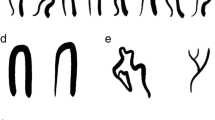Abstract
Purpose of Review
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by vascular complications, endothelial cell injury with subsequent dysfunction. Nailfold video capillaroscopy (NVC) is a non-invasive and reliable point-of-care method that can be used to directly visualize microscopic digital capillaries and monitor patients. In this review, we will describe NVC’s role in assessing SLE disease activity and prognosticating risk for developing vascular complications.
Recent Findings
In SLE, qualitative NVC changes like the presence of microhemorrhages and enlarged capillaries correlate with disease activity. These abnormalities are also associated with specific autoantibody profiles (e.g., anti-phospholipid and anti-U1-RNP antibodies). Recent studies show NVC changes may increase risks of digital ulceration, pulmonary arterial hypertension, and accelerated cardiovascular disease.
Summary
NVC may be used in personalized medicine as an adjunct in assessing SLE activity which may help prognosticate risk of serious complications, but more studies are needed to further delineate its role in specific SLE disease subtypes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic lupus erythematosus (SLE) is a complex multisystem autoimmune disease associated with many vascular complications [1, 2]. Vascular disease in SLE is characterized by endothelial cell damage and increased inflammatory mediators which further potentiate endothelial cell dysfunction [3,4,5]. SLE-related vascular complications (highlighted in Table 1) are associated with the highest risk of mortality in these patients. SLE-related vasculopathy includes microvascular abnormalities such as Raynaud’s phenomenon (RP), anti-phospholipid (aPL) antibody syndrome resulting in both micro/macrovascular thrombosis, and blood vessel inflammatory changes such as glomerular nephritis and cutaneous vasculitis.
The relationship between inflammatory and capillary changes was first made in the early nineteenth century by Maurice Raynaud in his assessment of patients who were subsequently found to have diseases compatible with systemic sclerosis (SSc)-spectrum disorders. Raynaud noted that his patients developed painful and reversible changes in blood circulation to their digits in response to cold temperatures, or in some patients, with no clear triggers [6]. These changes stemmed from microvascular alterations which have been directly visualized using different methods of capillaroscopy which have improved in their reliability and reproducibility over time [7].
Nailfold video capillaroscopy (NVC) is a simple point-of-care tool that directly visualizes the microvascular circulation in real time using the finger nailfolds [8••]. With the more prevalent use of digital capillaroscopy, NVC has become more readily available and utilized in the rheumatology clinic both in diagnosis of systemic autoimmune rheumatic diseases (SARDs) and in prospectively following patients with RP and suspected abnormal microcirculation [9]. Although its importance has been established in the diagnosis and follow-up in patients with similar connective tissue diseases such as systemic sclerosis (SSc) [10, 11], NVC’s clinical and prognostic role in stratifying patients with SLE has been less utilized. In this review, we aim to discuss the types of common NVC abnormalities present in patients with SLE and the specific subtypes of SLE associated with certain NVC abnormalities. We also aim to describe how NVC can be used as a tool to predict the development of serious complications in SLE such as pulmonary arterial hypertension (PAH), digital ulcers, and cardiovascular disease.
SLE Is Associated with Endothelial Cell Dysfunction and Damage Driven by Inflammation
One of the earliest recognized steps in the development of both micro- and macrovascular disease is endothelial cell damage with subsequent dysfunction [5]. In SLE patients, there are many components that promote the development of vascular disease early on—although they involve three main mechanisms: immune complex and oxidized lipoprotein deposition, abnormal angiogenesis, and thrombosis (reviewed in [12], Fig. 1). Upon endothelial cell damage, vascular endothelial growth factor (VEGF) is rapidly released, along with other mediators in an attempt to promote vessel healing and angiogenesis (Fig. 1). Indeed, circulating VEGF levels are elevated in patients with SLE and correlate with their disease activity—particularly in patients with lupus nephritis [13, 14] and in patients with oral ulceration [15]. VEGF levels correlate with increased circulating angiogenic T cells in patients with lupus nephritis, suggesting that these cells also play a role in endothelial dysfunction—although more studies are needed to determine their role(s) in SLE [16]. Interestingly, elevations in serum VEGF levels in SLE patients correlate with abnormal NVC patterns and with markers of endothelial cell damage in patients with SLE [9, 14, 17•].
Microvascular mechanisms involved in patients with SLE. Abbreviations: aPL (anti-phospholipid antibodies), EC (endothelial cell), eNOS (endothelial nitric oxide synthetase), ECp (endothelial cell precursor), LDL (low-density lipoprotein), NET (neutrophil extracellular trap), pDC (plasmacytoid dendritic cell), VEGF (vascular endothelial growth factor) (see text for details).
Unbalanced vascular remodeling is coupled with poor vessel healing. Endothelial cell precursors have a reduced lifespan [3, 4], and vascular mediators such as endocam [18] are elevated, further promoting vascular remodeling. Aberrant inflammatory signals in these patients in both the innate and adaptive immune responses also further potentiate the microvascular damage. Activated plasmacytoid dendritic cells releasing high levels of type I interferons directly contribute to accelerated vasculopathy [19]. Type I interferons not only promote endothelial cell apoptosis [20], but they also result in reduced levels of endothelial nitrous oxide synthase (eNOS), which is important in the maintenance of a healthy endothelial cell layer in the microvasculature [21]. Endothelial cell damage is also driven by B cell–derived autoantibodies such as anti-endothelial cell antibodies (AECA) as suggested by Van Paassen et al., as SLE patients more commonly developed AECA that induced endothelial cell apoptosis in vitro. Moreover, autoantibodies, immune complexes (ICs), and oxidized LDL particles [22] recruit and activate neutrophils to release neutrophil extracellular traps (NETs), which further promote vascular dysfunction [23, 24]. Finally, apoptotic bodies are less readily cleared [25] resulting in an abundance of nuclear antigens and antibodies that promote vascular dysfunction such as aPL antibodies. Indeed, a significant proportion of patients with SLE (approximately 30%) generate T cells recognizing β-2-glycoprotein I [26] which likely contribute to the generation of anti-β-2-glycoprotein I antibodies which in turn also promote endothelial cell damage. Hence, it is no surprise that patients with aPL antibodies with or without SLE commonly have abnormal NVC findings.
NVC Abnormalities Are Common in Patients with SLE
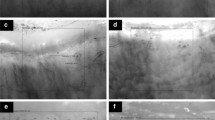
With NVC performed and recorded for over 25 years in many centers, it became evident that most patients with SLE had detectable capillaroscopic changes (73–94% of patients included), which ranged from patterns present in normal patients, albeit infrequently (e.g., tortuous or elongated capillaries, see Fig. 2) to grossly abnormal ones (see Fig. 2) [28, 29]. From these studies, it was clear that the normal “hairpin” capillary pattern was less common in many patients with active SLE disease [30, 31], although gross abnormalities such as giant capillaries or microhemorrhages were less frequently observed in SLE patients compared with patients with other diseases such as SSc or dermatomyositis [11, 32]. Further to this, some authors have described a specific “SLE pattern.” This pattern included elongated capillaries (> 300 μm in some studies [28, 33] or 500 μm in others [17, 31]) and was associated with more tortuous capillaries and a prominent subpapillary venous plexus [34] (Fig. 2b, left, middle, and right panels, respectively). Other authors confirmed this observation—namely elongated capillaries (> 300 μm) with an increased visibility of the subpapillary plexus—and estimated a 33/95% (sensitivity and specificity respectively) and a PPV and NPV of 71.43% and 80.77%, respectively [35]. Many of these changes are not unique to SLE, however, as diseases with a similar pathogenesis such as Sjogren’s syndrome develop comparable NVC changes [36]. Although this “SLE” pattern has been reproducible in different studies which assessed the frequency of this pattern in SLE patients compared with patients with other types of connective tissue diseases, there has only been a single study that has compared healthy patients with patients with SLE. In this study, NVC parameters from 123 healthy patients with RP were compared with 79 SLE patients with or without RP. SLE patients more commonly had tortuous capillaries with an increased capillary length (P = 0.0007 and P = 0.0005, respectively) [31], but many healthy patients with isolated RP also had these NVC findings as well (approximately 22%). Indeed, although tortuous changes were not different between SLE with or without RP and healthy patients, patients with SLE and more active RP more commonly had morphological changes present such as increased capillary apical and arterial diameter associated with capillary microhemorrhages (Fig. 2c) [31]. In a different study of patients with SLE and low disease activity, morphological parameters such as capillary diameter were comparable with healthy patients [37], suggesting that capillary abnormalities dynamically change in SLE and may be useful for determining overall disease activity particularly when the disease is not clinically quiescent. This notion is supported by a recent systematic review by Cutolo et al., which critically reviewed the prevalence of morphological abnormalities such as capillary enlargement and microhemorrhage in patients with SLE and how they relate to SLE-related disease activity [38••]. From these studies, it can be concluded that unlike SSc, there is no specific capillary pattern that can predict the development of SLE in patients with RP [11], although certain capillaroscopy changes are more associated with active disease.
Patients with SLE have nailfold video capillaroscopic abnormalities that are associated with increased disease activity. These images were obtained using a 200X Video Capillaroscope (DS Medica, Italy), as previously described [27•]. a Example of a normal capillaroscopy pattern from a healthy patient with Raynaud’s phenomenon. b A 19-year-old female with active SLE (arthritis, malar rash, and pleuritis). Note the presence of long (> 300 μm, left panel with solid arrows) and tortuous capillaries (middle panel) associated with a more visible subpapillary venous plexus (right panel, gray arrow). c Examples of a patient with SLE with frequent Raynaud’s phenomenon (RP) attacks and anti-phospholipid antibodies (left panel), and a patient with SLE, RP and arthritis with capillary enlargement (> 30-μm apical diameter, > 15-μm afferent diameter) and microhemorrhages who was subsequently found to be aPL antibody positive (right panel). d Patients with SLE and-U1-RNP autoantibodies with a “SSc-spectrum” capillaroscopy pattern have increased risk for complications such as digital gangrene (this patient) or pulmonary hypertension (see text). Note the presence of disorganized and decreased terminal capillary numbers (< 7 capillaries left and right panels) and enlarged (> 30-μm apical diameter, left panel) or giant (> 50-μm apical diameter, right panel) capillaries.
NVC Morphological Abnormalities Parallel the Severity of RP, Lupus Disease Activity and the Presence of aPL Antibodies
RP is a common clinical manifestation (up to 45%) in patients with SLE [39]. In particular, SLE patients with aPL more commonly develop RP than patients without these antibodies. Surprisingly, the presence of frequent RP in patients with SLE was more commonly associated with morphological abnormalities such as capillaries with an enlarged apical diameter [30] (Fig. 2c). In this study, capillary abnormalities in 29 SLE with or without RP were compared with 29 healthy controls [30]. Patients with SLE and RP suffering from more than 1 RP attack every week more commonly had enlarged capillaries compared to healthy controls (P < 0.0005) and patients with SLE without RP (P < 0.05). These findings were supported by a different study by Pavlov-Dolijanovic et al., where the capillaroscopy changes in 44 SLE patients with RP were compared with 36 SLE patients without RP. Patients with SLE and RP more commonly developed enlarged capillaries, capillary microhemorrhages, and avascular areas compared with those without [31]. In a different study, patients with critical digital ischemia/gangrene associated with RP also more commonly had dilated afferent diameter (> 15 μm) of the digital capillaries (P = 0.009) [40]. The presence of capillary microhemorrhage appears to be more associated with more active SLE disease activity [29, 31, 41], (Fig. 2c). Finally, patients with severe vascular abnormalities such as giant capillary loops or avascular areas were more frequently positive for anti-U1-RNP autoantibodies (P < 0.01), had RP (P < 0.001), or both (P < 0.001) [42]—suggesting that this subgroup of patients is more at risk for vascular complications associated with SSc.
Gross capillary morphological changes that are mild, such as the presence of longer capillaries that are more tortuous and/or have a prominent venous plexus, appear to correlate less than the presence of microhemorrhages and enlarged capillaries with SLE disease activity [43]. This observation is supported by several studies which correlated NVC morphological abnormalities with either serum VEGF levels (which parallel SLE disease activity) [17, 44] or with other biomarkers such as elevated anti-dsDNA titers [33]. Interestingly, there are conflicting reports for an association between NVC and lupus nephritis which may be attributable to how the different studies defined SLE disease activity or the specific serological profiles present in these studies [29, 33, 40, 43]. Systemic hypocomplementemia does not appear to be associated with NVC abnormalities [44]—suggesting that in SLE, NVC correlates more with vascular remodeling and inflammatory responses that culminate in endothelial cell damage rather than immune complex formation. Together, this may suggest that NVC abnormalities may be a predictor of cardiovascular complications in SLE, although more studies are needed.
One of the important mediators of endothelial cell damage in patients with SLE is the presence of aPL antibodies (Fig. 1). These pathogenic antibodies are commonly present in these patients with an estimated prevalence of 40–50% of patients with SLE [45]. One of the earliest capillaroscopy observations in patients with SLE was that patients with anti-cardiolipin (aCL) antibodies (a known aPL antibody) more frequently had abnormal capillaroscopy changes than those without them regardless of whether RP was present or not (54.5% and 20.7% (P < 0.05), respectively) [46]. Indeed, other studies subsequently confirmed this [33, 42, 47]. Further to this, patients with SSc with aPL antibodies also more frequently have more severe clinical vascular sequelae [48]—reinforcing the notion that aPL antibodies potentiate endothelial cell dysfunction, and microvascular damage which can be measured is in turn detected by NVC. It also may suggest that NVC may help predict macrovascular complications such as digital ulceration, PAH, and cardiovascular disease.
NVC Is Predictive of Macrovascular Complications in Patients with SLE
It is becoming evident that evolution of microvasculature in patients with SSc relates to visceral complications such as pulmonary arterial hypertension, lung fibrosis, or digital ischemia [10]. Although not as extensively studied as in SSc, NVC may be predictive of severe organ complications in SLE as well. Earlier studies in SLE patients identified patients with anti-U1-RNP autoantibodies at risk for developing NVC compatible with a “SSc-spectrum pattern” as defined by Cutolo et al, [27•] (i.e., the presence of giant capillary loops, frequent capillary hemorrhage, disorganization, and avascular areas (Fig. 2d)), developed visceral involvement such as esophageal dysmotility, and reduced lung diffusion capacity during the follow-up period [49]. In a subsequent longitudinal study involving serial NVC assessments, adult and juvenile SLE patients with anti-U1-RNP autoantibodies with a higher activity index had more abnormal NVC changes [43], which translated into end-organ complications such as lung fibrosis and pulmonary arterial hypertension [43].
Although relatively uncommon in patients with SLE (approximately 6% of patients) [31], recent studies have further supported the important predictive nature of the SSc pattern in patients with SLE. For example, in a recent retrospective study that compared the risk of developing PAH (as measured by right heart catheterization) in patients with SLE, patients with SLE that had a SSc pattern on NVC were at increased risk for develop PAH compared with those that did not (OR 6.393, P = 0.011) [50•]—especially when an anti-U1-RNP antibody is present [51]. Moreover, the “SS-pattern” may be predictive of other complications such as digital ulcers/gangrene (Fig. 2d) [52]. Finally, major NVC abnormalities may also be associated with a reduction in cardiac function, as recently suggested [53], and cardiovascular disease [54•]—further supporting the notion that NVC should be used prospectively to predict of vascular damage in patients with SLE.
Conclusions
In summary, NVC may be used to as an adjunct point-of-care tool in assessing patients with SLE-related disease activity. It may also be helpful in identifying patients at risk for developing end-organ complications such as pulmonary arterial hypertension, severe digital ischemia, and possibly reduced cardiac ejection fraction—although future prospective studies with both SLE patients and healthy controls are needed. Future studies linking the specific abnormal NVC patterns with the biology of different disease phenotypes may be instrumental in using NVC for personalizing clinical decisions in patients with SLE.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hermansen ML, et al. The risk of cardiovascular morbidity and cardiovascular mortality in systemic lupus erythematosus and lupus nephritis: a Danish nationwide population-based cohort study. Rheumatology (Oxford). 2017;56(5):709–15.
Tektonidou MG, et al. Subclinical atherosclerosis in systemic lupus erythematosus: comparable risk with diabetes mellitus and rheumatoid arthritis. Autoimmun Rev. 2017;16(3):308–12.
Castejon R, et al. Metabolic syndrome is associated with decreased circulating endothelial progenitor cells and increased arterial stiffness in systemic lupus erythematosus. Lupus. 2016;25(2):129–36.
Rajagopalan S, et al. Endothelial cell apoptosis in systemic lupus erythematosus: a common pathway for abnormal vascular function and thrombosis propensity. Blood. 2004;103(10):3677–83.
Taraborelli M, et al. Endothelial dysfunction in early systemic lupus erythematosus patients and controls without previous cardiovascular events. Arthritis Care Res. 2018;70(9):1277–83.
Raynaud M. De l’asphyxie locale et de la gangrène symétrique des extrémités. Paris: Leclerc; 1862.
Anders HJ, Sigl T, Schattenkirchner M. Differentiation between primary and secondary Raynaud’s phenomenon: a prospective study comparing nailfold capillaroscopy using an ophthalmoscope or stereomicroscope. Ann Rheum Dis. 2001;60(4):407–9.
Smith V, et al. An EULAR study group pilot study on reliability of simple capillaroscopic definitions to describe capillary morphology in rheumatic diseases. Rheumatology (Oxford). 2016;55(5):883–90 This is an important study that describes the reliability of evaluating capillary changes in rheumatologists with varying expertise.
Gorski S, et al. Microangiopathy in naifold videocapillaroscopy and its relations to sE- selectin, endothelin-1, and hsCRP as putative endothelium dysfunction markers among adolescents with Raynaud’s phenomenon. J Clin Med, 2019: 8(5): 567.
Avouac J, et al. Sequential nailfold videocapillaroscopy examinations have responsiveness to detect organ progression in systemic sclerosis. Semin Arthritis Rheum. 2017;47(1):86–94.
Koenig M, et al. Autoantibodies and microvascular damage are independent predictive factors for the progression of Raynaud’s phenomenon to systemic sclerosis: a twenty-year prospective study of 586 patients, with validation of proposed criteria for early systemic sclerosis. Arthritis Rheum. 2008;58(12):3902–12. This is a critical study that prospectively evaluated the development of connective tissue diseases in patients with Raynaud’s phenomenon with or without the presence of autoantibodies.
Liu Y, Kaplan MJ. Cardiovascular disease in systemic lupus erythematosus: an update. Curr Opin Rheumatol. 2018;30(5):441–8.
Heshmat NM, El-Kerdany TH. Serum levels of vascular endothelial growth factor in children and adolescents with systemic lupus erythematosus. Pediatr Allergy Immunol. 2007;18(4):346–53.
Kuryliszyn-Moskal A, et al. Vascular endothelial growth factor in systemic lupus erythematosus: relationship to disease activity, systemic organ manifestation, and nailfold capillaroscopic abnormalities. Arch Immunol Ther Exp (Warsz). 2007;55(3):179–85.
Aterido A, et al. Genome-wide pathway analysis identifies VEGF pathway association with oral ulceration in systemic lupus erythematosus. Arthritis Res Ther. 2017;19(1):138.
Zhao P, et al. Circulating angiogenic T cells are increased in lupus nephritis patients. Med Sci Monit. 2018;24:5384–90.
Moneib HA, et al. Assessment of serum vascular endothelial growth factor and nail fold capillaroscopy changes in systemic lupus erythematosus with and without cutaneous manifestations. J Dermatol. 2012;39(1):52–7 This is an interesting study which highlighted the correlation between NVC abnormalities and increased VEGF and SLE disease activity.
Icli A, et al. Endocan levels and subclinical atherosclerosis in patients with systemic lupus erythematosus. Angiology. 2016;67(8):749–55.
Clement M, et al. CD4+CXCR3+ T cells and plasmacytoid dendritic cells drive accelerated atherosclerosis associated with systemic lupus erythematosus. J Autoimmun. 2015;63:59–67.
Lee PY, et al. Type I interferon as a novel risk factor for endothelial progenitor cell depletion and endothelial dysfunction in systemic lupus erythematosus. Arthritis Rheum. 2007;56(11):3759–69.
Buie JJ, et al. IFN-alpha negatively regulates the expression of endothelial nitric oxide synthase and nitric oxide production: implications for systemic lupus erythematosus. J Immunol. 2017;199(6):1979–88.
Rhoads JP, et al. oxidized low-density lipoprotein immune complex priming of the Nlrp3 inflammasome involves TLR and FcgammaR cooperation and is dependent on CARD9. J Immunol. 2017;198(5):2105–14.
Pieterse E, et al. Neutrophil extracellular traps drive endothelial-to-mesenchymal transition. Arterioscler Thromb Vasc Biol. 2017;37(7):1371–9.
Rother N, et al. Acetylated histones in apoptotic microparticles drive the formation of neutrophil extracellular traps in active lupus nephritis. Front Immunol. 2017;8:1136.
Shao WH, Cohen PL. Disturbances of apoptotic cell clearance in systemic lupus erythematosus. Arthritis Res Ther. 2011;13(1):202.
Conti F, et al. Subclinical atherosclerosis in systemic lupus erythematosus and antiphospholipid syndrome: focus on beta2GPI-specific T cell response. Arterioscler Thromb Vasc Biol. 2014;34(3):661–8.
Cutolo M, et al. Nailfold videocapillaroscopy assessment of microvascular damage in systemic sclerosis. J Rheumatol. 2000;27(1):155–60 This is one of the seminal descriptions for capillaroscopy abnormalities in various rheumatological conditions including SLE.
Lambova S, Hermann W, Muller-Ladner U. Capillaroscopic pattern at the toes of systemic sclerosis patients: does it “tell” more than those of fingers? J Clin Rheumatol. 2011;17(6):311–4.
Shenavandeh S, Habibi S. Nailfold capillaroscopic changes in patients with systemic lupus erythematosus: correlations with disease activity, skin manifestation and nephritis. Lupus. 2017;26(9):959–66.
Caspary L, et al. Alterations of the nailfold capillary morphology associated with Raynaud phenomenon in patients with systemic lupus erythematosus. J Rheumatol. 1991;18(4):559–66.
Pavlov-Dolijanovic SD. N; Vujasinovic Stupar, NZ; Marcetic, DR; Sefik Bukilica, MN; Petrovic, RR, Is there a difference in systemic lupus erythematosus with and without Raynaud’s phenomenon? Rheumatol Int. 2013;33:859–65.
Kabasakal Y, et al. Quantitative nailfold capillaroscopy findings in a population with connective tissue disease and in normal healthy controls. Ann Rheum Dis. 1996;55(8):507–12.
Riccieri V, et al. Nailfold capillaroscopy changes in systemic lupus erythematosus: correlations with disease activity and autoantibody profile. Lupus. 2005;14(7):521–5.
Lambova SN, Muller-Ladner U. Capillaroscopic pattern in systemic lupus erythematosus and undifferentiated connective tissue disease: what we still have to learn? Rheumatol Int. 2013;33(3):689–95.
Wu PC, et al. Clinical applicability of quantitative nailfold capillaroscopy in differential diagnosis of connective tissue diseases with Raynaud’s phenomenon. J Formos Med Assoc. 2013;112(8):482–8.
Ohtsuka T. Nailfold capillary abnormalities in patients with Sjogren’s syndrome and systemic lupus erythematosus. Br J Dermatol. 1997;136(1):94–6.
Dancour MA, et al. Nailfold videocapillaroscopy in patients with systemic lupus erythematosus. Rheumatol Int. 2006;26(7):633–7.
Cutolo M, et al. Nailfold capillaroscopy in systemic lupus erythematosus: a systematic review and critical appraisal. Autoimmun Rev. 2018;17(4):344–52 This well-written and comprehensive systematic review discusses some of the important reports describing NVC changes in SLE patients.
Block JA, Sequeira W. Raynaud’s phenomenon. Lancet. 2001;357(9273):2042–8.
Ragab O, Ashmawy A, Abdo M, Mokbel A. Nailfold capilloroscopy in systemic lupus erythematosus. Egypt Rheumatol. 33:61–7.
Studer A, et al. Quantitative nailfold capillary microscopy in cutaneous and systemic lupus erythematosus and localized and systemic scleroderma. J Am Acad Dermatol. 1991;24(6 Pt 1):941–5.
Furtado RN, et al. Scleroderma-like nailfold capillaroscopic abnormalities are associated with anti-U1-RNP antibodies and Raynaud’s phenomenon in SLE patients. Lupus. 2002;11(1):35–41.
Ingegnoli F, et al. Evaluation of nailfold videocapillaroscopic abnormalities in patients with systemic lupus erythematosus. J Clin Rheumatol. 2005;11(6):295–8.
Ciolkiewicz M, Kuryliszyn-Moskal A, Klimiuk PA. Analysis of correlations between selected endothelial cell activation markers, disease activity, and nailfold capillaroscopy microvascular changes in systemic lupus erythematosus patients. Clin Rheumatol. 2010;29(2):175–80.
Love PE, Santoro SA. Antiphospholipid antibodies: anticardiolipin and the lupus anticoagulant in systemic lupus erythematosus (SLE) and in non-SLE disorders. Prevalence and clinical significance. Ann Intern Med. 1990;112(9):682–98.
Bongard O, et al. Association of anticardiolipin antibodies and abnormal nailfold capillaroscopy in patients with systemic lupus erythematosus. Lupus. 1995;4(2):142–4.
Kuryliszyn-Moskal A, et al. Clinical significance of nailfold capillaroscopy in systemic lupus erythematosus: correlation with endothelial cell activation markers and disease activity. Scand J Rheumatol. 2009;38(1):38–45.
Merashli M, Alves J, Ames PRJ. Clinical relevance of antiphospholipid antibodies in systemic sclerosis: a systematic review and meta-analysis. Semin Arthritis Rheum. 2017;46(5):615–24.
ter Borg EJ, et al. Clinical associations of antiribonucleoprotein antibodies in patients with systemic lupus erythematosus. Semin Arthritis Rheum. 1990;20(3):164–73.
Donnarumma JFS, et al. Nailfold capillaroscopy as a risk factor for pulmonary arterial hypertension in systemic lupus erythematosus patients. Adv Rheumatol. 2019;59(1):1 This recent report highlights the importance of the SSc-spectrum pattern in predicting the development of pulmonary arterial hypertension in patients with SLE.
Zhang N, et al. Pulmonary arterial hypertension in systemic lupus erythematosus based on a CSTAR-PAH study: baseline characteristics and risk factors. Int J Rheum Dis. 2019;22(5):921–8.
Rosato E, et al. Digital ulcers as an initial manifestation of systemic lupus erythematosus. Intern Med. 2011;50(7):767–9.
Chung HT, et al. Subclinical deterioration of left ventricular function in patients with juvenile-onset systemic lupus erythematosus. Lupus. 2015;24(3):263–72.
Cavazzana I, et al. Relationship between endothelial dysfunction, videocapillaroscopy and circulating CD3+CD31+CXCR4+ lymphocytes in systemic lupus erythematosus without cardiovascular risk factors. Lupus. 2019;28(2):210–6 The authors in this recent article link the presence of vascular T cells with abnormalities in patients with SLE.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Shahna Tariq declares that there are no conflicts of interest.
Jan Willem Cohen Tervaert declares that there are no conflicts of interest.
Mohammed Osman declares that there are no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Lupus
Rights and permissions
About this article
Cite this article
Tariq, S., Tervaert, J.W.C. & Osman, M. Nailfold Capillaroscopy in Systemic Lupus Erythematosus (SLE): a Point-of-Care Tool That Parallels Disease Activity and Predicts Future Complications. Curr Treat Options in Rheum 5, 336–345 (2019). https://doi.org/10.1007/s40674-019-00133-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40674-019-00133-x