Abstract
Contamination of heavy metals poses a significant threat to our different ecosystems and their biodegradation as organic contaminants is not possible as heavy metal ions cannot be mineralized to non-toxic forms. Instead, they can be biomobilized into other compounds. Cadmium is supposed to be the most toxic heavy metal occurring naturally in the environment. Fungal biosorption had become an answer to remove toxic metal ions from wastewaters or soils as they can be grown easily and inexpensively. Aspergillus niger is a biomass waste of citric acid production industry and can be used as a biosorbent for this purpose. The cadmium effect on A. niger ITCC 546 and ITCC 6117 has been investigated. The maximum biomass was observed at 475 mM containing medium compared to 500 and 525 mM. The higher concentration of Cd showed more inhibition of fungal cells. The protein synthesis was increased at 475 mM of Cd ions than in control samples. The total RNA expressed from treated fungal cells at 475 mM of Cd ions was in greater quantity than the RNA isolated at 500 mM, while the DNA from mycelia grown at 500 mM were more sheared and degraded than the DNA of 475 mM. The biosorption capability of A. niger ITCC 546 has been found to be much more than that of ITCC 6117.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The global requirement for metals has resulted in greater mining and metal-processing; the ever-increasing use of chemical fertilisers has lead to soil enrichment with heavy metals, as fertilizers contain high concentrations of heavy metals, e.g., high cadmium concentrations in phosphorus fertilisers (Bagot et al. 2006). Among mercury, cadmium and lead, Cd merits special attention due to its potential health hazard to all forms of life. Cadmium (Cd2+) is a heavy metal of great concern in the environment as it is considered a highly toxic element to nearly all organisms even at low concentrations (Cardoso et al. 2002; Guelfi et al. 2003). Kirkham (2006) reported that a particular Cd concentration may not be toxic to plants; however, the same level may become toxic to the other organisms. Cadmium toxicity particularly affects humans, because of their longevity and the accumulation of Cd in their organs by eating Cd-contaminated food (Tudoreanu and Phillips 2004).
At greater concentrations, heavy metals affect both the favourable rhizospheric microflora and crops (Ahmad et al. 2014a). Due to their extreme toxicity towards plants, aquatic life and humans, their removal from soils or wastewaters is essential. Cd is known to inhibit the growth processes and rapidly stimulate the biosynthesis of different cysteine-rich binding peptides and metallothioneins (Höfgen et al. 2001; Mejare and Blow 2001; Pal and Das 2005). In its presence, proteins such as superoxide dismutase (SOD), catalase (CAT) and glutathione reductase (GR) are known to behave differently in various species (Vitória et al. 2001; Guelfi et al. 2003). Purchase et al. (1997) has reported the occurrence of high intracellular carbohydrates and larger cell inclusions under elevated resistance of cadmium in rhizobial strains. Besides PGP activity, several reports have shown the response of rhizobium against heavy metals on the metabolic properties and stress tolerance of leguminous plants (Ahmad et al. 2014a; Abbas and Kamel 2004).
Bimolecular studies in filamentous fungi have been less extensively undertaken than in plants, bacteria and yeasts. Therefore, the aim of this work was to investigate the cadmium effect on biomass, protein content, DNA and RNA, and then biosorptive ability of the Aspergillus niger.
2 Materials and Methods
2.1 Microorganism and Growth Conditions
The strains of Aspergillus niger ITCC 546 and ITCC 6117 were obtained from Centre for Transgenic Plant Development (CTPD), Department of Biotechnology, Jamia Hamdard, New Delhi. The cultures were maintained as working cultures on potato dextrose agar (PDA) plates under sterile condition, and transferred to incubator already set at 28 ± 2 °C with a photoperiod of 10 h per day for proper growth. After 3–4 days of incubation, the plates were stored at 4 °C and used as mother culture in subsequent experiments.
2.2 Determination of Minimum Inhibitory Concentration (MIC)
The fungal strains were assessed for their resistance to Cd2+ by the agar plate dilution method. Freshly prepared PDA plates, amended with Cd(II) as CdCl2 at concentrations ranging from 400 to 600 mM, were inoculated with overnight grown cultures. Plates were incubated at 28 ± 2 °C for 4 days. The lowest concentration of Cd(II) inhibiting the growth on agar plates was described as minimum inhibitory concentration (MIC). A plate with no added Cd was treated as a control plate.
2.3 Fungal Cultivation, Treatments and Sampling
The cultivation of A. niger strains were performed using 250 mL shaking flasks containing 50 mL of potato dextrose broth (PDB) medium with sub-optimal concentrations of CdCl2 (475 and 500 mM). The broth with no supplemented cadmium was taken as control medium for fungal growth. The initial pH value of the medium was adjusted to 6.2 and then a loopful of fungal spores was inoculated. The cultures were aerated in a rotary shaker at 150 rpm and 28 °C for 4 days. After certain interval, cells were harvested by centrifugation and the fresh and dried biomass was analyzed by weight method.
2.4 Protein Isolation and its Measurement
Soluble protein content was estimated following the method of Barford (1976). One gram of fresh fungal tissue was ground in liquid nitrogen to form powder and then transferred to centrifuge tube containing 5 mL extraction buffer (50 mM Tris–HCl, pH 7.5, 0.07 % β-mercaptoethanol, 1 % triton X-100, 1 mM Phenylmethanesulfonyl fluoride (PMSF), 2 mM Ethylenediaminetetra acetic acid (EDTA)) vortexed for 5 min and centrifuged at 10,000 rpm for 10 min. 1.0 mL of supernatant was taken in microfuge tube to which equal amount of chilled 10 % trichloro acetic acid (TCA) was added. It was centrifuged at 5000 rpm for 5 min. The resulting supernatant was discarded and the pellet left was washed with acetone. It was then dissolved in 1.0 mL of 0.1 N NaOH. To a 0.1 mL aliquot, 5 mL of Bradford’s reagent was added and volume was made up to 6 mL and finally vortexed. After the maximum colour development, absorbance was recorded at 595 nm on UV–vis spectrophotometer (Model DU 640, Beckman, USA). The protein concentration was calculated by using BSA as the standard. The protein content was expressed as mg g−1 fw.
2.5 RNA Extraction and its Quantification
Total RNA of both fungal strains was extracted using RNeasy plant RNA isolation kit (Qiagen) as per the manufacturer’s instructions. The concentration of RNA was determined by measuring the absorbance at 260 nm (A260) in a spectrophotometer (Beckman-USA, DU 640B). The RNA yield was calculated using the following equation:
2.6 DNA Isolation and Quantification
Genomic DNA from mycelia was isolated following the protocol of Cappuccino and Sherman (1996) with slight modifications by Ahmad et al. (2014b). Fungal cultures were grown for 4 days at 28 ± 2 °C containing 100 mL of potato dextrose broth (PDB) with amended Cd salts. Mycelia were filtered and washed three times in phosphate buffer saline and 0.5 g of fungal biomass was finely powdered using a mortar and pestle, and mixed with lysis buffer. The mixture was, thereafter, transferred to the tube containing 50 μL β-mercaptoethanol. The mixture was incubated at 65 °C for 1 h with intermittent vortexing and cooled to room temperature. An equal volume of Phenol:chloroform:isoamyl alcohol (PCIA; 25:24:1) was added to the tube and vortexed gently for 15 min. The tube was then centrifuged (Beckman, Avanti™ 30) at 20,000 g for 20 min at 4 °C. The upper aqueous phase was collected in a fresh tube and 2/3rd volume of chilled iso-propanol was added to precipitate the DNA. The mixture was then vortexed smoothly and kept at −20 °C for 4–5 h followed by centrifugation at 6000 g to recover the DNA pellet. Finally, the pellet was washed with ethanol, air dried and dissolved in 100 μL Tris-EDTA (TE) buffer (pH 8.0). The quantification of genomic DNA was performed by measuring the absorbance at 260 nm (A260) in a spectrophotometer (Beckman-USA, DU 640B). The DNA yield was calculated using the following equation:
2.7 Biosorption Experiment
The cultivation of A. niger was performed using 250 mL shaking flasks containing 50 mL medium with different concentrations of CdCl2 (475 and 500 mM). The initial pH value of the medium was adjusted to 6.2 and then inoculated. The cultures were aerated in a rotary shaker at 150 rpm and incubated at 28 ± 2 °C for 40 h. At log phase, tissue (1 g) was harvested by centrifugation. The pellet was collected and digested in nitric acid solution (98 %) for 48 h. The amount of cadmium accumulated in the cells was determined by atomic absorption spectrophotometer (ZEEnit 95, Analytik Jena, Germany).
3 Results and Discussion
3.1 Minimum Inhibitory Concentration (MIC) of A. niger
Heavy metals tolerance was determined as the minimum inhibitory concentration (MIC) against the A. niger fungal strains. In this study, cadmium was used at concentrations ranging between 400 and 600 mM to evaluate the MIC of both strains. The MIC of A. niger ITCC 546 and ITCC 6117 towards cadmium was found to be 525 mM. Therefore, the concentration below the subMIC, was considered for further study.
It was generally found that the difference in the change of MIC values depends on the type of isolates of a particular species. However, in our case, the MIC was similar for both strains in comparison to Zafar et al. (2007), who reported a difference in the levels of metal tolerance by the two isolates of the Aspergillus genus. The similarity of MIC values might be due to the presence of the same type of resistance mechanisms that are exhibited by both fungal strains. It is further believed that the toxic metal tolerance primarily depends upon the association of ionic species of metals with the cell surface or the extra cellular polysaccharides, proteins and chitins present on the cell surface of the microorganisms (Volesky 1990).
3.2 Effect of Cd2+ Concentration on the Growth of A. niger
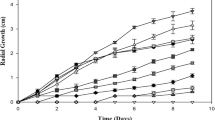
The inhibitory effect of supplemented Cd(II) on PDB on the growth of fungal biomass was evaluated by measuring the dry weight of A. niger mycelium. The growth of A. niger in the presence of different concentrations of Cd2+ is shown in Fig. 1. The maximum biomass was observed in the lowest CdCl2 containing medium while 500 mM containing medium have the least fungal growth.
Effect of Cd ions on the growth of fungal biomass of Aspergillus niger. Solid and dotted lines represents ITCC 546 and ITCC 6117, respectively. Cd ion concentration in PDB were ( ) 0, (
) 0, ( ) 475, (
) 475, ( ) 500 and (
) 500 and ( ) 525 mM. The results represent the mean of two strains in triplicate at each time point. Standard deviations were less than 5 %
) 525 mM. The results represent the mean of two strains in triplicate at each time point. Standard deviations were less than 5 %
Ezzouhri et al. (2009) reported that lag-phase may be prolonged due to the increased levels of concentration and toxicity of a heavy metal. Higher Cd ion concentration suppressed the fungal biomass growth by increasing the length of lag phase when compared with untreated samples. A reduction in the growth rate of fungi is a typical response towards toxic heavy metals (Gadd 1993). This usually happens because the concentration of Cd goes on increasing in the medium, the level of toxicity also increases and thus the fungi takes longer time to get acclimatized in the medium. Appanna et al. (1996) and Hassen et al. (1998) investigated the effect of multiple metal tolerances and showed that inhibition depended upon the metal and its concentrations in the medium.
3.3 Protein Concentration Under Cd Stress
To study the effect of Cd ions on the protein synthesis, the medium was augmented with Cd at variable concentrations viz. 0, 475, 500 and 525 mM. The protein synthesis, in both strains, had augmented at 475 mM of Cd ions than the controlled samples. However, as Cd concentration increased beyond 475 mM, it had a noticeable inhibitory effect on protein biosynthesis and beyond 500 mM, very less amount of total protein was obtained. A higher Cd(II) concentration resulted in a more serious inhibitory effect (Fig. 2).
Effect of Cd ions on total protein concentration of Aspergillus niger. Solid and dotted lines represents ITCC 546 and ITCC 6117, respectively. Cd ion concentration in PDB were ( ) 475, (
) 475, ( ) 0, (
) 0, ( ) 500 and (
) 500 and ( ) 525 mM. The results represent the mean of two strains in triplicate at each time point. Standard deviations were less than 5 %
) 525 mM. The results represent the mean of two strains in triplicate at each time point. Standard deviations were less than 5 %
Our results were in complete accordance with Weishuang et al. (2009) who also had similar results with Cd containing mediums, thereby suggesting that soluble protein has a positive role in heavy metal tolerance. In the presence of optimal metal ions, total protein content and dry cell weights only slightly fluctuated, which suggests that Cd2+ did not perturbed cell biomass and protein synthesis of the fungal cells. However, with the increase in Cd concentration, the total protein and biomass decreases sharply. Hassen et al. (1998) also revealed that metal ions exerts variable effects on protein biosynthesis, but varies from species to species. This may happen due to the chelation of heavy metals in the cytosol by peptides in heavy metal detoxification and tolerance. Depending upon type of heavy metal and its concentration, molds synthesize metallothioneins (MT) and phytochelatins (PC) for the chelation of heavy metals in the cytosol (Pal and Das 2005; Courbot et al. 2004). Although, correlation between the protein synthesis and Cd(II) has not yet been fully understood, however, the results obtained in the present study showed the increase of protein levels may be responsible by the synthesis of proteins involved in the detoxification mechanism.
3.4 Cadmium Effect on Fungal Nucleic Acids
The effect of Cd ions at different concentrations on RNA and DNA of fungal cells in the medium is depicted in Fig. 3. In both strains of A. niger, as compared to the RNA isolated from controlled cells, the total RNA isolated from treated fungal cells at 475 mM of Cd ions was in greater quantity (209 μg mL−1) while the RNA isolated from the biomass produced at 500 mM were in reduced quantity (87 μg mL−1). The amount of total RNA in the microbial cells increased as the Cd2+ concentration had increased upto 475 mM in the medium. However, as the concentration increased beyond limit, the total amount decreased markedly. In case of DNA isolated from untreated fungal cells, the total DNA quantity remained unchanged in both cases, i.e., at 0 and 475 mM, while the DNA isolated from mycelia grown at 500 mM were in lower quantity (62 μg mL−1) as well as more sheared and degraded in nature (Table 1).
The higher expression of RNA is in accordance with the increase in total protein content which has led to the synthesis of MTs and PCs. Tsekova et al. (2000) had shown a decrease in production of DNA with the reduction in growth of fungal biomass of A. niger and an enhanced production of lipids and polysaccharides. This might have occurred in order to deal with the Cd ion effect resulting in higher protein synthesis. Unlike the RNA, DNA shift can be measured as metal passes inside the nucleus of cells. In fact, genotoxicity was correlated with heavy metal load on different microorganisms.
3.5 Biosorption of Cadmium Ions by A. niger
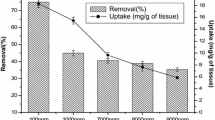
With increasing concentrations of cadmium, the biosorption potential of A. niger has increased upto 475 mM. The maximum biosorption has reached to 6.36 mg g−1 of Cd by the biomass of A. niger ITCC 546 while ITCC 6117 has adsorbed 4.94 mg g−1 of Cd ions (Fig. 4), which suggests that there is a modest correspondence between the metal tolerance and its biosorption. Cadmium biosorption by both Aspergillus niger strains used in the current study is higher compared to reports by few other researchers. Zafar et al. (2007) showed biosorption of 2.72–2.91 mg g−1 by Aspergillus species, whereas Filipovic-Kovacevic et al. (2000) reported 2.99 mg g−1 of biosorption of the same heavy metal by living biomass of Aspergillus niger after a contact time of 12 h.
Fungal strains showing higher resistance to Cd ions may, therefore, be used in metal recovery processes. A number of steps are involved in the adsorption mechanisms which deal with the transfer of cadmium from solution to the biosorbent surface (Weber 1985). Gadd (1988) had shown that the bulk transport of cadmium ions under the solution phase is usually speedy due to the continuous mixing and directional flow of ions and other constituents in the medium. The diffusion of the metal involves a passage through a hydrodynamic boundary layer around the biosorbent surface. The whole process of transferring metal ions by the active sites of the biomass needs only a few minutes to reach 90 % of the total metal adsorption (Tsezos et al. 1988).
4 Conclusion
Microorganisms face extended levels of metal pollution in their niches which may get even worse over the years. On the basis of observations, we can say that cadmium had a negative impact on growth of A. niger in a time as well as dose dependent manner. Overall, with increasing cadmium concentration, biomass and protein content decreased. However, Cd metal did not disturb the protein biosynthesis up to a certain extent and thus, there was a good correlation between microbial growth and RNA content. Fungal microorganisms when exposed to environmentally relevant heavy metal concentrations showed change in RNA as well as DNA level. Besides this, Aspergillus niger adsorbs cadmium in much more quantity, and therefore, may be used to recover cadmium from wastes.
References
Abbas SM, Kamel EA (2004) Rhizobiumm as a biological agent for preventing heavy metal stress. Asian J Plant Sci 3:416–424
Ahmad MM, Ali A, Alam P, Javed S, Abdin MZ, Khan MS (2014a) Toxicity, PGP activity, bioaccumulation of cadmium, copper and chromium (VI) in nitrogen-fixing rhizobacteria. Int J Plant Pathol 5:12–20
Ahmad MM, Ahmad M, Ali A, Hamid R, Javed S, Abdin MZ (2014b) Detection of Aspergillus flavus and Aspergillus parasiticus from aflatoxin-contaminated peanuts and their differentiation using PCR-RFLP. Ann Microbiol. doi:10.1007/s13213-014-0803-5
Appanna VD, Gazso LG, Pierre MS (1996) Multi metal tolerance in Pseudomonas fluorescens and its biotechnological significance. J Biotechnol 52:75–80
Bagot D, Lebeau T, Jezequel K (2006) Microorganisms for remediation of cadmium-contaminated soils. Environ Chem Lett 4:207–211
Barford MM (1976) A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye-binding. Anal Biochem 72:248–254
Cappuccino JG, Sherman N (1996) A laboratory manual: standard qualitative analysis of water, 4th edn. Addison-Wesely Longman, Boston, p 299
Cardoso PF, Molina SMG, Pereira GJG, Vitòria AP, Azevedo RA (2002) Response of rice inbred lines to cadmium exposure. J Plant Nutr 25:927–944
Courbot M, Diez L, Ruotolo R, Chalot M, Leroy P (2004) Cadmium-responsive thiols in the ectomycorrhizal fungus Paxillus involutus. Appl Environ Microbiol 70:7413–7417
Ezzouhri L, Castro E, Moya M, Espinola F, Lairini K (2009) Heavy metal tolerance of filamentous fungi isolated from polluted sites in Tangier, Morocco. Afr J Microbiol Res 3:35–048
Filipovic-Kovacevic Z, Sipos L, Briski F (2000) Biosorption of chromium, copper, nickel and zinc ions onto fungal pellets of Aspergillus niger 405 from aqueous solutions. Food Technol Biotechnol 38:211–216
Gadd GM (1988) Accumulation of metal by micro organisms and algae. In: Rehm H (ed) Biotechnology: a complete treatise, vol 6B. VCH, Verlagsgesellschaft, Weinheim pp. 401–430
Gadd GM (1993) Interactions of fungi with toxic metals. New Phytol 124:25–60
Guelfi A, Azevedo RA, Lea PJ, Molina SMG (2003) Growth inhibition of the filamentous fungus Aspergillus nidulans by cadmium: an antioxidant enzyme approach. J Gen Appl Microbiol 49:63–73
Hassen A, Saidi N, Cherif M, Boudabous A (1998) Effect of heavy metals on Pseudomonas aeruginosa and Bacillus thuringiensis. Bioresour Technol 65:73–82
Höfgen R, Kreft O, Wilmitzer L, Hesse H (2001) Manipulation of thiol contents in plants. Amino Acids 20:291–299
Kirkham MB (2006) Cadmium in plants on polluted soils: effects of soil factors, hyperaccumulation, and amendments. Geoderma 137:19–32
Mejare M, Blow L (2001) Metal-binding proteins and peptides in bioremediation and phytoremediation of heavy metals. Trends Biotechnol 19:67–73
Pal SK, Das TK (2005) Biochemical characterization of N-methyl N’-nitro-N-nitrosoguanidine-induced cadmium resistant mutants of Aspergillus niger. J Biosci 30:639–646
Purchase D, Miles RJ, Young TWK (1997) Cadmium uptake and nitrogen fixing ability in heavy metal resistant laboratory and field strains of Rhizobium leguminosarum biovar trifolii. FEMS Microbiol Ecol 22:85–93
Tsekova K, Dentchev D, Todorova D (2000) Effect of cadmium and copper on the production of citric acid by Aspergillus niger. Folia Microbiol 45:331–334
Tsezos M, Noh SH, Baird MHI (1988) A batch reactor mass transfer kinetic model for immobilized biomass biosorption. Biotechnol Bioeng 32:545–553
Tudoreanu L, Phillips CJC (2004) Modeling cadmium uptake and accumulation in plants. Adv Agron 84:121–157
Vitória AP, Lea PJ, Azevedo RA (2001) Antioxidant enzymes responses to cadmium in radish tissues. Phytochem 57:701–710
Volesky B (1990) Biosorption and biosorbents. In: Volesky B (ed) Biosorption of heavy metals. CRC Press, Boston
Weber WJJR (1985) Adsorption theory, concepts and models. In: Slejko FL (ed) Adsorption technology: a step-by-step approach to process evaluation and application. Marcel Dekker, New York, pp 1–35
Weishuang Z, Yingheng F, Yi H (2009) Soluble protein and acid phosphatase exuded by ectomycorrhizal fungi and seedlings in response to excessive Cu and Cd. J Environ Sci 21:1667–1672
Zafar S, Aqil F, Ahmad I (2007) Metal tolerance and biosorption potential of filamentous fungi isolated from metal contaminated agricultural. Soil Bioresour Technol 98:2557–2561
Acknowledgments
M.M.A. and A.A. is grateful for a RFSMS and SAP fellowship provided by the University Grants Commission (UGC), Govt. of India, New Delhi.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahmad, M.M., Ali, A., Khan, M.A. et al. Biomolecular Characteristics of Aspergillus niger Under Cadmium Metal Stress. Environ. Process. 2, 241–250 (2015). https://doi.org/10.1007/s40710-014-0047-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40710-014-0047-1