https://doi.org/10.2166/wst.2016.531
APHA (2016) Standard methods for the examination of water and wastewater. Washington, DC: American Public Health Association, 2012
Cervantes FJ, Vu-Thi-Thu L, Lettinga G, Field JA (2004) Quinone-respiration improves dechlorination of carbon tetrachloride by anaerobic sludge. Appl Microbiol Biotechnol 64:702–711. https://doi.org/10.1007/s00253-004-1564-z
Chin H, Elefsiniotis P, Singhal N (2005) Biodegradation of 2, 4-dicholophenoxyacetic acid using an acidogenic anaerobic sequencing batch reactor. J Environ Eng Sci 4(1):57–63. https://doi.org/10.1139/s04-044
Comfort SD, Inskeep WP, Macur RE (1992) Degradation and transport of dicamba in a clay soil. J Environ Qual 21(4):653–658. https://doi.org/10.2134/jeq1992.00472425002100040020x
Curtis GP, Reinhard M (1994) Reductive dehalogenation of hexachloroethane, carbon tetrachloride, and bromoform by anthraquinone disulfonate and humic acid. Environ Sci Technol 28:2393–3401. https://doi.org/10.1021/es00062a026
Da Silva MER, Firmino PIM, dos Santos AB (2012) Impact of the redox mediator sodium anthraquinone-2, 6-disulphonate (AQDS) on the reductive decolourisation of the azo dye reactive red 2 (RR2) in one-and two-stage anaerobic systems. Bioresour Technol 121(1–7):1–7. https://doi.org/10.1016/j.biortech.2012.06.099
Dos Santos AB, Traverse J, Cervantes FJ, Van Lier JB (2005) Enhancing the electron transfer capacity and subsequent color removal in bioreactors by applying thermophilic anaerobic treatment and redox mediators. Biotechnol Bioeng 89(1):42–52. https://doi.org/10.1002/bit.20308
Dutta A, Sarkar S (2015) Sequencing batch reactor for wastewater treatment: recent advances. Curr Pollut Rep 1(3):177–190. https://doi.org/10.1007/s40726-015-0016-y
Field JA, Brady J (2003) Riboflavin as a redox mediator accelerating the reduction of the azo dye mordant yellow 10 by anaerobic granular sludge. Water Sci Technol 48(6):187–193. https://doi.org/10.2166/wst.2003.0393
Field JA, Stams AJ, Kato M, Schraa G (1995) Enhanced biodegradation of aromatic pollutants in cocultures of anaerobic and aerobic bacterial consortia. J Microbiol 67(1):47–77. https://doi.org/10.1007/bf00872195
Ge T, Han J, Qi Y, Gu X, Ma L, Zhang C, Huang D (2017) The toxic effects of chlorophenols and associated mechanisms in fish. Aquat Toxicol 184:78–93. https://doi.org/10.1016/j.aquatox.2017.01.005
Ghoshdastidar AJ, Tong AZ (2013) Treatment of 2, 4-D, mecoprop, and dicamba using membrane bioreactor technology. Environ Sci Pollut Res 20(8):5188–5197. https://doi.org/10.1007/s11356-013-1498-z
González NV, Soloneski S, Larramendy ML (2006) Genotoxicity analysis of the phenoxy herbicide dicamba in mammalian cells in vitro. Toxicol in Vitro 20(8):1481–1487. https://doi.org/10.1016/j.tiv.2006.05.001
González-Cuna S, Galíndez-Mayer J, Ruiz-Ordaz N, Murugesan S, Piña-Escobedo A, García-Mena J, Santoyo-Tepole F (2016) Aerobic biofilm reactor for treating a commercial formulation of the herbicides 2, 4-D and dicamba: biodegradation kinetics and biofilm bacterial diversity. Int Biodeterior Biodegrad 107:123–131. https://doi.org/10.1016/j.ibiod.2015.11.014
Hamilton D, Crossley S (Eds.) (2004) Pesticide residues in food and drinking water: human exposure and risks. John Wiley and Sons. https://doi.org/10.1002/0470091614
Hamilton DJ, Ambrus A, Dieterle RM, Felsot AS, Harris CA, Holland PT, Wong SS (2003) Regulatory limits for pesticide residues in water (IUPAC technical report). Pure Appl Chem 75(8):1123–1155. https://doi.org/10.1351/pac200375081123
Kappler A, Haderlein SB (2003) Natural organic matter as reductant for chlorinated aliphatic pollutants. Environ Sci Technol 37:2714–2719. https://doi.org/10.1021/es0201808
Khan MZ, Khan SS (2011a) Aerobic granular treatment of 2, 4-dichlorophenol. Can J Chem Eng 89(4):914–920. https://doi.org/10.1002/cjce.20445
Khan MZ, Mondal PK, Sabir S, Tare V (2011b) Degradation pathway, toxicity and kinetics of 2, 4, 6-trichlorophenol with different co-substrate by aerobic granules in SBR. Bioresour Technol 102(13):7016–7021. https://doi.org/10.1016/j.biortech.2011.04.057
Khorsandi H, Ghochlavi N, Aghapour AA (2018) Biological degradation of 2, 4, 6-trichlorophenol by a sequencing batch reactor. Environ Process 5(4):907–917. https://doi.org/10.1007/s40710-018-0333-4
Koh YKK, Chiu TY, Boobis A, Cartmell E, Scrimshaw MD, Lester JN (2008) Treatment and removal strategies for estrogens from wastewater. Environ Technol 29(3):245–267. https://doi.org/10.1080/09593330802099122
Kuppusamy S, Jayaraman N, Jagannathan M, Kadarkarai M, Aruliah R (2017) Electrochemical decolorization and biodegradation of tannery effluent for reduction of chemical oxygen demand and hexavalent chromium. J Water Process Eng 20:22–28. https://doi.org/10.1016/j.jwpe.2017.09.008
Manu B, Chaudhari S (2002) Anaerobic decolorisation of simulated textile wastewater containing azo dyes. Bioresour Technol 82:225–231. https://doi.org/10.1016/S0960-8524(01)00190-0
Milligan PW, Häggblom MM (1999) Biodegradation and biotransformation of dicamba under different reducing conditions. Environ Sci Technol 33(8):1224–1229. https://doi.org/10.1021/es981117e
Mondal PK, Ahmad R, Usmani SQ (2010) Anaerobic biodegradation of triphenylmethane dyes in a hybrid UASFB reactor for wastewater remediation. Biodegradation 21(6):1041–1047. https://doi.org/10.1007/s10532-010-9364-x
Murphy CD, Clark BR, Amadio J (2009) Metabolism of fluoroorganic compounds in microorganisms: impacts for the environment and the production of fine chemicals. Appl Microbiol Biotechnol 84(4):617–629. https://doi.org/10.1007/s00253-009-2127-0
Navaratna D, Shu L, Baskaran K, Jegatheesan V (2012) Treatment of ametryn in wastewater by a hybrid MBR system: a lab-scale study. Water Sci Technol 66(6):1317–1324. https://doi.org/10.2166/wst.2012.318
Ratledge C (1992) Microbial oxidations of fatty alcohols and fatty acids. J Chem Technol Biotechnol 55(4):399–400. https://doi.org/10.1002/jctb.280550418
Rau J, Knackmuss HJ, Stolz A (2002) Effects of different quinoid redox mediators on the anaerobic reduction of azo dyes by bacteria. Environ Sci Technol 36(7):1497–1504. https://doi.org/10.1021/es010227+
Sangami S, Manu B (2017a) Fenton's treatment of actual agriculture runoff water containing herbicides. Water Sci Technol 75(2):451–461. https://doi.org/10.2166/wst.2016.538
Sangami S, Manu B (2017b) Optimization of Fenton’s oxidation of herbicide dicamba in water using response surface methodology. Appl Water Sci 7(8):4269–4280. https://doi.org/10.1007/s13201-017-0559-8
Shin EH, Choi JH, Abd El-Aty AM, Khay S, Kim SJ, Im MH, Shim JH (2011) Simultaneous determination of three acidic herbicide residues in food crops using HPLC and confirmation via LC-MS/MS. Biomed Chromatogr 25(1–2):124–135. https://doi.org/10.1002/bmc.1513
Speece RE (1996) Anaerobic biotechnology for industrial wastewaters. Archae Press, Nashville
Suflita JM, Horowitz A, Shelton D, Tiedje JM (1982) Dehalogenation: a novel pathway for the anaerobic biodegradation of haloaromatic compounds. Science 218(4577):1115–1117. https://doi.org/10.1126/science.218.4577.1115
Taraban RH, Berry DF, Berry DA, Walker HL (1993) Degradation of dicamba by an anaerobic consortium enriched from wetland soil. Appl Environ Microbiol 59(7):2332–2334. https://doi.org/10.1128/AEM.01201-16
Tratnyek PG, Scherer MM, Deng BL, Hu SD (2001) Effects of natural organic matter, anthropogenic surfactants, and model quinones on the reduction of contaminants by zero-valent iron. Water Res 35:4435–4443. https://doi.org/10.1016/S0043-1354(01)00165-8
Van der Zee FP, Cervante FJ (2009) Impact and application of electron shuttles on the redox (bio) transformation of contaminants: a review. Biotechnol Adv 27(3):256–277. https://doi.org/10.1016/j.biotechadv.2009.01.004
Weaver M, Zablotowicz RM, Locke MA (2004) Laboratory assessment of atrazine and fluometuron degradation in soils from a constructed wetland. Chemosphere 57(8):853–862. https://doi.org/10.1016/j.chemosphere.2004.08.013
Weinberg B, Teodosiu C (2012) Monitoring and assessment of herbicides removal by industrial wastewater treatment. Environ Eng Manag J 11(1):215–224. https://doi.org/10.30638/eemj.2012.028
William KV, Armbrust K (2002) Weed science Society of America. Herbicide handbook, Lawrence KS, USA
Yeruva DK, Jukuri S, Velvizhi G, Kumar AN, Swamy YV, Mohan SV (2015) Integrating sequencing batch reactor with bio-electrochemical treatment for augmenting remediation efficiency of complex petrochemical wastewater. Bioresour Technol 188:33–42. https://doi.org/10.1016/j.biortech.2015.02.014
Acknowledgments
The authors would like to thank MHRD, Government of India, for providing funds through institutional fellowship to carry out the research. We would also thank DST and SAIF, IIT Bombay, India, for providing the GC-HRMS analysis facility.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Sequential anaerobic-aerobic reactor was capable to mineralize dicamba
• Addition of AQS as redox mediator contributed to the enhanced methanogenic activity
• Increased anaerobic biotransformation of dicamba (70%) with high rate of biogas production
• System can remove 82% of dicamba (60 mg/L)
• Formation of aerobic granules and low effluent COD (<50 mg/L)
Electronic supplementary material
ESM 1
(JPG 220 kb)
Rights and permissions
About this article
Cite this article
Mahesh, G.B., Manu, B. Biological Treatment of 3,6-Dichloro-2-Methoxybenzoic Acid Using Anaerobic-Aerobic Sequential Batch Reactor. Environ. Process. 6, 493–509 (2019). https://doi.org/10.1007/s40710-019-00375-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40710-019-00375-w