Abstract
Photocatalytic degradation of two commercial dyes, Methylene Blue and Reactive Orange 4, in aqueous phase by mill scale (solid waste generated in steel plants) using advanced oxidation process was studied. The effects of various factors on the extent of degradation were investigated. These included: (1) the catalyst quantity, in the range 0.1–1.3 g/100 mL; (2) Methylene Blue concentration in the range 0.01–0.1 mM/100 mL; (3) Reactive Orange 4 concentration in the range 0.05–0.25 mM/100 mL; and (4) the concentration of hydrogen peroxide in the range 1–4/100 mL for Methylene Blue and 1–2/100 mL for Reactive Orange 4. The optimum catalyst dose in presence of hydrogen peroxide and UV illumination was found to be 0.8 and 0.3 g/100 mL for Methylene Blue and Reactive Orange 4, respectively. The decomposition rate was found to decrease with increasing dye concentration and increase with increasing hydrogen peroxide concentration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The textile dyeing and finishing processes in Bangladesh and all over the world produce significant daily amounts of wastewater, sewage sludge and solid waste materials. These effluents are coloured liquid wastes which also contain large concentrations of organic matter and are (in most cases in Bangladesh) directly discharged into the surrounding natural and irrigation channels, agricultural fields, and generally surface water bodies which finally end into rivers. The presence of dyes in surface and subsurface water can cause many water borne diseases and is aesthetically objectionable. Methods available for treatment of such wastewater include chemical treatments (Slokar and Marechal 1998; Gökçen and Özbelge 2006), electrochemical treatment (Jain et al. 2007), physical treatment (Ong et al. 2008; Tomaszewska and Morawski 2007), biological treatment and advanced oxidation processes (AOPs) (Mahmoud 2007). The advanced oxidation processes (AOPs) are considered very promising alternatives to conventional treatment. Hydroxyl radicals [HO•] generated in these AOP processes can, due to their high oxidation potential, degrade most organic compounds to carbon dioxide and water. Among the AOPs, Fenton, photo-Fenton and photo-Fenton like reactions have been successfully applied to treat dyes and textile effluents at a low cost (Feng et al. 2003; Litter et al. 2002; Torrades et al. 2004; Neamtu et al. 2003). In these reactions, H2O2 is added as the direct source of HO• and the reactions involved can be briefly described as:
Using photo generated Fe2+ the reaction process starts again (Litter et al. 2002). During the photochemical reaction of Fe(III)-oxalate complexes, superoxides hydroperoxyl radicals (O2 •-/HO2 •) are formed as the key intermediates. H2O2 thought to be formed from O2 •-/HO2 • participates in a classical Fenton reaction with Fe(II), formed by photo-reduction of Fe(III), producing HO•.
The effectiveness of mill scale as a photocatalyst has already been studied (Islam et al. 2013). In the present study, the photocatalytic decolorization of two commercial dyes, Methylene Blue (MB) and Reactive Orange 4 (RO4), has been investigated using mill scale (as source of iron oxide) by photo-Fenton process. Several key factors such as hydrogen peroxide concentrations, mill scale contents, dye concentrations were investigated.
2 Experimental
2.1 Materials
The molecular structure and other properties of MB and RO4 are shown in Table 1. Mill scale was collected from local steel industries. Solutions of dye were prepared using distilled water. All the chemicals were used without further purification.
2.2 Procedure
Varying amounts of mill scale were added to 100 mL of aqueous solution containing MB or RO4 and H2O2 or oxalic acid. The experimental conditions for the photodecomposition experiments are shown in Table 2. Before starting photo reaction, the solution was magnetically stirred in the dark for half an hour to establish absorption-desorption equilibrium. Photodegradation was carried out under two 8 W UV lamps (Fig. 1). The samples for analysis were withdrawn systematically and stored in the dark for subsequent analysis. The absorbance spectrum was determined with a UV–vis spectrophotometer (Optizen 3220UV, Korea). Degradation of MB and RO4 was monitored by recording the absorbance at λmax = 664 and at λmax = 490 nm, respectively, as a function of illumination time. The percent decolorization and rate constant values were calculated as follows (Balmer and Sulzberger 1999; Kansal et al. 2009):
where C0 is the initial concentration of dye, and Ct is the concentration of dye at reaction time t (min).
3 Results and Discussion
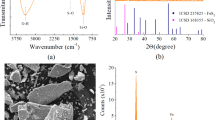
The crystalline phases of mill scale, as determined by X-ray powder diffraction, were wustite, magnetite, and hematite (Islam et al. 2013). The millscale was also observed under scanning electron microscope (Fig. 2).
3.1 UV–Vis Spectra of Dyes
Results of UV–Vis analysis on the decolorization of MB and RO4 with respect to the change in the intensity of absorption peak in UV illumination are shown in Figs. 3 and 4, respectively. The prominent peak for MB was observed at λmax, i.e., 664 nm and that for RO4 at 490 nm. The intensity of the peaks decreased gradually and finally disappeared indicating that both dyes had been degraded.
3.2 Decolorization of Dyes
3.2.1 MB
Degradation of MB in presence of 1.0 mM oxalic acid only was 11 % (curve a, Fig. 5) while the degradation under UV irradiation was only 12.5 % (curve b, Fig. 5). The extent of degradation increased to 20 % in presence of H2O2 only (curve c, Fig. 5) and to 38 % under UV and mill scale (curve d, Fig. 5). The degradation of MB was 61.9 % under UV and oxalic acid (curve e, Fig. 5) and 67 % under UV and H2O2 (curve f, Fig. 5). The extent of degradation increased to 88 % under UV, mill scale and oxalic acid (curve g, Fig. 5). Finally, when H2O2, mill scale and UV irradiation were used in combination, the removal of MB significantly increased to 98 % (curve h, Fig. 5). Thus, it was observed that either the combination of UV light, mill scale and H2O2 or the combination of UV light, mill scale and oxalic acid are required for complete degradation of MB.
3.2.2 RO4
The results of investigation on the decolorization of RO4 under different experimental conditions are shown in Fig. 6. It can be seen that only about 5.44 % decolorization was achieved when RO4 was irradiated under UV light alone (curve a, Fig. 6). The extent of decolorization was found to be 28 % when H2O2 and mill scale catalysts (curve b, Fig. 6) were used. But it was 82.89 and 91.65 %, respectively, when UV + oxalic acid (curve d, Fig. 6) and UV + H2O2 (curve e, Fig. 6) were used. Almost 95.89 % decolorization was observed when experiments were carried out for 60 min under UV light in the presence of mill scale and H2O2 (curve f, Fig. 6), whereas 66.83 % decolorization was achieved in the same duration (curve c, Fig. 6) with OA, UV and mill scale. It is clearly seen that mill scale is a better catalyst in presence of (UV + H2O2) for the degradation of RO4 dye also. The same trend was observed in earlier findings with azo reactive dyes (Lizama et al. 2002; Akyol et al. 2004). This was explained as: mill scale in presence of (UV + H2O2) has greater quantum efficiency than mill scale in presence of (UV + OA).
3.3 Effect of Mill Scale Content on the Degradation of Dyes in Presence of H2O2 and UV
Figure 7 shows the dependence of photo degradation of MB and RO4 on the quantity of mill scale under UV light in presence of 2 mL H2O2/100 mL aqueous solution. Mill scale content was taken as 0.1, 0.3, 0.5, 0.8, 1.0 and 1.3 g for 100 mL of solution. Almost 90 % degradation of MB and RO4 occurred in all cases within 30 min. The corresponding rate constant values of these experiments show that the decomposition rate of MB and RO4 increases with increasing mill scale content, reaches a maximum and then decreases again. When the Fe-ion concentration is too low, e.g., 0.1 g/100 mL, the decolorization rate is slow for both dyes. This is because, iron, in its Fe2+ and Fe3+ forms, acts as photo-catalyst (Xu et al. 2003) and the amount of ferrous or ferric ion influences the Fenton and photo-Fenton processes. An increase in mill scale content increases the decolorization rate. This is because an increase in mill scale content increases the amount of Fe-ion in the solution. Fe ion undergoes a reaction with hydroxyl ions to form Fe(OH)2+ which in turn produces more HO• radicals (Eqs. 1, 2 and 3). This HO• radical increases the rate of decomposition. On the other hand, when the iron powder concentration is too high, e.g., 1.3 g/100 mL for MB and 0.5 g/100 mL for RO4, the suspension gets covered with excessive oxide, and this prevents the penetration of UV light. This in turn decreases the formation of HO• radicals, lowering the photodecomposition ability. At higher dose of catalyst (1.3 g/100 mL), the suspension is almost covered with iron particles, so a higher dose is not useful both in view of aggregation as well as reduced irradiation field due to light scattering (Kansal et al. 2009). Optimum mill scale content is 0.8 and 0.3 g/100 mL for MB and RO4, respectively.
3.4 Effect of H2O2 Variation on the Decomposition of Dyes
MB and RO4 degradation was carried out with 1 and 2 mL H2O2. Figure 8 displays the effect of the hydrogen peroxide dosage for decolorizing MB and RO4. It can be seen that the higher the H2O2 concentration, the faster the colour removal. Using concentrations of H2O2 of 1 mL, colour degradation rate was 0.020, 0.055 for 0.1 mM MB and 0.1 mM RO4, respectively. The rate increased to 0.025 and 0.10 at H2O2 of 2 mL. The H2O2 concentration is directly related to the number of hydroxyl radicals generated and the rate increased due to the effect of the additionally produced hydroxyl radicals (Herney-Ramirez et al. 2010).
3.5 Degradation of Different Concentrations of Dyes in the Presence of H2O2 and Mill Scale
After optimizing the catalyst dose (0.8 g/100 mL for MB and 0.3 g/100 mL for RO4 with 2 mL H2O2/100 mL), the photocatalytic degradation of both dyes was carried out by varying the initial concentrations of the dye, from 0.01 to 0.1 mM for MB and 0.05–0.25 mM for RO4. All the rate constant (k) values are plotted in Fig. 9. It can, thus, be seen that the decomposition rate decreased with an increase in dye concentration. This can be explained as: the higher the initial concentration, the higher the adsorbed organic substances on the surface of the catalyst. There are, therefore, only few active sites for adsorption of OH−, and thus, the generation of OH• is reduced. As the concentration of MB or RO4 increases at constant intensity of UV illumination, the path length of photons entering the solution decreases, so only fewer photons reach the catalyst surface. As a result, the production of holes or hydroxyl radicals that can attack the pollutants are limited. Therefore, the relative number of HO• attaching to the compound decreases and the photodegradation efficiency decreases (Modirshahla et al. 2007; Mohamed et al. 2012; Hayat et al. 2010; Martyanov et al. 1997).
4 Conclusions
From the experiments conducted in this study, it can be concluded that: (1) The combination of UV+ mill scale + H2O2 or combinations of UV + mill scale + OA give better results for the degradation of dyes. (2) The optimum mill scale content in presence of UV and H2O2 was found to be 0.8 and 0.3 g/100 mL for MB and RO4, respectively. (3) For both dyes, the decomposition rate increased with increasing H2O2 and decreased with increasing dye concentration.
References
Akyol A, Yatmaz HC, Bayramoglu M (2004) Photocatalytic decolorization of Remazol red RR in aqueous ZnO suspensions. Appl Catal B Environ 54(1):19–24
Balmer ME, Sulzberger B (1999) Attrazine degradation in irradiated iron/oxalate systems: effect of pH and oxalate. Environ Sci Technol 33:2418–2424
Feng J, Hu X, Yue PL, Zhu HY, Lu GQ (2003) Discoloration and mineralization of reactive red HE-3B by heterogeneous photo-Fenton reaction. Water Resour 37:3776–3784
Gökçen F, Özbelge TA (2006) Pre-ozonation of aqueous azo dye (Acid Red-151) followed by activated sludge process. J Chem Eng 123:109–115
Hayat K, Gondal MA, Khaled MM, Ahmed S (2010) Kinetic study of laser-induced photocatalytic degradation of dye (alizarin yellow) from wastewater using nanostructured ZnO. J Environ Sci Health 45(1):1413–1420
Herney-Ramirez J, Vicente MA, Madeira LM (2010) Heterogeneous photo-Fenton oxidation with pillared clay-based catalysts for wastewater treatment: A review. Appl Catal B Environ 98:10–26
Islam SMKN, Kurny ASW, Gulshan F (2013) Photocatalytic efficiency of mill scale for the degradation of textile dye by photo Fenton and photo-ferrioxalate system under UV and sunlight. Environ Ecol Res 1(3):129–134
Jain R, Varshney S, Sikarwar S (2007) Electrochemical techniques for the removal of Reactofix Golden Yellow 3 RFN from industrial wastes. J Colloid Interface Sci 313:248–253
Kansal SK, Kaur N, Singh S (2009) Photocatalytic degradation of two commercial reactive dyes in aqueous phase using nanophotocatalysts. Nanoscale Res Lett 4(7):709–716
Litter M, Emilio C, Jardim W, Mansilla H (2002) EDTA destruction using the solar ferrioxalate advanced oxidation technology (AOT) comparison with solar photo-Fenton treatment. J Photochem Photobiol A Chem 151:121–127
Lizama C, Freer J, Baeza J, Mansilla HD (2002) Optimized photodegradation of reactive blue 19 on TiO2 and ZnO suspensions. Catal Today 76(2–4):235–246
Mahmoud BY (2007) Photocatalytic degradation of methyl orange by gold silver nano-core/shell nano-shell. J Phy Chem Solids 68:413–419
Martyanov IN, Savinov EN, Parmon VN (1997) A comparative study of efficiency of photooxidation of organic contaminants in water solutions in various photochemical and photocatalytic systems. 1. Phenol photoxidation promoted by hydrogen peroxide in a flow reactor. J Photochem Photobiol A Chem 107(1–3):227–231
Modirshahla N, Behnajady MA, Ghanbary F (2007) Decolorization and mineralization of C.I. Acid Yellow 23 by Fenton and photo-Fenton processes. Dye Pigment 73:305–310
Mohamed RM, Khalid IAM, Baeissa ES, Al-Rayyani MA (2012) Photocatalytic degradation of Methylene Blue by Fe/ZnO/SiO2 Nanoparticles under visible light. J Nanotechnol 329082:5
Neamtu M, Yediler A, Siminiceanu I, Kettrup A (2003) Oxidation of commercial reactive azo dye aqueous solutions by the photo-Fenton and Fenton like processes. J Photochem Photobiol A 161:87–93
Ong SA, Toorisaka E, Hirata M, Hano T (2008) Granular activated carbon-bio film configured sequencing batch reactor treatment of C.I. Acid Orange 7. Dye Pigment 76:142–146
Slokar YM, Marechal AMLE (1998) Methods of decoloration of textile waste waters. Dye Pigment 37:335–339
Tomaszewska SM, Morawski AW (2007) Photodegradation of azo dye Acid Red 18 in a quartz labyrinth flow reactor with immobilized TiO2 bed. Dye Pigment 75:60–66
Torrades F, Garcia-Montano J, Garcia-Hortal JA, Domenech X, Peral J (2004) Decolorization and mineralization of commercial reactive dyes under solar light assisted photo-Fenton conditions. Sol Energy 77:573–581
Xu X, Zhao H, Wang YDW (2003) 2-Chlorophenol oxidation kinetic by photo-assisted Fenton process. J Environ Sci 15:475–81
Acknowledgments
The authors are grateful to Ministry of Science and Technology, Government of the Peoples Republic of Bangladesh for providing fund and Bangladesh University of Engineering and Technology for providing facilities to carry out this research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Islam, S.M.N., Kurny, A.S.W. & Gulshan, F. Degradation of Commercial Dyes Using Mill Scale by photo-Fenton. Environ. Process. 2, 215–224 (2015). https://doi.org/10.1007/s40710-014-0055-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40710-014-0055-1