Abstract
The present study investigates the addition of several commercially available cationic (FO 4000 SSH and ADIFLOC series) and anionic (LT-25 Magnafloc) polyelectrolytes in a fully automated, pilot-scale Membrane Bioreactor (MBR), aiming to the development of an integrated methodology for the control of membrane fouling in MBR treatment systems. For this purpose, a series of batch experiments were conducted in order to evaluate their effectiveness on membrane fouling mitigation, using a typical hollow fiber, commercially available microfiltration membrane. Reversible fouling was assessed in terms of sludge filterability tests, whereas irreversible fouling was assessed in terms of SMPs (Soluble Microbial Products) concentration measurements. Results showed that the addition of cationic polyelectrolytes FO 4000 SSH series and ADIFLOC series were found capable to reduce both reversible and irreversible fouling. More specifically, the FO 4350 SSH type exhibited a remarkable performance, regarding both sludge filterability enhancement and SMP removal, when added at the concentration of 10 mg/L. Further comparison with the anionic polyelectrolyte LT-25 Magnafloc reinforced the allegation that cationic polyelectrolytes are preferable for control of membrane fouling, mainly due to charge neutralization mechanism.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Membrane Bioreactors (MBRs) are increasingly used over the last few years in municipal or industrial wastewater treatment. However, their widespread application is still limited mainly by the fouling of used membranes, which remains the most significant problem, affecting the efficiency and operation of this treatment process (Liang et al. 2006; Meng et al. 2009). Fouling is defined as the undesirable deposition and accumulation of microorganisms, colloids, solutes and cell debris within or/and on the membrane surface (Wu et al. 2012; Zhang et al. 2011), resulting to permeate flux decline, which in turn decreases the time intervals for membrane cleaning and eventually replacement, hence leading to higher operating costs. Soluble Microbial Products (SMP), also known as soluble Extracellular Polymeric Substances (sEPS), are considered to be among the major compounds responsible for membrane fouling. These are large molecular weight bio-polymeric compounds, which are released by bacteria. They consist of proteins, polysaccharides, lipo-polysaccharides, lipo-proteins or complex mixtures of these biopolymers with a variety of other functional groups, such as carboxyl, amino and/or phosphate (Dizge et al. 2011).
Most of the strategies, which are employed for fouling mitigation, are focusing on the (bio)chemical modification of mixed liquor in the aeration tank, by the addition of specific chemicals, e.g., coagulants or adsorbent agents, or by the application of ultrasound, electric field, ozone, and/or by the appropriate membrane surface modification. The latter may include the appropriate physical coating/adsorption, the application of grafting methods, the use of specific patterned (custom-made) membranes, the application of plasma treatment, chemical reactions on the membrane surface, or the surface modification with novel nanoparticles (Gkotsis et al. 2014).
The appropriate dosing of coagulants into MBR systems has been reported to assist greatly the reduction of fouling, due to the reduction of organics in the supernatant liquor, or to the formation of larger flocs, which may limit the blockage of membranes pores (Zhang et al. 2015). In literature, a wide variety of coagulant dosages have been identified as optimal in terms of membrane fouling mitigation and nutrient removal, ranging from few mg/L or even some dozens of mg/L (Ma et al. 2014; Shon et al. 2005), but also up to 500 mg/L (Song et al. 2008). The commonly used coagulant reagents fall into three main categories: inorganic monomers, inorganic polymers and organic polymers. When compared to inorganic monomeric coagulants, the polymeric coagulants present a number of advantages, including lower addition of dosage, smaller influence upon pH changes and lower sludge production; therefore, they have been widely used in water and wastewater treatment (Yu et al. 2015). Thus, apart from the typical coagulation reagents, such as simple Fe or Al salts, inorganic polymeric chemical reagents, as well as organic polyelectrolytes are commonly used as coagulant of flocculant agents, respectively, in MBR systems in order to enhance the filterability properties of membranes (Dizge et al. 2011). According to their charge, polyelectrolytes can be further classified into cationic, anionic, or non-ionic types. More specifically, cationic organic polyelectrolytes are extensively used for the prevention of filter media fouling during the conventional filtration process (Huyskens et al. 2012; Dizge et al. 2011). Among them, poly-acrylamides have been reported to reduce significantly SMP and noticeably alter the structure of created flocs, resulting in much longer operation period of filtration process (Ji et al. 2014). PolyDADMAC ((poly)diallyl-dimethyl-ammonium chloride) polyelectrolytes have also been used for the mitigation of filter media fouling (i.e., of membranes), as well as for the additional removal of contaminants. Kim et al. (2014) used a series of cationic polyelectrolytes to treat laundry wastewater and showed that the addition of polyDADMAC decreased the fouling of PVDF microfiltration membranes by a factor of 10 at pH 11.
However, to the author’s best knowledge, rather few research studies were conducted to compare and investigate the effect of cationic or anionic polyelectrolytes on the mitigation of fouling of membrane bioreactors. This study is part of a research project that aims to the development of a systematic and integrated methodology for the fouling mitigation and control during membrane bioreactors operation. For this purpose, more than 40 inorganic and organic, Al- and Fe-based, pre-polymerized and conventional, commercially available and laboratory prepared (Tolkou and Zouboulis 2014) coagulation and flocculation reagents were tested in mixed liquor samples of a fully automated, pilot-scale MBR system (Gkotsis et al. 2015). The mitigation of fouling was evaluated in terms of filterability tests (indicative of reversible fouling) and of SMP concentration measurements (indicative of irreversible fouling). In this paper, the results are presented regarding specifically the addition and comparison of specific, commonly applied organic cationic and anionic polyelectrolytes.
2 Material and Methods
2.1 Pilot-Scale MBR Operation
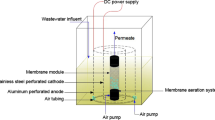
The pilot-scale MBR system, in which all the experiments were carried out, is presented in Fig. 1. This treatment system consists of four sub-units: (a) wastewater feed unit, (b) bioreactor, (c) membrane (side-stream) filtration unit, and (d) permeate collection unit. The operation of this system includes two subsequent stages. First, the bioreactor was inoculated with activated sludge, which was received from the municipal wastewater treatment plant of Thessaloniki (located in the area of Sindos, near Gallikos river), and then, the system was operated continuously in order to achieve steady-state conditions in the bioreactor. In the second stage, the organic polyelectrolytes were added in a series of batch experiments. During these experiments, the polyelectrolytes were added in a single drop mode in mixed liquor samples, which were obtained from the aeration tank on a daily basis.
Experimental set-up of the pilot-scale MBR system (Gkotsis et al. 2015): a wastewater feed unit, b bioreactor, c membrane filtration unit, and d permeate collection unit
In both phases synthetic municipal wastewater (Table 1) was led by a peristaltic pump to the aeration tank, where the concentration of dissolved oxygen was monitored by a dissolved oxygen meter in the range of 2–3 mg/L. This is the “standard” synthetic wastewater composition proposed by OECD for performing relevant biological wastewater treatment laboratory experiments. However, the concentrations of the synthetic wastewater components (peptone water, meat extract etc.) were selected to be much higher (×10) in this case, than those proposed by the OECD guidelines (OECD 2010), in order to obtain a satisfactory F/M ratio (approximately 0.2). The effluent of the aeration tank passed through the (separate) membrane system, while part of the activated sludge was returned to the aeration tank, also using another peristaltic pump. The air needed for biomass aeration and for the cleaning of membrane was supplied by an air compressor, whose pressure was appropriately reduced to the desired value, by means of an air pressure reducer. Gas and liquid flow rates were measured by gas and liquid flow meters, while level sensors were used in order to control the liquid level in the membrane tank.
The permeate was withdrawn from the upper end of the membrane by another peristaltic pump, while a high-resolution pressure transmitter was placed at the outlet of the membrane in order to record the Trans-Membrane Pressure (TMP) during the MBR operation. The permeate collection unit was the final recipient of produced permeate, a part of which was used for backwashing the membrane filtration unit by the use of another peristaltic pump. Membrane backwashing steps of 1 min were regularly performed every 10 min of membrane filtration. It is noteworthy to highlight the automated operation of the pilot-scale MBR system: the operation of all peristaltic pumps, the dissolved oxygen meter, the level sensors and the pressure transmitter were controlled by several Programmable Logic Controllers (PLCs), which are widely used for automation of typical industrial processes by other researchers as well (Melidis et al. 2016). This programming allowed the automatic backwashing of membranes through pneumatic electro-valves. The membrane used in the pilot-scale MBR was a microfiltration, hollow fiber membrane made of polypropylene (PP), with a pore size of 0.1 μm and a surface area of 0.75 m2 (ZENA-P50 model, supplied by ZENA Membranes Inc.).
2.2 Organic Polyelectrolytes
Several commercially available cationic (FO 4000 SSH and ADIFLOC series) and one anionic (LT-25 Magnafloc) polyelectrolytes were tested in order to evaluate membrane fouling mitigation (Table 2). The FO 4000 SSH polyelectrolytes included cationic polyacrylamides of medium and high positive charge. The ADIFLOC series included middle, high and very high MW homo-polymer compounds of DADMAC (diallyl-dimethyl-ammonium chloride). In order to investigate the effect of negative charge on membrane fouling, an anionic polyelectrolyte (polyacrylamide LT-25 Magnafloc) was also tested.
In this study, reversible (or temporary) fouling was assessed in terms of filterability tests, which were conducted before and after the addition of polyelectrolytes in mixed liquor samples, obtained from the MBR aeration tank on a daily basis. It is widely accepted that during filtration the Dissolved Organic Matter (DOM) compounds, mainly comprising SMP, would be adsorbed onto and/or into the used membrane, tending to block membrane pores and forming a partly irreversible gel structure on the membrane surface (Patsios and Karabelas 2011). Therefore, irreversible (or permanent) fouling was assessed in terms of SMP concentration measurements in the present study.
2.3 Filterability Tests with the TTF Method (Time-To-Filter Method)
The Time-To-Filter (TTF) method is a well-established method, which can be used as an easy and relatively rapid way to assess sludge filterability (De la Torre et al. 2008; Rosenberger and Kraume 2002). A 90-mm Buchner funnel is used with Whatman #1, #2, or equivalent filter papers (Fig. 2). A short description of the procedure is following: after pouring 200 mL of mixed liquor on the Buchner funnel, the time required to obtain 100 mL of filtrate was recorded at the vacuum pressure of 510 mbar (TTF100). Low TTF100 times indicate high sludge filterability, whereas high TTF100 times indicate low sludge filterability. In our study, except for the TTF100, the time required to obtain 20, 40, 60 and 80 mL of filtrate was also recorded, in order to plot a full profile of the recorded times, which can contribute to a better comparison and understanding of the obtained results.
2.4 SMP Concentration Measurements
The Phenol-Sulfuric Acid method (DuBois et al. 1956) is the most widely used colorimetric method for the determination of carbohydrate concentration in aqueous solutions. The principle of this method is that carbohydrates, when dehydrated by reaction with concentrated sulfuric acid, produce furfural derivatives. Further reaction between furfural derivatives and phenol develops detectible color. A short description of the standard procedure is following: 1 mL aliquot of a carbohydrate solution was mixed with 1 mL of 5 % aqueous solution of phenol in a test tube. Subsequently, 5 mL of concentrated sulfuric acid were added rapidly to the mixture. After allowing the test tubes to stand for 10 min, they were vortexed for 30 s and placed for 20 min in a water bath at room temperature for color development. Then, light absorption at 490 nm was recorded on a spectrophotometer. Reference solutions were prepared in identical manner as aforementioned, except that the 1 mL aliquot of carbohydrate was replaced by deionized water. A Hitachi UV/Vis spectrophotometer was used for these measurements. The Phenol-Sulfuric Acid method was applied after the centrifugation of the mixed liquor samples.
3 Results and Discussion
The results of batch experiments (as described in previous section 2.1) are presented in terms of the ratios: TTFpol./TTFblank and SMPpol./SMPblank. TTFpol./TTFblank is the ratio of the TTF100 recorded after the addition of polyelectrolyte in the mixed liquor sample, to the TTF100 recorded before this addition (i.e., the blank measurement). It is evident that the lower this ratio is, the more the sludge filterability is enhanced. SMPpol./SMPblank is the ratio of the SMP concentration after the addition of the polyelectrolyte in the mixed liquor sample, to the SMP concentration before this addition (i.e., the respective blank measurement). In the same way, the lower this ratio is, the more effective the tested polyelectrolyte is in terms of SMP removal. The green horizontal line in each figure (Figs. 3, 4, 5, 6 and 7) represents the blank ratio value (i.e., TTFblank/TTFblank, or SMPblank/SMPblank), which is always equal to 1.
In the first run of batch experiments the effect of cationic polyelectrolyte FO 4000 SSH series on sludge filterability and SMP removal was examined at two different concentrations, i.e., 5 and 10 mg/L (Figs. 3 and 4). As shown in these figures, the addition of all types of cationic polyelectrolytes from the FO 4000 SSH series enhanced sludge filterability and reduced SMP concentration in the mixed liquor samples, in agreement with Huyskens et al. (2012), who attributed this phenomenon to the formation of larger flocs, due to a charge neutralization mechanism, i.e., when a cationic polyelectrolyte is added to the mixed liquor of the aeration tank and adsorbs onto the microbial flocs, which are dominantly negatively charged, then the surface charge of these flocs changes from negative to circum-neutral. These neutralized flocs may attract easier to each other, eventually producing larger flocs. During this flocculation process, the smaller size particles are presumably entrapped within the flocs’ structure. This flocculation mechanism can explain the restriction observed for both reversible and irreversible fouling. Relevant specific Oxygen Uptake Rate (SOUR) measurements, published in the literature have shown that the activity of microorganisms is not noticeably affected by the addition of polyelectrolytes (Hwang et al. 2007). Besides, polyelectrolytes with net positive charge can flocculate not only small debris, but also the SMPs. Thus, it is believed that these chemicals can also mitigate membrane fouling by reducing, apart from fine particles, also the SMP concentrations in the mixed liquor, leading to higher cake layer porosity (Yoon 2015).
However, although the addition of cationic polyelectrolytes from FO 4000 SSH series enhanced sludge filterability and decreased SMP concentration, it is interesting to notice that the same category (but different types) of this flocculant agent series might give conflicting results, when comparing the reversible with the irreversible fouling. For example, at the concentration of 5 mg/L, the FO4350SSH type enhanced significantly the sludge filterability (Fig. 3a), while its performance in terms of SMP removal was relatively poor (Fig. 3b). On the contrary, the addition of the FO4290SSH and FO 4490 SSH types effectively reduced SMP (Fig. 3b), but at the same time they did not enhance sludge filterability (Fig. 3a). These results suggest that the size (or percentage) of electrical charge in the chemical structure of applied polyelectrolyte (see Table 2) might be also of significant importance.
Nevertheless, at the concentration of 10 mg/L there was a correlation in the behavior between the TTF100 and the SMP concentration measurements for the case of FO 4350 SSH type, as after its addition in the mixed liquor sample, sludge filterability was remarkably enhanced, while a greater part of SMPs was also removed (Fig. 4).
In the second run of batch experiments, four types of the cationic polyelectrolyte ADIFLOC series were examined at the concentration of 10 mg/L as well (Fig. 5), in order to evaluate and compare their effectiveness in terms of fouling mitigation with the previous cationic polyelectrolyte FO 4350 SSH, which was the optimum polyelectrolyte type found, as indicated from the first run of batch experiments. The choice of this group of polyelectrolytes was based on the fact that the ADIFOC series include a number of novel, homo-polymer compounds of DADMAC (diallyl-dimethyl-ammonium chloride), which recently entered the global polymer market and are expected to provide promising results in the field of coagulation-flocculation process, mainly due to their higher charge density (Ariffin et al. 2012).
The obtained results showed that the addition of cationic polyelectrolyte ADIFLOC can also be used to mitigate effectively membrane fouling. However, although all ADIFLOC types significantly enhanced the sludge filterability (Fig. 5a), only the KD 453 and 550 types simultaneously removed a large part of SMP (Fig. 5b). Between them, the ADIFLOC KD 453 type exhibited the best results.
Further comparison of the cationic polyelectrolyte ADIFLOC KD 453 with the FO 4350SSH type (Fig. 6), showed that the latter can be used as the best of all cationic polyelectrolytes reagents examined, as it was exhibiting the optimum results both in the filterability tests and in the SMP removal measurements. It is possible that in comparison with polyDADMAC, polyacrylamides can favour the formation of larger microbial flocs, which can further enhance both sludge filterability and SMP removal by their specific adsorption onto activated sludge (bio)flocs. According to Ariffin et al. (2012), the utilization of polyDADMAC alone can produce small and scattered flocs, which grew into larger flocs, when polyacrylamide was added and gave better flocculation results.
In the 3rd experimental run, an anionic polyelectrolyte (LT-25 Magnafloc) was added in the mixed liquor of the aeration tank at concentrations 5, 10 and 15 mg/L, in order to investigate the effect of negative charge on sludge filterability and SMP removal, and compare it with the optimum cationic polyelectrolyte FO 4350 SSH, as indicated from the 2nd run of experiments (Fig. 7).
The addition of cationic polyelectrolyte FO 4350 SSH was found to produce better results in comparison with the anionic polyelectrolyte LT-25 Magnafloc for all the examined concentrations (5, 10 and 15 mg/L), typically used in wastewater treatment applications. In fact, the addition of anionic polyelectrolyte LT-25 Magnafloc had the opposite result, especially as far as the reversible fouling is concerned, because sludge filterability was not enhanced; on the contrary, it was found to be decreased (Fig. 7a). The observed negative influence of anionic polyelectrolyte addition (LT-25 Magnafloc) can be attributed again to the charge neutralization mechanism or, better, to the absence of it. It must also be noted that the optimum results for FO 4350 SSH are observed at 10 mg/L, suggesting that there is a limit to the amount of coagulant that should be added in the mixed liquor. The SMPs concentration decreases as the polyelectrolyte dosage increases to a certain point, but it can be increased again with the presence of excess polyelectrolyte. The zeta-potential of particles is expected to increase from (naturally) negative to positive, as the cationic polyelectrolyte dosage increases, due to the net positive charge of polyelectrolyte. Since all the commercial membranes, commonly used in MBR systems, present negative zeta-potential values, the polyelectrolyte dosage must be carefully controlled in order to prevail any charge reversal (Lee et al. 2007; Yoon 2015). It is evident from the presented results that the optimum FO 4350 SSH concentration that does not cause charge reversal of particles, thus maximizing the coagulation of fine particles and SMP, is 10 mg/L.
In most practical cases, the hydrophobic colloidal particles, existing in most wastewaters, are negatively charged, and thus, either inorganic flocculants (i.e., metal salts carrying positive charges) or cationic polyelectrolytes are preferably to be used. The flocculation could occur simply as a result of reduced surface charge of the particles (due to the reduction of zeta potential-values), and hence, of decreased electrical repulsion force between the colloidal particles (Lee et al. 2014). This mechanism allows the formation of van-der-Waals force of attraction to encourage the initial aggregation of colloidal and fine suspended materials to form micro-flocs (Lee et al. 2014). It is obvious that the addition of an anionic polyelectrolyte could not have this impact on the negatively charged microbial flocs. Selected measurements showed that zeta-potential of the activated sludge was −13 ± 2 mV, while after the addition of polyelectrolytes it was slightly increased to −10 ± 2 mV.
The superior performance of cationic polyelectrolyte FO 4350 SSH over the anionic polyelectrolyte LT-25 Magnafloc, in terms of sludge filterability enhancement, is also evidently shown in Fig. 8. In this figure, all the respective measurements made with the TTF method are presented (i.e., TTF20, TTF40, TTF60, TTF80 and TTF100). It is noteworthy to observe that all these curves, which correspond to the addition of the anionic polyelectrolyte LT-25 Magnafloc, are above the green coloured curve, which represents the blank measurements (high TTFs).
Regarding the relevant operation cost increase, cationic polyelectrolytes, such as polyacrylamides and homo-polymer compounds of DADMAC, have been used as coagulation agents in wastewater treatment processes for several years, because of their low cost and high efficiency (Ji et al. 2014). Typical cost of such polyelectrolytes ranges between 2 and 5 €/kg, while the current commercial cost of FO 4350 SSH was 3.5 €/kg. Filterability tests showed that the optimum dose of polyelectrolyte was 1 g/kg MLSS on dry basis. Following the latter, the polyelectrolyte addition should be estimated as 1 g polyelectrolyte/kg MLSS produced, to maintain constant the required polyelectrolyte concentration in the bioreactor. Since in our experiments the MLSS production was 0.3 kg MLSS/kg BOD5, the polyelectrolyte cost was estimated at 1.05€/103 kg BOD5, i.e., quite small in comparison with other chemicals running costs.
4 Conclusions
In this study, several commercially available cationic and anionic polyelectrolytes were used in order to mitigate the fouling of hollow fiber membranes in a fully automated, pilot-scale MBR system and contribute to the development of an integrated methodology, regarding the control of membrane fouling of MBR systems. The results showed that the addition of cationic polyelectrolytes FO 4000 SSH series and ADIFLOC series reduced the reversible, as well as the irreversible fouling. The comparison between the optimum cationic polyelectrolytes of each series (FO 4000 SSH and ADIFLOC) showed that the cationic polyelectrolyte FO4350SSH type exhibited better performance than the respective ADIFLOC KD 453 type, indicating that the chemical structure of polyacrylamides might promote the formation of larger microbial (bio)flocs in comparison with that of DADMAC (diallyl-dimethyl-ammonium chloride). Further comparison of this cationic polyelectrolyte type with the anionic one LT-25 Magnafloc reinforced the allegation that cationic polyelectrolytes can be preferably used, mainly due to the presence of charge neutralization mechanism.
References
Ariffin A, Razali MAA, Ahmad Z (2012) PolyDADMAC and polyacrylamide as a hybrid flocculation system in the treatment of pulp and paper mills waste water. Chem Eng J 179:107–111
De la Torre T, Lesjean B, Drews A, Kraume M (2008) Monitoring of transparent exopolymer particles (TEP) in a membrane bioreactor (MBR) and correlation with other fouling indicators. Water Sci Technol 58:1903–1909
Dizge N, Koseoglu-Imer DY, Karagunduz A, Keskinler B (2011) Effects of cationic polyelectrolyte on filterability and fouling reduction of submerged membrane bioreactor (MBR). J Membr Sci 377:175–181
DuBois M, Gilles K, Hamilton J, Rebers P, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Gkotsis PK, Banti DC, Peleka EN, Zouboulis AI, Samaras PE (2014) Fouling issues in membrane bioreactors (MBRs) for wastewater treatment: major mechanisms, prevention and control strategies. Process 2:795–866
Gkotsis PK, Zouboulis AI, Mitrakas MM, Peleka EN, Tolkou AK, Zamboulis DX (2015) Wastewater treatment in MBRs; the application of coagulants for membrane fouling control. Proc. of IWA Water & Industry Conference, June 7–10, Västerås, Sweden
Huyskens C, De Wever H, Fovet Y, Wegmann U, Diels L, Lenaerts S (2012) Screening of novel MBR fouling reducers: benchmarking with known fouling reducers and evaluation of their mechanism of action. Sep Purif Technol 95:49–57
Hwang BK, Lee WN, Park PK, Lee CH, Chang IS (2007) Effect of membrane fouling reducer on cake structure and membrane permeability in membrane bioreactor. J Membr Sci 288:149–156
Ji J, Li J, Qiu J, Li X (2014) Polyacrylamide-starch composite flocculant as a membrane fouling reducer: key factors of fouling reduction. Sep Purif Technol 131:1–7
Kim HC, Shang X, Huang JH, Dempsey BA (2014) Treating laundry wastewater: cationic polymers for removal of contaminants and decreased fouling in microfiltration. J Membr Sci 456:167–174
Lee WN, Chang IS, Hwang BK, Park PK, Lee CH, Huang X (2007) Changes in biofilm architecture with addition of membrane fouling reducer in a membrane bioreactor. Process Biochem 42:655–661
Lee CS, Robinson J, Chong MF (2014) A review on application of flocculants in wastewater treatment. Process Saf Environ Prot 92:489–508
Liang S, Song LF, Tao GH, Kekre KA, Seah H (2006) A modeling study of fouling development in membrane bioreactors for wastewater treatment. Water Environ Res 78:857–863
Ma B, Yu W, Liu H, Qu J (2014) Effect of low dosage of coagulant on the ultrafiltration membrane performance in feedwater treatment. Water Res 51:277–283
Melidis P, Ntougias S, Vasilatou V, Skouteris G, Azis K, Diamantis V, Alexandridis A (2016) Biofouling aspects and critical flux evaluation in an intermittently aerated and fed submerged membrane bioreactor. Environ Process. doi:10.1007/s40710-016-0159-x
Meng FG, Chae SR, Drews A, Kraume M, Shin HS, Yang FL (2009) Recent advances in membrane bioreactors (MBRs): membrane fouling and membrane material. Water Res 43:1489–1512
OECD (2010) OECD Guidelines for the Testing of Chemicals (Technical Report 209). Activated Sludge, Respiration Inhibition Test (Carbon and Ammonium Oxidation). Guideline 16
Patsios SI, Karabelas AJ (2011) An investigation of the long-term filtration performance of a membrane bioreactor (MBR): the role of specific organic fractions. J Membr Sci 372:102–115
Rosenberger S, Kraume M (2002) Filterability of activated sludge in membrane bioreactors. Desalination 151:195–200
Shon HK, Vigneswaran S, Ngo HH, Ben Aim R (2005) Is semi-flocculation effective as pretreatment to ultrafiltration in wastewater treatment? Water Res 39:147–153
Song KG, Kim Y, Ahn KH (2008) Effect of coagulant addition on membrane fouling and nutrient removal in a submerged membrane bioreactor. Desalination 221:467–474
Tolkou AK, Zouboulis AI (2014) Synthesis and coagulation performance of composite poly-aluminum-ferric-silicate-chloride coagulants in water and wastewater. Desalin Water Treat 53:3309–3318
Wu B, Kitade T, Chong TH, Uemura T, Fane AG (2012) Role of initially formed cake layers on limiting membrane fouling in membrane bioreactors. Bioresour Technol 118:589–593
Yoon SH (2015) Membrane bioreactor processes: principles and applications. CRC Press, USA
Yu Z, Song Z, Wen X, Huang X (2015) Using polyaluminum chloride and polyacrylamide to control membrane fouling in a cross-flow anaerobic membrane bioreractor. J Membr Sci 479:20–27
Zhang H, Gao J, Jiang T, Gao D, Zhang S, Li H, Yang F (2011) A novel approach to evaluate the permeability of cake layer during cross-flow filtration in the flocculants added membrane bioreactors. Bioresour Technol 102:11121–11131
Zhang Z, Wang Y, Leslie G, Waite T (2015) Effect of ferric and ferrous iron addition on phosphorus removal and fouling in submerged membrane bioreactors. Water Res 69:210–222
Acknowledgments
The financial support through the co-financed by the European Union and the Greek State Program EPAN-II (OPCII)/ ESPA (NSRF):‘SYNERGASIA II’, Project (FOULMEM) “New processes for fouling control in membrane bioreactors” (11SYN 8–1084), is gratefully appreciated. An initial version of the paper was presented in Greek at the 3rd Common Conference ‘Integrated Water Resources Management in the New Era’ of the Hellenic Hydrotechnical Association, the Greek Committee on Water Resources Management and the Hellenic Water Association, organized at the School of Rural and Surveying Engineering, National Technical University of Athens, Athens, Greece, 10–12 December 2015. Since then it has been largely extended and improved.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gkotsis, P., Peleka, E., Zamboulis, D. et al. Wastewater Treatment in Membrane Bioreactors: The Use of Polyelectrolytes to Control Membrane Fouling. Environ. Process. 4 (Suppl 1), 9–21 (2017). https://doi.org/10.1007/s40710-016-0168-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40710-016-0168-9