Abstract
We analyze the relationship between evolutionary theory and classification of higher taxa in the work of three ichthyologists: Albert C.L.G. Günther (1830–1914), Edward Drinker Cope (1840–1897), and Theodore Gill (1837–1914). The progress of ichthyology in the early years following the Origin has received little attention from historians, and offers an opportunity to further evaluate the extent to which evolutionary theorizing influenced published views on systematic methodology. These three ichthyologists held radically different theoretical views. The apparent commensurability of claims about relationships among groups of fishes belies differences in what the relationships actually were supposed to be. As well, interpreting classification as genealogical did not lead to agreement about taxonomic methodology; instead, applying evolutionary theory raised new axes of disagreement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
When the views entertained in this volume on the origin of species, or when analogous are generally admitted, we can dimly foresee that there will be a considerable revolution in natural history. Systematists will be able to pursue their labours as at present; but they will not be incessantly haunted by the shadowy doubt whether this or that form be in essence a species. (Darwin, 1859, p. 484)
The impact of evolutionary theory on taxonomy and systematics in the years immediately following Darwin’s publication of On the Origin of Species has been difficult to assess. Mayr (1982, p. 242) claimed that “the acceptance of evolution had singularly little influence on the theory of taxonomic sequencing.” Systematists continued to seek “taxonomic sequences”—linear sequences of organisms, which Mayr considered necessary in practice (to organize specimens in collections and the order of taxa on printed pages). In Mayr’s view, systematists simple relabeled the endpoints of the scale. Rather than a scale from least to most perfect, the terms of the old scala naturae, sequences ranged from primitive to more highly evolved organisms. Following Hull (1965) and Cain (1958), Mayr (1982, 1998) further attributed the supposed lack of change to an essentialist concept of species. More recent scholarship has thoroughly undermined what Amundson (2005) called the “essentialism story” (Winsor, 2003, 2006; Wilkins, 2009), raising the need for a new assessment of the relationship between evolutionary theory and systematics prior to the Modern Synthesis.
Winsor (2021) has recently analyzed a dispute between Darwin and Huxley about the relevance of evolution to systematics. Huxley claimed that the question of evolution was not relevant to the science of classification:
But it is important to remember that the classification of animals and plants stands on its own basis and is entirely independent of physiological considerations. For the purposes of the classifier it is wholly immaterial whether, as some maintain, “species” are immutable, and have taken their origin independently of one another, directly from the hand of the Creator; or whether, as others think, they are indefinitely modifiable, and have all resulted from the changes induced by external influences upon some common stock. (Huxley, 1871, p. 369); originally published in 1857
In contrast, Darwin proposed that the natural classification ought to be explicitly understood in genealogical terms:
In regard to Classification, & all the endless disputes about the “Natural System which no two authors define in same way, I believe it ought, according to my heteredox notions, to be simply genealogical.—But as we have no written pedigrees, you will, perhaps, say, that this will not help much; but I think it ultimately will, whenever heteredoxy becomes orthodoxy, for it will clear away an immense amount of rubbish about the value of characters &—will make the difference between analogy & homology, clear—The time will come I believe, though I shall not live to see it, when we shall have very fairly true genealogical trees of each great kingdom of nature. DarwinCorrespondenceProject (2021), Darwin to Huxley, 26 September 1857
In Winsor’s analysis, Darwin’s view is informed by his experience thinking through not only the mutability of species, but the processes of branching, divergence, and extinction (Ospovat, 1995). Winsor also argues that the Darwin-Huxley dispute reflects a disciplinary gulf between natural history—primarily concerned with species and varieties—and comparative anatomy—primarily concerned with higher taxa.Footnote 1
In this paper, we analyze the relationship between evolutionary theory and classification of higher taxa in the work of three ichthyologists: Albert C.L.G. Günther,Footnote 2 (1830–1914), Edward Drinker Cope (1840–1897), and Theodore Gill (1837–1914). The progress of ichthyology in the early years following the Origin has received little attention from historians, and offers an opportunity to further evaluate the extent to which evolutionary theorizing influenced published views on systematic methodology. As will be seen, these three ichthyologists held radically different theoretical views. The apparent commensurability of claims about relationships among groups of fishes belies differences in what the relationships actually were supposed to be. As well, interpreting classification as genealogical did not lead to agreement about taxonomic methodology; instead, applying evolutionary theory raised new axes of disagreement.
2 Albert Günther
2.1 Origin and development
Albert Carl Ludwig Gotthilf Günther’s entry into the field of zoology followed early training for the ministry, where he had obtained both an M.A. and a Ph.D., as well as taking holy orders in 1852 (L. et al., 1915; Smith et al., 1927). His interest in natural history subsequently led him to the pursuit of a degree in medicine, and his studies included time at the University of Berlin, where he worked under Müller (1846b), who had earlier published an influential classification of fishes based largely on internal anatomy.
Günther visited the British Museum (Natural History)Footnote 3 in 1856, where he met Richard Owen and John Edward Gray. In 1857, they invited him to prepare a catalogue of the reptiles and amphibians at the Museum, and by 1862 he had been appointed to a full-time position on the Museum staff. Günther became Assistant Keeper of the Zoological Department of the Museum in 1872, a position for which he solicited a testimonial from Charles Darwin (Burkhardt & Smith, 1985, p. 361). Günther was promoted to Keeper in 1875, and held that post until his retirement in 1895.
Though based in the British Museum for the bulk of his professional career, Günther’s training in the German context was firmly on the comparative anatomy side of the split between natural history versus morphology and physiology (Winsor, 2021). However, throughout his life Günther was an avid outdoorsman and naturalist, and he developed an extensive network of collaborations with amateur naturalists; his grandson and biographer would characterize Günther as a naturalist who laid less emphasis on internal characters than would a typical comparative anatomist (Gunther, 1975). Having studied under Müller, Günther avoided the split between morphology versus physiology that characterized some areas of German biology (Rieppel, 2016).
Indeed, Günther’s interests were wide-ranging, and resulted in the publication of over 400 papers, most of which dealt with reptiles, amphibians, and fishes. His seminal contribution to ichthyology was undoubtedly the eight-volume catalogue of fishes in the British Museum, published between 1859 and 1870 (Günther, 1981). Jordan (1922, vol. 1, p. 270) considered Günther’s catalogue to be “the foundation of modern Ichthyology.” The work followed on a catalogue of snakes that J. E. Gray had commissioned. Whereas Gray had envisioned a listing of the snake species present in the collections of the British Museum, including only brief remarks about taxonomic synonyms, Günther summarized information about all species known from specimens properly described in museums. The catalogue of fishes further developed the idea of what a museum catalogue could be, by systematically covering international, state-of-the-art knowledge of fishes. As each volume was released, gaps in knowledge were revealed, so that the catalogue provided a framework for ichthyologists to direct their efforts at collection and description (Gunther, 1975).
Günther (1880) wrote a general text on ichthyology, one of the first of its kind—the laudatory report in the Quarterly Review concluded by describing it as “a book the like of which, we believe, does not elsewhere exist—and one which, if it will not interest a majority of our readers, cannot fail to please all who have any taste for Natural History and a desire to know more of it” (Günther 1882, p. 266). His shorter contributions on fishes generally dealt with new species coming into the Museum collections, and only rarely included discussions of the broader issues of the time.
Günther’s personality and interactions with his contemporaries were complicated, and available evidence indicates that he could be stubborn and difficult to deal with. His interactions with Francis Day provide an illustration of his attitudes towards those with whom he disagreed, which varied from indifference to aloof disregard (Whitehead & Talwar, 1976). Gunther (1975) presented evidence of Günther’s tendency to harshness, for example in critiques—published in his own vehicle, The Zoological Record—of sloppy taxonomic work. Conversely, many workers were impressed by Günther’s hospitality towards visitors at the Museum. Jordan (1922, vol. 1, p. 271) notes that “Günther had the reputation of being a crusty critic, sometimes needlessly severe,” but towards Jordan “he was always kind and considerate, as well as to my students.”Footnote 4 Gunther (1975) detailed Günther’s network of correspondence with naturalists at under-supported institutions around the world, with demonstrations of heart-felt gratitude for Günther’s support. Cope described Günther as an “old friend” in a letter to his wife during an 1878 visit to London (Osborn 1931, p. 253). But the two differed on many points of classification, particularly Cope’s view that Günther placed too much emphasis on external characters. As will be seen below (Subsect. 4.1), Günther’s in-print interactions with Gill were rather pointed.
2.2 Günther’s systematics
In Günther’s view, higher taxa are not absolute—not “real” in the sense of Agassiz (1859) (Winsor, 1976, 1991)—but a device to express claims about relative affinity: “I do not attach any value to the terms subfamilies, suborders, &c., except as expressions of the relative degree of affinity” (Günther 1872, p. 560). What precisely Günther meant by “affinity” is unclear. Günther never explicitly incorporated evolutionary ideas into his practice, and seems to have been skeptical of evolutionary theories. For example, in 1866, p. 600, Günther’s discussion of the fish species of Central America included the comment that despite the time that must have elapsed since there had been a connection between the oceans “the specimens examined from the opposite coasts of the isthmus were absolutely identical,” with “no indication that any of these forms had undergone modification or degenerated into a climatic or local variety.” Günther expanded on this point in 1869, p. 399, comparing the “artificial communication” between the seas to a temporary inundation of freshwater from the Baltic Sea into the Arctic: “By far the greater part of the animals became extinct; but a few survive, however, in spite of the greatly altered physical conditions, without altering their specific characters, still agreeing with the typical forms in every point, except in size, remaining smaller, leaner, almost starved.” (emphasis original). Günther thus seems to have thought that populations might undergo some degree of modification in the face of an external cause, but that this degeneration was of limited scope.
Nineteenth century taxonomists who did not adopt an evolutionary framework may have intended the term affinity to refer to a real, discoverable property of groups of organisms. The pattern of affinities might be postulated as itself a brute fact, or perhaps might be thought of as a pattern whose explanatory basis is unknown. In the mid-nineteenth century “affinity” might also be used to describe a method of taxonomic practice (e.g. Whewell (1847), following de Candolle (1813)).
In Günther’s practice, the relationships of taxa could be evaluated in terms of grade: relationships of “higher” and “lower”, reflecting degree of development (from primitive to advanced). Whereas some nineteenth century systematists explicitly interpreted grade as a relationship independent of affinity (Novick, 2016; Quinn, 2017), Günther used reasoning about grade to evaluate claims about affinity. To illustrate, we will analyze Günther’s work on the relationships of major groups of fishes.
Prior to the classifications published by Günther, the classification of Müller (1844, 1845, 1846b, a) was perhaps the most influential (Patterson, 1977, p. 584). Müller had recognized six subclasses of fishes within the broad class Pisces, placing great emphasis on the soft anatomy (see Table 2).
Günther’s (1872) classification of fishes is shown in Table 3. Table 1 lists common names of taxa with groupings by each of Müller, Günther, Cope, and Gill, for comparison.
The progression of subclasses in Günther’s classification reflects relative grade, from what Günther considered the lowest, most primitive fishes (Leptocardii, the lancelets i.e. Amphioxus) to the most advanced (Palæichthyes). The naturalness of, and relationships within, the Palæichthyes were matters of dispute (as will be seen below), and in particular the placement of Dipnoi (lungfishes) raised difficulties.
By grouping Müller’s (2)–(4), Günther emphasized the distinctness of two of the groups that he retained: subclasses Cyclostomata (lampreys and hagfishes, Müller’s (5)) and Leptocardii (6). These groups stood out (to Günther and to others) as the most primitive fishes. Indeed, in discussing the brains of fishes, Günther (1880, p. 96) characterized the Leptocardii as possessing “the most simple condition of the nervous central organ known in Vertebrates.”
The 1880 text then described the various developments from the teleosts to the ganoids, and placed the Dipnoi between the ganoids (sturgeons, gars, bowfin) and Chondropterygii (sharks and rays), in which “the brain...is more developed than that of all other fishes” (Günther, 1880, pp. 96–100). Günther found similar evidence of this sequence of development in the optic nerves (p. 104), respiratory organs (pp. 135–149) and heart (pp. 150–154). Variations in the reproductive organs of fishes were also used by Günther to confirm the sequence of development, stating that the “genital organs of Ganoids show similar diversity of structure as those of Teleosteans [bony fishes], but on the whole they approach the Batrachian [amphibian] type,” and emphasizing internal fertilization in the chondropterygians (Günther, 1880, pp. 157–169). Günther’s discussion of the skeleton admits the trend towards increasing ossification that would presumably place the ganoids and Chondropterygii between the Cyclostomata and the teleosts, but adds the qualification “in this respect.”
The concept of grade informed what characters were taken to be useful in studying affinity. Indeed, Günther linked reasoning about progressive development from fishes to amphibians to the (by then common) injunction that many different characters must be used:
Perhaps future palæontologists will be able to demonstrate as complete a series of transitional stages from the Fish to the Amphibian as that obtained by the study of the living and therefore more accessible forms of Hæmatocrya. Zoologists have had to abandon the attempt to separate the two classes by one or several absolute characters; and it is only the concurrence of either decidedly ichthyic or amphibian characters by which they refer a creature to the one or the other class. (Günther, 1866, p. 561)
No single character could be used to classify all fishes versus all amphibians, because the degree of advance of different organ systems can come apart. That is, (in Günther’s view) the Dipnoi have a higher degree of development of the pulmonary system than do Chondropterygii: the Dipnoi have lungs, and so resemble the amphibian condition. However, the Chondropterygian brain has a higher degree of development than does that of Dipnoi. Arranging taxa along multiple lines or in circles could solve the problem, and this solution was adopted by many systematists (Novick, 2016; Winsor, 2015). Günther instead suggested that the problem can be solved by considering more than two systems within each taxon, identifying characters that as a whole determine the placement of the group in question.
While Günther’s classification was based on the advancement of several traits towards the amphibian type, he did not assert that the developments observed among the groups indicated descent or genetic relationship. Bowler (1996, p. 220) has claimed that the challenge of placing the Dipnoi was a source of Günther’s skepticism about evolution. For one thing, comparing recent to ancient Dipnoi suggested that forms remain stable over extremely long periods of time:
The Dipnoi offer the most remarkable example of persistence of organization, not in Fishes only, but in Vertebrates. On a former occasion we have shown that numerous recent species of fishes have survived from the period of the geological changes which resulted in the separation of the Atlantic and Pacific by the Central American isthmus. In Ceratodus we have now found a genus which, so far as evidence goes, persisted unchanged from the Mesozoic era; and in the Sirenidae, a family the nearest ally of which lived in the Palaeozoic epochs. (Günther 1872, p. 561); emphasis in original
The persistence of forms over vast stretches of time was not necessarily a cause for skepticism about evolution. Indeed, to Huxley, the phenomenon was a mark in favor of Darwin’s theory as opposed to other modes of evolution: “It is one remarkable peculiarity of Mr. Darwin’s hypothesis that it involves no necessary progression or incessant modification, and that it is perfectly consistent with the persistence for any length of time of a given primitive stock, contemporaneously with its modifications” (Huxley 1863, p. 144). Bowler’s interpretation of Günther as an evolutionary skeptic seems to assume that the evolutionary theory in question is progressivist.
A somewhat different challenge is that the most advanced fishes appear early in the fossil record. It was striking that of all the known fishes, the most advanced (Palæichthyes) were also the most ancient:
Geologically, as a subclass, they were the predecessors of Teleosteous fishes; and it is a remarkable fact that all those modifications which show an approach of the ichthyic type to the Batrachians are found in this subclass. (Günther, 1880, p. 313)
The problem here is not to explain the fact that a primitive stock persists over a long period of time. Rather, the challenge is to explain why the higher form did not originate earlier, given that its precursor was present over the long period of time.
There is also an additional challenge: to explain how the most advanced group of fishes appeared without being preceded by a gradual, progressive series of evolution.
The defenders of the doctrine of evolution hold that a space of time like that which elapsed from the Devonian epoch to our period, is a drop in the sea, when compared with the time required for the development of life. From this point of view the Ganoid fauna of the Devonian...must have been preceded by a long and varied ichthyic series. (Günther, 1872, p. 560)
No evidence of the hypothesized ancient series had been produced. However, Günther (p. 560) left open the possibility that future discoveries could substantiate the existence of the long series. Thus Bowler’s (1996, p. 220) characterization of this passage as presenting “evidence against the possibility of evolution” is too strong. Bowler’s characterizations of Günther as “indifferent” to Darwinism (p. 32) and to evolution (p. 387) are more apt.
In Günther’s view, systematists could simply avoid the use of evolutionary theory when constructing classifications. Indeed, Günther (1975) argued that the rapid pace of discovery of specimens and the influx of biogeographic information was such that systematists like Günther considered themselves too busy for abstract theoretical disputes.
3 E.D. Cope
3.1 Origin and development
Edward Drinker Cope is a better known figure in the history of zoology than either Günther or Gill.Footnote 5 His contributions consist of over 1,300 scientific papers dealing with virtually all the practical and philosophical issues of his time. Cope was raised in a Quaker household that held to the traditional view of special creation. His father wanted Cope to take up farming, but eventually succumbed to his son’s interest in science. Cope was 19 when he published his first scientific paper (Cope, 1859). Cope did not receive traditional college training in the natural sciences, but did study anatomy for a year under Joseph Leidy at the University of Philadelphia. Unlike Günther and Gill, Cope only briefly held academic or institutional positions during his career, instead supporting his extensive travels and field work with personal funds or through temporary attachments with various geological surveys.
Through the course of his travels, Cope became acquainted with many of the leading naturalists of preceding and succeeding generations, including Louis Agassiz, Spencer Fullerton Baird, Henry Fairfield Osborn, William King Gregory, and David Starr Jordan. Cope’s relationships with fellow naturalists appear to have been amiable, though not always based on scientific agreement; a glaring exception was his famously volatile interactions with O. C. Marsh (Brinkman, 2010; Shor, 1974).Footnote 6An anecdote related by Jordan (1922, vol. 1, p. 179) illustrates Cope’s sometimes generous nature, but also a possessive attitude with his prized collections: “[Cope] invited us to his home and offered every facility in the way of books and advice, except that he naturally did not show the great collection of fish skeletons he had lately purchased from Josef Hyrtl.” Cope and Gill enjoyed a friendly relationship that can be traced back to 1859, and Gill’s (1897) tribute following Cope’s death demonstrates his admiration for Cope’s contributions despite their scientific differences. Not the least of these differences was the interpretation of evolution, and, as Cope (Osborn et al., 1931, Letter from Cope to his sister, Jan. 1863, p. 109) put it, “Gill & I argue the subject [evolution] continually.”
Cope held views on evolution that differed dramatically from those presented by Darwin, and by the end of the century he had become one of America’s leading “non-Darwinian” evolutionary theorists (Bowler, 1983). Gould (2002) argued that some orthogenecists were pluralists, holding that both orthogenetic and selection theories were necessary to explain that the course of evolution is generally progressive but with exceptions and irregularity. Whereas Charles Otis Whitman attributed evolution’s progressive character to adaptation by natural selection, William B. Scott held that an orthogenetic theory was required to explain the progression—natural selection alone would result in more random fluctuation.
Mayr (1982, p. 50) characterized these non-Darwinian theorists as positing “the existence of a non-physical (perhaps even non-material) force which drove the living world upward towards ever-greater perfection (orthogenesis).” Recent work has sought to re-evaluate the relationship between materialism and non-Darwinian theories of evolution. Ulett (2014) claims that most of the non-Darwinian evolutionary theorists of this period were materialists, and were not committed to vitalism. Shanahan (2011) compares Cope’s orthogenesis to the physical concept of inertia. Ulett points to Cope’s use of mechanical examples when presenting his theory to a general audience. Just as the form of a coat-sleeve is shaped by the movements of the wearer’s arm, the form of an organism is shaped by the organism’s movements (Cope 1896, pp. 370–371),Footnote 7 On the other hand, it is important to note that positing non-physical forces need not be inherently mysterious or unscientific. Ceccarelli (2019) emphasizes the role of the organism’s psychology in Cope’s theory, and points to eighteenth century hylozoist theories, such as that of Erasmus Darwin—which Cope himself referenced (1896, p. 505).
Cope’s views are often described as Lamarckian or Neo-Lamarckian; some clarification of the terms “Lamarckian” and “Neo-Lamarckian” is needed. Cope (1891, p. 15) used both terms to refer to views that he endorsed, and claimed that there existed two “opposite schools of evolutionists, which correspond in the main with the Neolamarckian and Neodarwinian.” He further noted: “Although particular men may not hold all the affirmations of either side, they form two distinct and consistent bodies of doctrine.”
Cope believed that some characters acquired during life could be transmitted to offspring, a claim that would become increasingly at issue in early twentieth century biology. Of course, Darwin also accepted the inheritance of acquired characters, and relied more heavily on this mechanism in later editions of the Origin. Thus, it is historically problematic to use “Lamarckian” to describe any theory that relies on the inheritance of acquired characters, while also characterizing Lamarckian theories as alternatives to Darwinian theories. The label “neo-Lamarckian” might seem to resolve the difficulty, by opposing neo-Lamarckian theories (which include the inheritance of acquired characters) to neo-Darwinian theories (which reject the inheritance of acquired characters). It is crucially important, however, to avoid collapsing the diversity of late nineteenth century evolutionary theorizing into two categories, with acquired characters the key issue at stake. As Burkhardt (2013) has demonstrated, belief in the inheritance of acquired characters was commonplace in Lamarck’s day, was not particularly associated with Lamarck, and was only a small part of Lamarck’s theorizing. Further, Corsi (2011) explicates crucial changes in Lamarck’s theorizing during his lifetime, arguing that on Lamarck’s mature view traits are not in themselves transmitted or inherited—only processes, such as distribution of organic fluids, which may influence the development of parts.Footnote 8
The labels “Lamarckian” and “neo-Lamarckian” may also refer to theories that posit an inherent tendency towards progressive evolution; Cope certainly held such a theory.Footnote 9 Cope’s commitment to progressivist evolution can be traced to sources both scientific and philosophical. Cope saw in his paleontological studies a steady advancement in the specializations of animals, presumably directed towards man, that he could not accept resulted from random variation and natural selection (Bowler, 1989). Similarly, Cope’s religious views made it difficult for him to accept life as a process driven by random forces (Bowler, 2017). Cope left the Quaker church shortly after his father’s death in 1875, but never abandoned his conviction that the world had been created with an underlying purpose and direction. Cope’s comments on the result of evolution through the conscious action of the organism are revealing:
...the control of mind over matter is seen, although this kind of will is not free, but acts under the dominion of reasons, or motives. This is the outcome of Neolamarckian philosophy, which proves the supremacy of the mind, and is therefore theistic, and entirely subversive of atheism. Osborn et al. 1931, p. 544; letter from Cope, Oct. 1888
Cope allowed in his early papers that natural selection could account for development of specific characters and species, but felt that genera and higher groups were the result of the process of “acceleration and retardation” directed towards definite ends by “the will of the Creator” (Cope 1868, pp. 243–244). At this stage, as Bowler (1977) has argued, Cope held that natural selection could explain the adaptive characters of species, but the overall rational structure of the natural system demonstrated the Creator’s plan. In his later writings, Cope would further diminish the role of natural selection in evolution. Cope began an 1878 paper with an explicit statement about the role of selection:
The origin of variation in animal structure is, par excellence, the object of the doctrine of evolution to explain. There can be little doubt that the law of natural selection includes the cause of the preservation of certain modifications of preëxistent structure, in preference to others, after they have been brought into existence. In what manner or by what process the growing tissues of young animals have been so affected as to produce some organ or part of an organ which the parent did not or does not possess, must be explained by a different set of laws. These have been termed originative, while those involved in natural selection are restrictive only. (Cope 1878b, p. 40; see also Cope 1889, p. 1059)
Cope’s paper proceeds to explain the progressive evolution of animals via the powers of movement and consciousness, so that the history of life has been “a succession of conquests over the restraints imposed by physical surroundings” (Cope 1878b, p. 47). Bowler (1977) has argued that Cope’s mature theory was more flexible, and in particular was more suited to explain patterns of divergence—adaptive radiations triggered by exposure to different conditions. The transition from fishes to amphibians is thus of particular importance (Cope 1878b, pp. 41, 48), as are the characters of the cardiovascular system that correspond to aquatic, semi-aquatic, and terrestrial lifestyles. Cope connected this reasoning about evolutionary processes to his classificatory practice, to which we now turn.
3.2 Cope’s systematics
Cope’s (1871b) classification was characterized by Günther as introducing “radical changes into the system” (Günther 1873, p. 93). Cope divided the fishes into three classes: Leptocardi, Dermopteri (lampreys and hagfishes), and Pisces (with five subclasses) (Table 4). The Dermopteri (lampreys and hagfishes) and Leptocardi (lancelets) were retained from Müller’s system ((5) Marsipobranchii (Cyclostomi), and (6) Leptocardii), but further isolated from other taxa by Cope’s grouping of all [remaining] fishes as Class Pisces.
Cope followed up on Rudolph Kner’s suggestions that the ganoids represented an unnatural assemblage, redistributing Müller’s (3) Ganoidei among two different subclasses. Cope (1871b, p. 581) pointed out that Müller united the ganoids on the basis of two soft tissue characters that were apparently shared uniquely. Kner had shown that one of the apparently unique characters (the connection of optic nerves via a band rather than by crossing) was also present in some fishes that Müller had considered teleosts, and that the other supposed shared character (the bulbus arteriosus having multiple valves) was not in fact a shared similarity: there were marked differences in the structure within the ganoids, comparable to the differences among ganoids and teleosts. Cope identified skeletal similarities and differences that also ran across Müller’s proposed separation of the ganoids from the teleosts.
Cope’s discussion of the relationships among the various groups of fishes was clarified by the publication, with minor modifications of his original system, of diagrams in 1885a (Figs. 1 and 2). Cope distributed Günther’s Palæichthyes among four subclasses, the “Holocephali” (chimaerids), “Selachii” (sharks and rays), “Dipnoi” (lungfishes), and Huxley’s “Crossopterygia” (lobe-finned fishes), including the extant bichirs, but also several forms that were extinct or thought to be extinct, such as the coelacanths. The sturgeons, paddlefishes, gars, and bowfin were placed within the subclass “Actinopteri,” which also included the bony fishes, and replaced Müller’s more restricted (1) Teleostei.
Cope’s (1885a) depiction of major groups of vertebrates as (in Cope’s words) a phylogenetic diagram
By 1885, Cope had slightly re-organized Class Pisces into four subclasses: Holocephali, Dipnoi, Elasmobranchi (Selachii and Ichthyotomi, in Fig. 1), and Hyopomata (a consolidation of Crossopterygia and Actinopteri), each originating in the early Palæozoic. Cope considered the division into four subclasses in light of his concern for the direction of evolutionary change: “If one type be derived from the other it is not certain which is ancestor, and whether the process has been one of advance or retrogression” (Cope 1885a, p. 236). The order Ichthyotomi he considered “technically” within Elasmobranchi, but speculated that Elasmobranchi may have descended from Ichthyotomi. The relationships of Dipnoi, Holocephali, Ichthyotomi, and Selachii (Elasmobranchi other than Ichthyotomi) are thus not as clear as the schema depicted in Fig. 1. Cope was convinced that Ichthyotomi contained the ancestor of the Hyopomata ((Cope, 1885a), p. 144), and that the Holocephali were the primitive fishes (Cope 1884, p. 585). But to Cope, the grouping of Ichthyotomi and Selachii as subclass Elasmobranchi was compatible with multiple hypotheses of the descent of the four subclasses. On some of these hypotheses, the subclass Elasmobranchi is polyphyletic, having arisen from multiple distinct evolutionary lineages. Polyphyletic groups are considered invalid in modern (post-Hennigian) systematics; the term “polyphyletic” implies a group is not real and the grouping is erroneous. In contrast, in the late nineteenth century the origin of natural groups via multiple independent lines of evolution was considered a real possibility. Indeed Bather (1927) suggested that many of the major groups might be polyphyletic and that the natural classification must reflect this apparent fact.
Though Cope referred to these diagrams as “phylogenetic”, it is important to note that some of the conventions of modern (post-Hennig) phylogenetic trees and taxonomy do not apply. The polyphyletic origin of Elasmobranchi—the independent origins of Selachii and Ichthyotomi from Holocephali, in Fig. 1—is one example. Another example is Cope’s suggestion that (some of) the Hemibranchi and Percomorphi descend directly from Holostei, in addition to or instead of Haplomi. He viewed this suggestion as compatible with Fig. 2. In Cope’s view, names of taxa can refer to natural groups despite including sub-groups with multiple distinct origins.
Cope’s classification of the major groups of fishes demonstrated his concern for directionality. Cope had defined degeneration in 1885a: “Degeneracy may be defined as loss of parts without corresponding development of other parts” (p. 141). This theoretical claim about the nature of degeneration complemented the view that evolution has central “stems”—main lines, like the central trunk of an oak tree (Bowler 1996, p. 163). Dohrn (1875) had proposed that evolution progressed towards higher forms (vertebrates and ultimately humans), but that from this central line various side branches had split off and ceased to follow the central progressive trend. For example, Dohrn considered the hagfishes a degenerate offshoot from the main line (Bowler 1996, pp. 206–207). Dohrn linked the tendency to degenerate to what he considered to be inactive modes of life, such as parasitism (Cope 1885a, p. 142).
Cope posited multiple branchings within the fishes, and considerable degeneration from the main line leading to terrestrial vertebrates:
The descent of the fishes in general has witnessed, then, a contraction of the limbs to a very small compass, and their substitution by a system of accessory radii. This has been an ever-widening divergence from the type of the higher Vertebrata, and from this standpoint, and also a view of the “loss of parts without complementary addition of other parts,” may be regarded as a process of degradation”. (Cope 1885b, p. 238)
Cope’s discussion of limbs also clarified that what he considered “higher” conditions are not necessarily those that are most adaptive:
The limbs of the Pisces are as well adapted to their environment as are those of the land Vertebrata, but from an embryological standpoint, their structure is inferior. The primitive rays are less modified in the fin than in the limb; and limbs themselves display a constantly increasing differentiation of parts, commencing with the Batrachia and ending with the Mammalia. (Cope 1885a, p. 148)
Adaptation, on the other hand, was about specialization:
Paleontology has provenFootnote 10 what had been already surmised, that the development of animal organisms has been on lines of increasing specialization of parts. That is, in lines of increasingly perfect adaptations of structures to ends, or functions. (Cope 1885a, p. 141)
Cope’s views about degeneration, adaptation, and the general directions of evolutionary change within lines of descent helped resolve tensions about the origins of the “higher” fishes and of the “higher vertebrates” from within fishes. Evolutionary progress could occur at different rates—including negative rates, and potentially very rapid rates—across different lines of descent. Cope did not see as problematic for evolutionary theory that the origin of the major groups of fishes is “lost in the obscurity of the early Palaeozoic” (1885b, p. 236). Moreover, in Cope’s view, Batrachia (amphibians) arose from Dipnoi; but Dipnoi was not the most advanced group of fishes. The higher vertebrates (Batrachia, reptiles, birds, and mammals) arose as an offshoot of a group (Dipnoi) that was relatively “low”, both with respect to its Batrachian descendants and to the other major groups of fishes. Separately, the most specialized fishes arose within another group: the Hyopomata.
Cope’s (1885a) Hyopomata included the Crossopterygia (which connected back to the main line of vertebrate development), Chondrostei, and Actinopteri (which roughly corresponded to Müller’s (1) Teleosts, but included some of his (3) Ganoids). The Actinopteri included three large divisions: the Holostei (gars and bowfin), the Physostomi (teleosts with a connection between the swimbladder and the esophagus), and the Physoclysti (teleosts without the swimbladder connection). The three groups “represent three series of the true fishes which indicate lines of descent”, with the Holostei originating first (see Fig. 2) (Cope 1885b, p. 238). The physostomes and physoclysts diverged from the holosteans and represented “further divergencies [sic] from the other vertebrate classes, or away from the general line of ascent of the vertebrate series as a whole” (Cope 1885b, p. 238).
Within the physostomes, Cope posited several distinct lines of descent. The line containing the eels (Ichthyocephali to Lyomeri) was viewed by Cope to diverge from the holostean type by a succession of losses, including the pectoral and pelvic fins, and loss of ossification in several skeletal elements. The series of Haplomi (a group including pikes) to Lophobranchii (seahorses and pipefishes) was also characterized as progressing through downward steps characterized by the loss, among other things, of spinous fin rays and de-ossification of the branchial apparatus. The series that ends in Nematognathi (catfishes) acquired more complicated vertebrae, hearing, and armor, which Cope (1885b, p. 239) considered progressive evolution (though he identified three genera within Nematognathi as “distinctly degenerate”).
The fishes that diverged the most from the main vertebrate line were the Physoclysti, and put to test Cope’s views about “grade”—progressive versus retrogressive evolution. The Physoclysti (a grouping of bony fishes) were united by a soft-tissue character: the lack of an opening between the gas bladder and the alimentary canal. Cope sought to reconcile this grouping with new hard-tissue discoveries. In particular he sought to arrange the Physioclysti in a sequence of development of fin bones (Cope 1878a, p. 297).
The end gained is specialization, but whether the series can be called either distinctively progressive or retrogressive is not so clear. The development of osseous spines, rough scales and other weapons of defense, together with the generally superior energy and tone which prevail among the Physoclysti, characterize them as superior to the Physostomi, but their departure from the ascending line of the Vertebrata has another appearance. (Cope 1885b, pp. 238–239)
Despite the flexibility of Cope’s framework to account for changes in evolutionary direction, Cope evidently had misgivings about identifying the Physoclysti as the “highest” fishes, given that they are not the fishes that gave rise to the “higher” vertebrates. Normally, complexity would be a mark of progressive evolution; but in this case, the added complexity was of a nature that did not resemble the ways in which humans are complex. Cope evidently considered this a problem for defining progressive versus retrogressive evolution. In discussing the meaning of taxonomic levels, Cope (1871c, p. 229) cited Agassiz’s (1857) definitions with approval (“far nearer a representation of nature than any other ever given”). Orders are defined “by the degree of complication of that class-structure”. Cope’s decision to discuss fishes mainly at the level of orders may therefore relate to his focus on developing a theory of progressive versus retrogressive evolution that could reconcile the distinct views of grade in terms of departure from the main steam, degree of complexity, and loss of parts.
Cope was evidently not troubled by the possibility of polyphyletic groups—cases in which a natural group descended from multiple distinct events. He suggested that the Physoclysti arose “from Holostean ancestors, both with and without the intervention of Physostomous forms” (p. 239). Patterson (1977, p. 591) considered Cope’s discussion of the various lines of descent to be the first clear proposal of possible polyphyletic origins of the teleosts, citing Cope’s (1871a, p. 453) claims that Nematognathi descended from a Chondrostein in the same way that Isopondyli descended from a Crossopterygian. Cope professed doubts about the particular details of each scenario, but did not seem troubled by the idea of connecting orders via multiple distinct lines of descent.
The need for polyphyletic scenarios stemmed partly from Cope’s enthusiasm for identifying linear evolutionary trends across extended series of ancestors and descendants (Bowler, 1983, 2017; Patterson, 1977). Notably, Cope’s “phylogenetic tree” (Fig. 2) places 25 orders in a handful of linear sequences, separated by a handful of branches. Today’s phylogenetic trees attempt to resolve the order of branching of lineages; Cope’s phylogenetic trees attempt to resolve the order of ancestor–descendant sequences. Ultimately, Cope’s arrangement of fishes into various series consistent with the development or degeneration of the characters that he emphasized was based less on genetic descent as we currently understand it than on his own views of development through the efforts of the organism, and it may be more helpful to view his trees as explicating developmental series rather than phylogeny (Ceccarelli, 2019). Indeed, Cope’s classifications relied in large part on analyses of the development of the limbs that had been initiated by Gegenbauer, whose work Cope cited as a “landmark in the history of modern theories of creation” (Cope 1871b, p. 582; emphasis original).
Cope’s focus on process also shows up in his harsh assessment of Günther’s arrangement of the amphibians for the British Museum:
the characters are treated as of equal importance in all cases, producing a kind of dichotomous system, each group being equal and similar to others, and presenting none of that successional relation which we know so well characterizes nature’s groups. The unfavorable impression is strengthened by a further examination into the structure, and the system is found to be little better than if it had been based, dictionary-fashion, on the first letters of their names. (Cope 1870, p. 199)
The problem, according to Cope, was that Günther’s classification does not depict what Cope thought was the evolutionary process itself: ancestors and descendants evolving in directional series. Instead, Günther had produced a set of (mostly) dichotomous branches, with no information about what are the ancestral taxa (indeed leaving it ambiguous whether there are relationships of ancestry at all).
Cope also identified Günther’s methodology—his choice of characters—as problematic. In a dispute with Gill (analyzed below, Sect. 4.2), Cope argued that natural groups can ultimately be defined on the basis of a single character (Cope 1871c, pp. 226–229). This is because “genera form series indicated by successional differences of structural character, so that one extreme of such series is very different from the other, by the regular addition or subtraction of characters, step by step.” (Cope 1869, p. 397). Here Cope cited “St. Hilaire, Owen, Agassiz.” If the history of evolution is a step-wise addition of single characters, then, Cope argued:
If we analyze the “sum of agreements” of given groups, we cannot affirm that all of those separate characters which constitute that sum have been always, in past time, coëxistent. In fact, we know that they have not been so, and that the differences of groups consist in the abstraction of single characters from, or addition of single characters to, this “sum.” Hence of the history of this “sum” is the history of the single characters which compose it, and each of them has a special value of its own, which cannot be sunk in a state of association. (Cope, 1871c, p. 227)
Given that we lack perfect knowledge of the lines of evolution, however, and given the gaps in the fossil record, it is often necessary in practice to consider multiple characters. In Cope’s view, the characters that will be useful in classification are those that relate to the directional evolution of the series that the taxon is part of, for example features of the circulatory system in the general vertebrate line (Cope, 1885a), the cranial structure in fishes, and the fins within the teleosts (Cope, 1887). The Leptiocardii have a simple tube for a heart, the Marsipobranchii and Pisces have two chambers, amphibians and reptiles have three chambers, and mammals four (Cope, 1887, p. 1015). Cope (1868, pp. 256–256) described details of 28 structures in the circulatory systems of the major groups of vertebrates, for example the presence, placement, and form of particular veins.
Cope criticized Günther for weighing what Cope considered to be the important characters equally with unimportant characters. The initial decision of what characters to select reflects an implicit weighting, and Cope (1870, p. 199) also criticized Günther’s procedure of selecting characters that are important to the life history of the species. To Cope, adaptation was not equivalent to progressive evolution, and played a limited role in the evolutionary process (Cope, 1869, p. 398).
Despite these differences, there is little evidence that Günther and Cope carried on a hostile relationship. Günther was generally positive in his notices of Cope’s papers in the Zoological Record, referring to them as “valuable” contributions, but prescribing caution in the adoption of Cope’s classifications Günther (1867, 1868, 1931, p. 253). For those to whom Günther did feel animosity, his treatment was not so tactful. Theodore Gill, to whom we now turn, was not among the American ichthyologists who enjoyed friendly relations with Günther (1975).
4 Theodore Gill
4.1 Origin and development
Theodore Nicholas Gill, like Cope, was drawn to the study of natural history at an early age, and despite the protestations of his family (Dall, 1916; Jordan, 1931). Gill was trained in Latin and Greek by a private tutor, followed by a brief effort to study law, and finally returned to his first interest upon receipt of a scholarship to the Wagner Free Institute of Science in Philadelphia. Gill’s contacts in Philadelphia included William Stimpson, who recommended him to Spencer Baird at the Smithsonian Institution in Washington, DC. Gill visited the Smithsonian for the first time in 1857, and began a series of projects under the guidance of Baird. In 1861, Joseph Henry invited Gill to take charge of the Smithsonian’s scientific library. Gill moved with the Smithsonian’s books to the Library of Congress in 1866, but throughout his tenure as librarian he maintained an office at the Smithsonian, where he would hurry after work to conduct his zoological studies. Starting in 1860, Gill was also a professor, in various capacities, at Columbian University (now George Washington).
Gill was in many ways a stereotypical museum worker, and participated in only limited field work early in his career. Even at the museum, Gill was apparently particular about his study materials. As told by Jordan, “specimens he did not care to handle except in the form of dry and clean skeletons; it was therefore a familiar joke to bring him a fish and say that he ‘might be interested in it because he had probably never seen one before’.” (Jordan, 1922, 1, p. 175) In addition to his work with skeletons, Gill brought to bear on his studies a thorough knowledge of the literature, and most who came in contact with him remarked upon his encyclopedic knowledge and phenomenal memory. Gill’s over 400 publications touched on a variety of scientific, historical, and social issues, but his special interest was ichthyology, and it was in that field that he applied the bulk of his efforts.
Gill had a reputation as a helpful and magnanimous member of the scientific community. As related by Jordan, Gill “most hospitably received all young naturalists who coveted his personal acquaintance or desired aid from his universal store of biological knowledge. His expression was friendly—often mildly quizzical—and his natural impulse was always toward kindly criticism” (Jordan, 1931, p. 286). Among Gill’s admirers were George Brown Goode, W. K. Gregory, and Jordan himself, who dedicated his 1905 textbook of ichthyology to Gill: “Ichthyologist, Philosopher, Critic, Master of Taxonomy” (Jordan, 1922, 1, p. 176).
However, Gill could be a harsh critic. William O. Ayres, a California ichthyologist, was among those who was harshly judged by Gill, ultimately abandoning his researches of fish (Leviton & Aldrich, 1981). Jordan (1922, vol. 1, p. 144) described Gill’s interactions with Günther: “with Dr. Günther of London, whose genius ran in a totally different channel, he was in chronic collision about matters in which either one may have been technically right from his own point of view.” The quarrel between Günther and Gill seems to have been confined to comments within their publications. Günther’s species and genera frequently fell victim to Gill’s revisions, and may have inspired Günther’s comments in the first volume of the Zoological RecordFootnote 11:
Mr. Gill would much advance Ichthyology by giving us serviceable descriptions, instead of limiting himself to synoptical tables with minute pseudo-generic subdivisions. As regards his frequent critical remarks on synonyms, it would be very useful if he would state whether he arrived at his conclusions from an examination of the typical specimens; but frequently it is not even evident whether he has known the species from autopsy. (Günther, 1864)
From one museum worker to another, failing to examine the crucial specimens—or even any specimens at all—would have been a serious charge.Footnote 12 The comments were made in regard to Gill’s (1864) “Synopsis of the Pleuronectoids of the Eastern Coast of North America. Gill’s classification of 1872 received a noncommittal notice from Günther.
Gill, if anything, was more pointed in his criticisms of Günther. Gill (1872a, p. xx) was particularly critical of the sequence of Günther’s classification, attributing it to “metaphysical or psychological considerations.” Gill was generally professional in his tone in the scientific literature but an 1881 review of Günther’s (1880) ichthyology text, published in a popular magazine, was not so subtle. In addition to a multitude of factual errors, Gill charged Günther with inappropriately claiming credit for work done by Gerard Krefft. Gill (1881, p. 120) also berated Günther for failing to adopt an evolutionary framework, stating that “The author...has been unable to any considerable degree to discard what he has once accepted and to bring himself into relations with the science of the present, but adheres tenaciously to beliefs formed in a much less advanced state of knowledge, and in spite of conclusive evidence against their tenability.” Gill concluded:
Darwin has given to the world his immortal work and revolutionized the methods and objects of biological investigation, while laborers almost innumerable have elucidated the various branches of ichthyology—the anatomy, embryology, the past history, the systematic relations, the species, the geographical distribution of forms, the faunas of the world. All these have been in vain for Dr. Günther. ...Unquestionably, the most prominent characteristic of the present time is the acceptance of evolution and its ramification into all the details of biological investigation and classification. But in the ‘Introduction to the Study of Fishes’ no allusion has been made to this principle, and the author’s treatment of his subject indicates that it has been practically ignored. (Gill, 1881, p. 122)
The criticism here is of omission: Günther’s failure to use diverse sources of evidence, as well as failure to explain to the general reader that by 1880, the theory of evolution was central to the practice of systematics. Gill (p. 122) also criticized what he took to be Günther’s actual methodology: “A certain type has been assumed as ‘highest’ on account of vague psychological conceptions and, with this as an initial form, others are successively taken up, till the author has lost his bearings and recklessly dealt with the remainder.” As will be seen, Gill extended his criticism to use of the concepts of “high” and “low” more generally.
4.2 Gill’s systematics
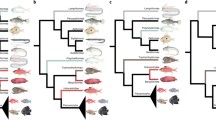
Like Darwin, (but unlike Huxley in the period 1857–1867), Gill thought that the natural classification should reflect the degree of evolutionary relatedness of taxa. Gill elucidated this idea in a set of five principles of classification, with principle three declared as beyond serious doubt (p. 288): “the animals and plants of the present epoch are the derivatives with modification of antecedent forms to an unlimited extent” (Gill, 1872b, p. 286). From principle three, followed principle four: “an arrangement of organized beings in any single series is, therefore, impossible, and the system of sequences adopted by genealogists may be applied to the sequence of groups of natural objects” (Gill, 1872b, p. 286). In a list prepared for the arrangement of fishes at the Smithsonian, Gill arranged the fishes into a “quasi-genealogical tree” (Fig. 3), based on ancestor–descendant relationships suggested by his studies (Gill, 1872a, p. xliii).
Gill’s (1872a, p. xliii) “quasi-genealogical tree” of major groups of fishes. Note that some of the conventions of modern (post-Hennig) phylogenetic trees do not apply. For example, nodes cannot be rotated
Gill, following Cope and Haeckel, broke up Günther’s class Pisces. Gill placed the living fishes into three distinct classes: Leptocardii, Marsipobranchii, and Pisces, the latter including three subclasses, the Elasmobranchii, Ganoidei, and Teleostei (Tables 5 and 6). In a later paper, Gill emphasized the validity of placing the fishes into more than one class based on abundant taxonomic evidence, attributing earlier efforts to encompass all the fishes into a single class to the “vague idea that what are called lower forms are more elastic and exhibit a wider range of variation than superior ones, and by assuming that all low forms of any branch, however much they differ in structure, are constituents of a natural group to be compared with several more restricted higher ones”. (Gill, 1873, p. 77; emphasis original)
Unlike Cope, Gill chose to retain Müller’s subclass Ganoidei, stating that while he was “prepared to admit that the extremes of the Ganoids are more dissimilar than one of those extremes and the typical physostome Teleosts, it is not yet apparent that the relations between Ganoids and Teleosts are as intimate as those between the contiguous orders of the latter series” (Gill, 1872a, p. xi). As the supposedly “lowest” (or “most generalized”—see below) Teleosts, Gill offered Polypterus (bichirs) and Acipenser (sturgeons), whereas the “most teleosteoid” Ganoids were exemplified by Amia (bowfin). Gill denied that any unambiguous Teleosts had been demonstrated to have the soft tissue characters that Müller used to distinguish the Ganoids from Teleosts (namely the connection of optic nerves, and the bulbous arteriosus having multiple valves).
Other significant differences with Cope’s classification include Gill’s incorporation of the Holocephali into a common subclass with the sharks and rays, and a reduction, through combination, of the number of orders Cope introduced to define the bony fishes, most notably in Apodes, the eels. Gill (1872a, pp. xxxviii–xxxix) noted that he was only able to examine skeletons of two eel species, but emphasized that all eels have the “same common form”, similar brains, and a number of skeletal characters (greatly increased number of vertebrae, lack of ventrals, simple structure of the rays of the fins, and restricted branchial apertures).
Gill included Roman numerals on the tree diagram to emphasize what he saw as the best sequence for addressing the orders of fishes, from most generalized (or “quasi-eldest”—see below) to most specialized. However, Gill explained that the natural classification itself would not constitute a single sequence; the sequence I–XXII reflected the fact that the book (and its table of contents) must present information in a linear order, from line to line, page to page.
A difficult problem is the arrangement, in a linear series, of forms so as to best express their relationships. This is perhaps most aptly effected by taking, in the first place, the most generalized type known (a), and follow that by the one (or two or more) most closely allied to it (a1), then by the nearest to that (a2), and thus to the end of the series...; then we may recommence with the one next most nearly related to (a) the first type, and project another series (b, b1, &c). It will be evident that the last term of the first series (ax) will often be much less nearly related to the first term of its own series (a) than is the first term of the second series (b); and of course, that it,—(ax) the last term of the first series,—so far from being intermediate between the two (a and b), must be the most remote from the first term, if we are right in the appreciation of the relative affinities of the succeeding series, since both are the descendants of the same original progenitor. (Gill, 1872b, pp. 288–289)
Based on this concept of relationship, Gill concluded that “it is now beyond the province of doubt that such a phenomenon as the chain of beings, which existed in the imaginations of Lamarck and De Blainville, does not exist in nature” (Gill, 1872b, p. 289).
Gill presented a similar argument in his list prepared for the Smithsonian, but introduced language from human genealogies to illustrate:
The most convenient mode of arranging forms in a linear succession appears to be in series,—that is, taking a number of types and arranging them successively, having regard to the forms next most allied, till the series is exhausted; and then recommencing anew with that series whose first member is most nearly allied to one of the preceding:—in other words, following a genealogical system and assimilating it to a scheme, where we would have a given ancestor, and then (1) eldest son, (1a) eldest grandson, (1b eldest great-grandson, etc.; and after giving all terms of such lineage, we would recommence with the (2) second son and proceed with descendants in like manner. (Gill, 1872a, p. xlii)
Simply substituting the term “ancestor” for “most generalized type known” glosses over some critical challenges that would be much discussed by later systematists (Ebach et al., 2008; Eldredge & Cracraft, 1980; Patterson, 1977; Rieppel, 2010; Sober, 1988). One problem is that modern-day taxa cannot be equated with hypothesized ancestral forms, because the modern-day taxa result from the continued evolution of a lineage that existed subsequent to the ancestral form. A further problem is that we cannot expect that the fossils that we find are direct ancestors of modern taxa (or of other fossil forms). While sequences like Gill’s (1)—(1a)—(1b)...can be hypothesized, any fossil that we actually find may have resulted from a lineage that split off without leaving known descendants. To the degree that the fossil record is incomplete, we should expect that many or indeed all of the fossils that we find are not direct ancestors of other known taxa. As Patterson (1977) argued, trust in paleontological evidence to reconstruct phylogenies can be problematic when fossil forms are interpreted as direct ancestors. For example, a fossil interpreted as (1a) would be expected to have features intermediate between a fossil interpreted as (1) and a fossil (or modern form) interpreted as (1b). This expectation will be misleading in the case that the supposed intermediate form in fact represents a distinct lineage that split from (1) and that is not directly ancestral to (1b).
Gill may have intended to signal that putative ancestors and the living, most generalized forms couldn’t really be substituted freely; he repeatedly included language softening the comparison to human genealogies (“quasi-eldest” 1872b, pp. 289, 294, 1872a, pp. xxxvi; “quasi-genealogical” 1872a, p. xlii; “quasi-relations” 1872a, p. xlii; “quasi-youngest” 1872a, p. xxxvi; “quasi-younger” 1872a, p. xxxvi). As Gill pointed out, the last terms of each ancestor-descendent series (ax and bx) will not be intermediate—we should not expect to find a living form with both fish-like characters and amphibian-like characters, intermediate between fishes and amphibians. Rather, we might expect to find living forms that descend from lineages that split off from the lineage that ultimately produced amphibians.
Gill seemed more willing to interpret fossil forms as direct ancestors. Following Haeckel and Gegenbaur, Gill identified Dipnoans as the transitional form linking fishes to amphibians. That is, the earliest amphibian evolved from an “ancestral stock” identified as Dipnoan, and from which the modern Dipnoi (lungfishes) also descended:
Let the Dipnoan be considered as the eldest representative of the ancestral stock equally of the Fishes and of the Batrachians, from which the respective forms have descended, diverging more and more in the course of time. Of course, the Dipnoan will be more nearly related to the Batrachians than the Fishes diverging from the same stem—as the grandparent is more nearly related to the children of two sons than such grandchildren by the different sons are to each other. (Gill, 1872a, p. xxxv)
Gill allowed that taxa may be what modern systematists would call paraphyletic—for example, his name “Dipnoi” refers to the ancestral stock and its fish descendants (the modern Dipnoi), but not to the amphibian descendants. Gill (1872a, p. xxxv) insisted that Dipnoi are Fishes, so that “Fishes” includes the ancestral Dipnoi but not their amphibian descendants. Note that care must thus be taken in interpreting Gill’s quasi-genealogical trees (e.g. Fig. 3) together with his Tables 5 and 6 of taxa. For example, Superorder Hyoganoidei includes Rhomboganoidei (gars) and Cycloganoidei (bowfin) but not the Teleosts.
Gill considered the Dipnoans (living and ancestral) “the most generalized” fishes (compared to Teleosts).Footnote 13 He commented that those who view the Dipnoans as the “highest” fishes do so by virtue of the belief that Dipnoans represent the transitional form between fishes and the “higher” tetrapods. However, this leads to confusion when the term “higher” is also taken to be an indication of degree of specialization:
Inasmuch, for example, as the Dipnoan is (1) the most generalized, and therefore (2) more nearly related to the Batrachian than the typical fishes, because (1) of that nearer affinity, and (2) the recognition of the quadruped type as “highest,” it is called “higher” than the fishes.
Perhaps there are no words in science that have been productive of more mischief and more retarded the progress of biological taxonomy than those words, pregnant with confusion, High and Low, and it were to be wished that they might be erased from scientific terminology. They deceive the person to whom they are addressed; they insensibly mislead the one who uses them. Psychological prejudices and fancies are so inextricably associated with the words that the use of them is provocative of such ideas. The words generalized and specialized, having become almost limited to the expression of the ideas which the scientific biologist wishes to unfold by the other words, can with great gain be employed in their stead. (Gill, 1872a, p. xxxvi)
Thus, like Cope, Gill sought to clarify the language of grade. But unlike Cope, Gill thought that once the concept of degree of specialization was distinguished from the language of grade, the terms “higher” and “lower” should simply be abandoned. For example, he noted that whales are extremely specialized among mammals, and that “the Aye-aye exhibits in its dentition excessive specialization and deviation from the primitive type (as exhibited in its own milk teeth) of the Primates” (Gill, 1870, p. xxi). He considered it a reductio of the grade concept to consider whales as the highest mammals and Aye-ayes as the highest primates.
Whereas Cope sought to retain a grade concept distinct from specialization, Gill sought to identify groups on the basis of shared ancestry. In his view, degree of specialization might provide evidence about ancestry but was not itself the direct target of a natural classification. In general, specialized character states (e.g. the Aye-aye’s large, continuously growing incisors separated by a wide margin from the other teeth) are expected to arise from more generalized character states. Distinguishing general from specialized may be helpful in reasoning about ancestral character states, without positing an axis of primitive–to–advanced taxa.
In Gill’s view, degree of specialization could also play a role in choosing what groups to name. Gill retained paraphyletic names such as “Fishes” (excluding amphibians and other vertebrate descendants of ancestral fishes) because the terrestrial vertebrates showed marked specialization away from the general fish form. He also sought to depict degree of generalization on the quasi-genealogical trees themselves: “In all cases (except the Vertebrates and Molluscoids), the branch to the left—major as well as minor—indicates the supposed most generalized type of the two or more more springing or diverging from the same common stem” (p. xlii). Gill’s “to the left” is towards the bottom of the page (see pp. 289–290 of Gill , 1872b).Footnote 14
Both Gill’s retention of paraphyletic names and his depiction of degree of specialization on phylogenetic trees contrast with modern (post-Hennigian) cladistic methodology. Yet Gill’s taxonomic methodology reflected an awareness of what would later be called symplesiomorphy—similarity owing to retention of ancestral features. As he put it, the early links in a descendent sequence (a1) will closely resemble the early links in a separate descendent sequence (b1), while the ends of the chain (ax) are likely to be very different from the initial steps (a1) (Gill, 1872b, pp. 288–289). In the event that not much morphological evolution has transpired over the course of a chain (a), then the end of that chain (ax) may closely resemble the earliest steps in the other chain (b). The most generalized living forms could be treated as “quasi-eldest” in the sense that (Gill thought) they likely resemble the ancestral form in important respects—they retain many ancestral features. Many of the ancestral features will have been lost in other lineages, but some will have been retained. Thus, information about the modern Dipnoi may provide information about early links in other chains descended from the same ancestral stock (such as the Crossopterygia, with whom the Dipnoi share “much closer bonds” compared to amphibians). Gill was reasoning about which similarities would provide information about ancestral states at different levels in the tree of branching genealogical sequences. In this sense, as Panchen (1992) has claimed, Gill’s methodology resembled the cladistic framework later defended by Brundin (1966) and Hennig (1966). However, Gill’s willingness to identify fossils as direct ancestors would be problematic in a cladistic framework (Patterson, 1977).
Gill’s insistence that taxonomists ought to start with the most generalized forms did prove helpful in reasoning about what characters must be considered (a central issue in the history of taxonomy—(Huelsenbeck et al., 1994; Quinn, 2016; Rieppel & Kearney, 2007; Whewell, 1837)). Each proposed more specialized sequence could be taken in turn, aiming to identify characters “peculiar to and common to certain forms” (Gill, 1872a, p. viii).Footnote 15 However, Gill (1872a, pp. xx–xxii) recognized the dangers of relying on the “irrelative specialization of isolated parts” and argued that taxonomists must examine the “sum of the parts”.Footnote 16
The injunction to use many characters was not unusual (de Candolle, 1813; Whewell, 1840; Mill, 1843), but Gill’s comments about the sum of parts were spurred by a particular exchange with Cope. Gill (1870, p. 267) argued that “the affinities of such organisms are only determinable by the sum of their agreements in morphological characteristics, and not by the modifications of any single organ.” Cope responded:
If we analyze the sum of the agreements of given groups, we cannot affirm that all of those separate characters which constitute that sum have been always, in past time, coexistent. In fact, we know that they have not been so, and that the differences of groups consist in the abstraction of single characters from, or addition of single characters to, this “sum.” Hence the history of this “sum” is the history of the single characters which compose it, and each one of them has a special value of its own, which cannot be sunk in a state of association....
Every structural feature possesses some systematic value, and when our knowledge extends over a greater number of forms than the system at present includes, the definitions of our groups will rest upon single characters only, and the history of the origin of those characters will be the history of the origin of the groups. (Cope, 1871c, pp. 227–228)
Cope (1883b, 1896) would later reconstruct ancestor-descendent sequences of fossil mammals, building on the idea that such sequences displayed regular tends towards specialization, for example of the teeth (Bowler, 1996, pp. 343–356). The discovery of an (apparent) directional sequence in the rich fossil record of horses inspired Cope and others to interpret evolution linearly (Bowler, 2017; Manias, 2017). The regularity of the horse sequence suggested that, as more fossils were discovered, the gaps between forms would be reduced to the logical limit: individual characters.
The fossil record included ample fish fossils, but these were not very amenable to treatment as regular, linear sequences of ancestors and descendants. Moreover, crucially, Gill worked from specimens of living forms. He suggested that Cope might be correct in principle, but that in practice, consideration of multiple characters was necessary, because there is no way to know in advance which are the single characters that pick out natural groups. In Gill’s words, the groups are determinable only by consideration of multiple characters. Single characters might be used to diagnose natural groups, after the groups are known. Gill further argued that Cope implicitly made this distinction in his practice, and announced “I am happy to believe that there is only an apparent and no real difference between my eminent friend [Cope] and myself” (1872b, p. 287).
In fact, Gill’s reliance on modern forms led him to approach classification much differently than Cope did. Patterson (1977) argued that late-nineteenth century ichthyology was actually impeded by attempts to incorporate evolutionary theory. In Patterson’s view, the problem was the methodology of putting paleontology first: specifically, identifying fossils as direct ancestors and as transitional forms.Footnote 17 The appearance of success in the case of sequences of mammals such as the horse seemed to justify theoretical views about directional evolution that later proved to be misleading (Bowler, 2017). Another effect was support for the idea of identifying ancestor–descendant sequences: identifying particular species (living and fossil) as ancestors. In contrast to Cope, Gill reasoned about hypothesized but unobserved sequences, of which modern forms are all ends of the chain. Gill’s reasoning about the hypothesized sequences led him to the view that living forms would not look like intermediate forms, even if they retained many features from the ancestral forms (i.e. if they were “living fossils”). Even the most generalized (“quasi-eldest”) modern forms have a history of character evolution, and their uniquely acquired characters must be carefully disentangled from characters retained from the ancestral forms.
5 Conclusion
Günther, Cope, and Gill’s classifications demonstrate diverse approaches to taxonomy and to evolutionary theory. The comparative reception of each ichthyologist’s classification demonstrates changes in the relationship between taxonomy and evolutionary theory.
Günther’s ideas provided the framework for systematic studies of the fishes at the British Museum and in Europe, until George Albert Boulenger began his systematic studies at the Museum in 1883 (Gregory, 1907; Stearn, 1981). The system that Boulenger applied to the fishes in his uncompleted revision of Günther’s Catalogue was described by Jordan as “distinctly modern, and with the writings of the contemporary ichthyologists of Europe and America, it is fully representative of the scientific era ushered in by the researches of Darwin” (Jordan, 1905, p. 402). Jordan also credited Boulenger’s use of ample material, particularly osteological evidence. Gill, too, welcomed Boulenger’s (1904) views on fish systematics, writing: “It was a bad and unscientific method that has paralyzed science in Europe for these many years, and let us hope the new work may force it into the background, if not wholly eradicate it” (Gill, 1905, p. 661). Similarly, Boulenger’s colleague, Arthur Smith Woodward, began a (1891–1901) catalogue of the fossil fishes at the British Museum that was evolutionary in its format, and incorporated some of the ideas of Cope and Huxley on those groups, but introduced many new ideas into the classification of the fossil groups (Patterson, 1977, pp. 597–599). The studies of Charles Tate Regan completed the transition to evolutionary studies at the British Museum. Regan “was more interested in problems of higher classification, geographical distribution and phylogeny than had been his predecessors, who had been disbelieving, skeptical, neutral or lukewarm about the theory of evolution” (Stearn, 1981, p. 171). Regan’s conclusions formed the basis of fish classifications through at least the middle of the twentieth-century.Footnote 18 The impact of Günther’s classification, then, was short-lived, even in his own Museum.
Although some late nineteenth and early twentieth century ichthyologists criticized Günther for declining to incorporate evolutionary theorizing, there was no clear consensus about how an evolutionary framework ought to have affected systematic work. Both Jordan (1905) and Gill (1881) claimed that Darwin’s theoretical work was crucial for modern systematics, but neither discussed Darwin’s (1859) own chapter on classification. As Winsor (1991) pointed out, it would be wrong to assume that systematists who ignored evolutionary theory must consequently make wrong assessments of affinity. Indeed Patterson (1977) argued that evolutionary thinking hindered progress, because ichthyologists searched for direct ancestors in the fossil record.
Cope’s arrangement of the fishes stemmed from the expectation that classifications should reflect ancestor–descendant sequences and that fossil taxa can be identified as direct ancestors. Cope’s theoretical views about directional evolution led him to place orders of fishes into linear sequences, with subdivisions of the higher classes arranged in accordance with the linear sequences. The groups of fishes that did not fit into the line of development from the Leptocardi through the Holocephali to the Dipnoi and ultimately to the amphibians necessarily represented offshoots from the main line of evolution. Cope’s view of evolution facilitated a classification based on modification of a few, specific anatomical features, and the result was a series of ramifications reflecting several different lines of development away from the main vertebrate line. When groups proved difficult to arrange in direct sequences, Cope did not hesitate to posit polyphyletic origins and parallel evolution. This approach proved popular (Bather, 1927).
Of the three ichthyologists, Cope is the better known historical figure today. At least in the immediate future of ichthyology, however, Gill’s contributions to taxonomy—both methodology and the actual classifications—were considered crucial. Gill’s classification was largely accepted by the American workers who followed, although his retention of the Ganoidei was a notable exception. Gill’s conclusions about the major groups of fishes were still reflected in the classification of Leo Berg in 1940 (Greenwood et al., 1966, p. 345). Gregory considered Gill’s work to be the “basis of all subsequent work of the American school” (1907, p. 439), and singled out Gill’s disagreement with Cope on character analysis in particular (Osborn et al., 1931, p. 501). Jordan, whose 1905 classification defined the views of the “American School,” considered Gill to have “a keener appreciation of the meaning of structure in classification and in evolution than that shown by any other naturalist,” and adopted, in large part, the basic plan of Gill’s classification (Jordan, 1922, p. 175).
It is not true that not much happened in the development of theory of classification in the period between Darwin and the Synthesis (cf. Mayr 1982). Rather, this period has been comparatively understudied by historians of taxonomy (Nyhart, 1999), despite the potential value of such study for understanding the development of biological disciplines and the later cladistic revolution. We hope to have raised critical questions for further work foregrounding the development of systematic theories. We hope especially to motivate further work on the influence of the diverse array of nineteenth century evolutionary ideas on systematic work.
Notes
Huxley later adopted genealogical classification, in response to Haeckel. See di Gregorio (1984).
In his (1975) A Century of Zoology at the British Museum Through the Lives of Two Keepers, 1815–1914 Albert E. Gunther noted: “While Albert Gunther lived the family wrote its name as he did, with the “ü”, which is, by rule, maintained in scientific literature.” A.E. Gunther (A.C.L.G. Günther’s grandson) chose to omit the umlaut when referring to his grandfather: “In a biography written over sixty years after Albert Gunther’s death it would hardly be in keeping to retain a form no longer in use.” The Dictionary of National Biography (1927, 2004) lists A.C.L.G. Günther with the umlaut, offering “Gunther” as an alternative form in brackets, though the Anglicized form is used in-text in the 2004 edition. We here follow the usage in the scientific literature, which continues to retain the umlaut.
The museum that was named the British Museum (Natural History) in Günther’s day legally separated from the British Museum in 1963, and was renamed the Natural History Museum in 1992.
Whitehead and Talwar (1976, p. 84) report that Günther, when contacted for advice by the publishers of Macmillan & Co., questioned Jordan’s ability to write a proposed book on western European fishes, calling Jordan a “compiler” and stating that he was “an adherent of the rules of zoolog. nomenclature established by American naturalists to supersede the nomenclature followed in Europe.”
A thorough, though hagiographic, treatment of all aspects of Cope’s life has been presented by Osborn in Cope: Master Naturalist (1931). Davidson’s (1997) biography is far more balanced in its assessment of the man, but largely avoids Cope’s taxonomic theory and practice. We have also drawn biographical information from Osborn’s (1930) shorter sketch, and from Gill (1897).
Ulett also notes that orthogeneticists’ theories may seem unscientific because they devised many technical terms that sound bizarre to us, such as bathmysm, physiogenesis, kinetogenesis, aristogenesis, genepistasis, halmatogenesis, and kyesamechania (Ulett 2014, p. 125). It is worth remembering that strange-sounding technical terms occur in other historical episodes in biological theorizing. Writing on the systematists of the Modern Synthesis, Kruseman (1950) discusses the uses of the terms Jordanont, Linneont, Oecotype, Oecospecies, comparium, commiscuum, convivum, Rassenkreis, and isoreagent. Simpson (1944) is also a fertile source of examples (hypsodonty, homeosis, megaevolution, horotelic, brachytelic, tachytelic, quantum evolution).
See Corsi (2006) for further analysis, in particular of Lamarck’s materialism, vitalism, and his changing views on spontaneous generation.
Levit and Olsson (2006) provide an extremely useful taxonomy of orthogenetic theories.
Cope here cites Cope (1883a).
Günther was editor of the Record of Zoology (known as the Zoological Record from 1870 to present) through 1864 and 1869 and continued to write the section on fishes through 1872.
For example, Gill would have been aware of his Smithsonian colleagues’ frustration with Rafinesque’s haphazard approach to ichthyology and mammology, which resulted in species and genera whose identities were difficult or impossible to verify (Baird, 1857; Girard, 1857) The crux of the problem was that Rafinesque generated names that were not linked to specimens; sometimes his taxa were not based on any direct examination of specimens. Agassiz (1854) wrote that “Nothing is more to be regretted for the progress of natural history in this country than that Rafinesque did not put up somewhere a collection of all the genera and species he had established, with well-authenticated labels...” Jordan (1877) later reported that some of Rafinesque’s species were based on drawings made by Audobon as a prank, to deliberately trick Rafinesque. The taxonomic havoc caused by Rafinesque was a powerful impetus for the museum community’s focus on the value of specimens and direct examination of specimens, an ethos that continues to this day (Woodman, 2016).
Bowler (1996, p. 232) cites this passage to indicate that Gill considered the Dipnoans “among the most primitive fish”. Gill uses the phrase “most generalized” when referring to living forms that have diverged the least from a (hypothesized) “primitive stock”, but does not here refer to living Dipnoans as “primitive”.
Panchen (1992, p. 31) claims that in Gill’s trees, “the horizontal axis is not a measure of morphology or anything else”, and that Gill’s trees resemble cladistic analysis in this respect. However, Gill repeatedly discussed the relative degree of specialization of groups and consistently placed groups that he considered more generalized on the left versus right branch.
See Hennig (1966) pp. 119–122.
See Sepkoski (2009, 2012) for analysis of attempts to synthesize paleontology and evolutionary theory (and their discontents). On a standard historiographic narrative, during the period between Darwin’s 1859 publication and the Modern Synthesis, the theoretical development of such a paleobiology was hindered by under-appreciation of the power of selection, enthusiasm for orthogenetic theories, and pessimism about the fossil record. As Bowler (2009, p. xiii) remarked and Allmon (2020) has demonstrated, a re-evaluation of the history of paleontology is necessary to avoid assumption that theoretical progress has been hindered by failure to adopt neo-Darwinist views.
Regan’s key systematic papers were published in series between 1903 and 1923 primarily in Annals and Magazine of Natural History See also Greenwood et al. (1966, p. 345).
References
Agassiz, L. (1854). Notice of a collection of fishes from the southern bend of the Tennessee River. American Journal of Science and Arts, 17(49), 352–369.
Agassiz, L. (1857). Contribution to the natural history of the United States of America, Vol. 1. Little Brown and Company.
Agassiz, L. (1859). An essay on classification. Longman, Brown, Green, Longmans, & Roberts, and Trübner & Co.
Allmon, W. D. (2020). Invertebrate paleontology and evolutionary thinking in the US and Britain, 1860–1940. Journal of the History of Biology, 53(3), 423–450.
Amundson, R. (2005). The changing role of the embryo in evolutionary thought: Roots of evo-devo. Cambridge University Press.
Baird, S. (1857). Reports of explorations and surveys, to ascertain the most practicable and economical route for a railroad from the Mississippi River to the Pacific Ocean, Vol. 8. Beverley Tucker.
Bather, F. A. (1927). Biological classification: Past and future. Quarterly Journal of the Geological Society of London, 83(2), l19–l20.
Boulenger, G. (1904). Teleostei. In S. Harmer & A. Shipley (Eds.), The Cambridge natural history, Vol. VII, (pp. 539–727). Macmillan and Co. Limited.
Bowler, P. J. (1977). Edward Drinker Cope and the changing structure of evolutionary theory. Isis, 68(2), 249–265.
Bowler, P. J. (1983). The eclipse of Darwinism: Anti-Darwinian evolution theories in the decades around nineteen hundred. Johns Hopkins University Press.
Bowler, P. J. (1989). Evolution: The history of an idea. University of California Press.
Bowler, P. J. (1996). Life’s splendid drama. University of Chicago Press.
Bowler, P. J. (2009). Evolution: The history of an Edea (3rd ed.). University of California Press.
Bowler, P. J. (2017). American palaeontology and the reception of Darwinism. Studies in History and Philosophy of Science Part C: Studies in History and Philosophy of Biological and Biomedical Sciences, 66, 3–7.
Brinkman, P. D. (2010). The second Jurassic dinosaur rush: Museums and paleontology in America at the turn of the twentieth century. University of Chicago Press.
Brinkman, P. D. (2015). The ‘Chicago idea’: Patronage, authority, and scientific autonomy at the Field Columbian Museum, 1893–97. Museum History Journal, 8(2), 168–187.
Brinkman, P. D. (2016). Edward Drinker Cope’s final feud. Archives of Natural History, 43(2), 305–320.
Brundin, L. (1966). Transarctic relationships and their significance, as evidenced by chironomic midges with a monograph on the subfamilies Podonominae and Aphroteniinae and the austral Heptagyiae. Kungliga Svenska Vetenskapsakademiens Handlingar, 11(1), 1–472.
Burkhardt, R. W. (2013). Lamarck, evolution, and the inheritance of acquired characters. Genetics, 194(4), 793–805.
Burkhardt, F., & Smith, S. (Eds.). (1985). A calendar of the correspondence of Charles Darwin, 1821–1882. Garland Publishing Inc.
Cain, A. (1958). Logic and memory in Linnaeus’s system of taxonomy. Proceedings of the Linnaean Society of London, 170(1–2), 185–217.
Ceccarelli, D. (2019). Between social and biological heredity: Cope and Baldwin on evolution, inheritance, and mind. Journal of the History of Biology, 52(1), 161–194.
Cope, E. D. (1891). Alfred Russell Wallace. In Evolution in science, philosophy, and art: Popular lectures and discussions before the Brooklyn Ethical Association. D. Appleton and Company.
Cope, E. D. (1859). On the primary divisions of the Salamandridae, with descriptions of two new species. Proceedings of the Academy of Natural History of Philadelphia, 11, 122–128.
Cope, E. D. (1868). On the origin of Genera. Proceedings of the Academy of Natural History of Philadelphia, 20, 147–148.
Cope, E. D. (1869). Synopsis of the Cyprinidae of Pennsylvania. Transactions of the American Philosophical Society, 13(3), 351–410.
Cope, E. D. (1870). On the system of Batrachia Anura of the British Museum catalogue. American Journal of Science, 3(1), 198–203.
Cope, E. D. (1871). Contribution to the ichthyology of the Lesser Antilles. Transactions of the American Philosophical Society, 14, 445–483.
Cope, E. D. (1871). Observations on the systematic relations of the fishes. The American Naturalist, 5(8/9), 579–593.
Cope, E. D. (1871). On the homologies of some of the cranial bones of the Reptilia, and their bearing on the systematic arrangement of the class. Proceedings of the American Association for the Advancement of Science, 19, 194–247.
Cope, E. D. (1878). On the classification of the extinct fishes of the lower types. In F. W. Putnam (Ed.), Proceedings of the American Association for the Advancement of Science (pp. 292–300). Salem Press.
Cope, E. D. (1878). The relation of animal motion to animal evolution. American Naturalist, 12, 40–48.
Cope, E. D. (1883). On the evidence for evolution in the history of the extinct Mammalia. Science, 2(30), 272–279.
Cope, E. D. (1883). On the trituberculate type of molar tooth in the Mammalia. Proceedings of the American Philosophical Society, 21(114), 324–326.
Cope, E. D. (1884). On the structure of the skull in the Elasmobranch genus Didymodus. Proceedings of the American Philosophical Society, 21(116), 572–590.
Cope, E. D. (1885). On the evolution of the Vertebrata, progressive and retrogressive. The American Naturalist, 19(2), 140–148.
Cope, E. D. (1885). On the evolution of the Vertebrata, progressive and retrogressive (continued). American Naturalist, 19(3), 234–247.
Cope, E. D. (1887). Zittel’s manual of paleontology. The American Naturalist, 21(11), 1014–1019.
Cope, E. D. (1889). On inheritance and evolution. American Naturalist, 23(276), 1058–1071.
Cope, E. D. (1896). The primary factors of organic evolution. The Open Court Publishing Company.
Corsi, P. (2011). Jean-baptiste lamarck: From myth to history. In S. B. Gissis & E. Jablonka (Eds.), Transformations of Lamarckism: From subtle fluids to molecular biology (pp. 9–18). MIT Press.
Corsi, P., Gayon, J., Gohau, G., & Tirard, S. (Eds.). (2006). Lamarck, philosophe de la nature. Presses Universitaires de France.
Dall, W. H. (1916). Biographical memoir of Theodore Nicholas Gill 1837–1914. National Academy of Sciences Biographical Memoirs, 8, 313–343.
Darwin Correspondence Project (2021). https://www.darwincorrespondenceproject.ac.uk/letter/DCP-LETT-684.xml
Darwin, C. (1859). On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. John Murray.
Davidson, J. P. (1997). The bone sharp: The life of Edward Drinker Cope. Academy of Natural Sciences.
Davis, H. W. C., & Weaver, J. R. H. (Eds.). (1927). Dictionary of national biography 1912–1921. Oxford University Press.
de Candolle, A. P. (1813). Théorie élémentaire de la botanique; ou, exposition des prinicpes de la classification naturelle et de l’art de décrire et d’étudier les végétaux. Déterville.
Di Gregorio, M. A. (1984). T. H. Huxley’s place in natural science. Yale University Press.
Dohrn, A. (1875). Der Ursprung der Wirbelthiere und das Princip des Functionswechsels: genealogische Skizzen. Engelmann.
Ebach, M., Morrone, J. J., & Williams, D. M. (2008). A new cladistics of cladists. Biology and Philosophy, 23(1), 153–156.
Eldredge, N., & Cracraft, J. (1980). Phylogenetic patterns and the evolutionary process. Columbia University Press.
Gill, T. (1864). Synopsis of the pleuronectoids of the eastern coast of North America. Proceedings of the Academy of Natural History of Philadelphia, 16, 214–220.
Gill, T. (1870). On the relations of the orders of mammals. Proceedings of the American Association for the Advancement of Science, 19, 267–270.
Gill, T. (1872). Arrangement of the families of fishes, or class Pisces, Marsipobranchii, and Leptocardii, Vol. 11. Smithsonian Institution.
Gill, T. (1872). On the characteristics of the primary groups of the class of mammals. In J. Lovering (Ed.), Proceedings of the American Association for the Advancement of Science, Vol. 20, (pp. 284–306). John Wilson and Sons.
Gill, T. (1873). On the limits of the class of fishes. American Naturalist, 7, 71–79.
Gill, T. (1881). Günther’s ichthyology. Nation, 33(841), 120–122.
Gill, T. (1893). Families and subfamilies of fishes. Memoirs of the Natural Academy of Sciences, 6, 125–138.
Gill, T. (1897). Edward Drinker Cope, naturalist—a chapter in the history of science. Proceedings of the American Association for the Advancement of Science, 41, 1–30.
Gill, T. (1905). A new introduction to the study of fishes. Science, 21(539), 653–661.
Girard, C. (1857). Researches upon the Cyprinoid fishes inhabiting the fresh waters of the United States of America, west of the Mississippi Valley, from specimens in the Museum of the Smithsonian Institution. Proceedings of the Academy of Natural Sciences of Philadelphia, 8, 165–213.
Gould, S. J. (2002). The structure of evolutionary theory. Belknap Press.
Greenwood, P. H., Rosen, D. E., Weitzman, S. H., & Myers, G. S. (1966). Phyletic studies of Teleostean fishes, with a provisional classification of living forms. Bulletin of the American Museum of Natural History, 131(4), 339–455.
Gregory, W. K. (1907). The orders of Teleostomous fishes. Annals of the New York Academy of Sciences, 17(3), 437–508.
Günther, A. C. (1866). Memoir on the fishes of the states of Central America collected by messrs. Salvin Godman and Capt. Dow. Proceedings of the Zoological Society of London, 600–604.
Günther, A. C. (1864). The record of zoological literature. Pisces, 82(1), 144.
Günther, A. C. (1867). The record of zoological literature. Pisces, 4, 157.
Günther, A. C. (1868). The record of zoological literature. Pisces, 5, 139.
Günther, A. C. (1869). An account of the fishes of the states of Central America, based on collections made by Capt. J. M. Dow, F. Godman, Esq., and O. Salvin, Esq. The Transaction of the Zoological Society of London, 6(7), 377–494.
Günther, A. C. (1872). Description of Ceratodus, a genus of Ganoid fishes, recently discovered in rivers of Queensland, Australia. Philosophical Transactions of the Royal Society of London, 161, 511–571.
Günther, A. C. (1873). Zoological record. Pisces, 8, 89–112.
Günther, A. C. (1880). An introduction to the study of fishes. Adams & Black.
Günther, A. C. L. G. (1882). Review of an introduction to the study of fishes. Quarterly Review, 153, 241–266.
Gunther, A. E. (1975). A century of zoology at the British Museum through the lives of two keepers, 1815–1914. Dawsons of Pall Mall.
Günther, A. C. (1981). Catalogue of the fishes in the British Museum. A.J. Reprints Co.
Hennig, W. (1966). Phylogenetic systematics. University of Illinois Press.
Huelsenbeck, J. P., Swofford, D. L., Cunningham, C. W., Bull, J. J., & Waddell, P. J. (1994). Is character weighting a panacea for the problem of data heterogeneity in phylogenetic analysis? Systematic Biology, 43, 288–291.
Hull, D. (1965). The effect of essentialism on taxonomy—two thousand years of stasis. British Journal for the Philosophy of Science, 16(61), 1–18.
Huxley, T. H. (1871). Principles and methods of palaeontology. In Annual report of the board of regents of the Smithsonian Institution, (pp. 363–388). Government Printing Office.
Huxley, T. H. (1863). On our knowledge of the causes of the phenomena of organic nature. Robert Hardwicke.
J. L., W. M., & S. V. (1915). Obituary notices of fellows deceased. Proceedings Linnean Society London, 88(608), i–xxxviii.
Jordan, D. S. (1931). Theodore Nicholas Gill. In Dictionary of American biography, vol. VII, (pp. 285–286). Charles Scribner’s Sons.
Jordan, D. S. (1877). Contributions to ichthology: Based primarily on the collections of the United States National Museum: Review of Rafinesque’s memoirs on North American fishes. Bulleton of the United States National Museum, 1(9), 1–53.
Jordan, D. S. (1905). A guide to the study of fishes, Vol. 1. Henry Holt and Company.
Jordan, D. S. (1922). The days of a man. World Book Co.
Kruseman, W. (1950). Gradation of language in biological systematics. Synthese, 8(3), 175–181.
Levit, G. S., & Olsson, L. (2006). “Evolution on rails’’: Mechanisms and levels of orthogenesis. Annals for the History and Philosophy of Biology, 11, 97–136.
Leviton, A. E., & Aldrich, M. L. (1981). William O. Ayres, pioneer ichthyologist in California. In American Society of Ichthyologists and Herpetologists 61st Annual Meeting.
Manias, C. (2017). Progress in life’s history: Linking Darwinism and palaeontology in Britain, 1860–1914. Studies in History and Philosophy of Science Part C: Studies in History and Philosophy of Biological and Biomedical Sciences, 66, 18–26.
Matthew, H. C. G., Harrison, B., & Long, R. J. (Eds.). (2004). Oxford dictionary of national biography (Vol. 24). Oxford University Press.
Mayr, E. (1982). The growth of biological thought: Diversity, evolution, and inheritance. Harvard University Press.
Mayr, E. (1998). The role of systematics in the evolutionary synthesis. In E. Mayr & W. B. Provine (Eds.), The evolutionary synthesis: Perspectives on the unification of biology (pp. 123–136). Harvard University Press.
Mill, J. S. (1843). A system of logic, ratiocinative and inductive: Being a connected view of the principles of evidence and the methods of scientific investigation, Vol. 1. John W. Parker.
Müller, J. (1845). Über den Bau und die Grenzen der Ganoiden und über das natürliche System der Fische. Archiv für Naturgeschichte. Gedruckt in der Druckerei der Königlichen Akademie der Wissenschaften
Müller, J. (1844). Über den Bau und die Grenzen der Ganoiden und über das natüraliche System der Fische. Berlin Akademie der Wissenchaften.
Müller, J. (1846). Further remarks on the structure of the Ganoidei. Scientific Memoirs, 4, 543–558.
Müller, J. (1846). On the structure and characters of the Ganoidei, and on the natural classification of fish. Scientific Memoirs, 4, 499–558.
Novick, A. (2016). On the origins of the Quinarian system of classification. Journal of the History of Biology, 49(1), 95–133.
Nyhart, L. K. (1999). Life’s splendid drama: Evolutionary biology and the reconstruction of life’s ancestry, 1860–1940 by Peter J. Bowler. Isis, 90, 607.
Osborn, H. F. (1930). Biographical memoir of Edward Drinker Cope 1840–1897. National Academy of Sciences Biographical Memoirs, 13(3), 129–317.
Osborn, H. F., Cope, E. D., & Warren, H. A. (1931). Cope: Master naturalist. The life and letters of Edward Drinker Cope, with a bibliography of his writings classified by subject. Princeton University Press.
Ospovat, D. (1995). The development of Darwin’s Theory: Natural history, natural theology, and natural selection. Cambridge University Press.
Panchen, A. L. (1992). Classification, evolution, and the nature of biology. Cambridge University Press.
Patterson, C. (1977). The contribution of paleontology to Teleostean phylogeny. In M. K. Hecht, P. C. Goody, & B. M. Hecht (Eds.), Major patterns in vertebrate evolution (pp. 579–635). Plenum Press.
Quinn, A. (2016). Phylogenetic inference to the best explanation and the bad lot argument. Synthese, 193(9), 3025–3039.
Quinn, A. (2017). Charles Girard: Relationships and representation in nineteenth century systematics. Journal of the History of Biology, 50(3), 609–643.
Rieppel, O. (2010). The series, the network, and the tree: Changing metaphors of order in nature. Biology and Philosophy, 25(4), 475–496.
Rieppel, O. (2016). Phylogenetic systematics: Haeckel to Hennig. CRC Press.
Rieppel, O., & Kearney, M. (2007). The poverty of taxonomic characters. Biology and Philosophy, 22(1), 95–113.
Sepkoski, D. (2009). The emergence of paleobiology. In D. Sepkoski & M. Ruse (Eds.), The paleobiological revolution: Essays on the growth of modern paleontology (pp. 15–42). University of Chicago Press.
Sepkoski, D. (2012). Rereading the fossil record: The growth of paleobiology as an evolutionary discipline. University of Chicago Press.
Shanahan, T. (2011). Phylogenetic inertia and Darwin’s higher law. Studies in History and Philosophy of the Biological and Biomedical Sciences, 42(1), 60–68.
Shor, E. N. (1974). The fossil Feud between Cope and OC Marsh (between). Exposition Press.
Simpson, G. G. (1944). Tempo and mode in evolution. Columbia University Press.
Smith, G., Davis, H. W. C., & Weaver, J. R. H. (1927). The dictionary of national biography, 1912–1921. Oxford University Press.
Sober, E. (1988). Reconstructing the past: Parsimony, evolution, and inference (1st ed.). MIT Press.
Stearn, W. T. (1981). The Natural History Museum at South Kensington: A history of the British Museum (Natural History) 1753–1980. William Heinemann Ltd.
Ulett, M. A. (2014). Making the case for orthogenesis: The popularization of definitely directed evolution (1890–1926). Studies in History and Philosophy of Science Part C: Studies in History and Philosophy of Biological and Biomedical Sciences, 45, 124–132.
Whewell, W. (1837). History of the inductive sciences, Vol. 3. John W. Parker.
Whewell, W. (1840). Philosophy of the inductive sciences, founded upon their history, Vol. 1. John W. Parker.
Whewell, W. (1847). The philosophy of the inductive sciences: Founded upon their history, 2nd ed., Vol. 1. John W. Parker.
Whitehead, P., & Talwar, P. (1976). Francis Day (1829–1889) and his collections of Indian fishes. Bulletin of the British Museum (Natural History), 5, 1–189.
Wiley, E. O., Siegel-Causey, D., Brooks, D., & Funk, V. (1991). The compleat cladist. The University of Kansas Museum of Natural History.
Wilkins, J. (2009). Species: A history of the idea. University of California Press.
Winsor, M. P. (2021). “I would sooner die than give up”: Huxley and Darwin’s deep disagreement. History and Philosophy of the Life Sciences, 43(2), 53. https://doi.org/10.1007/s40656-021-00409-3
Winsor, M. P. (1976). Starfish, jellyfish, and the order of life: Issues in nineteenth century science. Yale University Press.
Winsor, M. P. (1991). Reading the shape of nature: Comparative zoology at the Agassiz museum. University of Chicago Press.
Winsor, M. P. (2003). Non-essentialist methods in pre-Darwinian taxonomy. Biology and Philosophy, 18, 387–400.
Winsor, M. P. (2006). The creation of the essentialism story. History and Philosophy of the Life Sciences, 28, 149–174.
Winsor, M. P. (2015). Considering affinity: An ethereal conversation (part two of three). Endeavour, 39(2), 116–126.
Woodman, N. (2016). Pranked by Audobon: Constantine S. Rafinesque’s description of John James Audobon’s imaginary Kentucky mammals. Archives of Natural History, 43(1), 95–108.
Acknowledgements
We thank William Kimler, Ted Pietsch, and Gareth Nelson for encouragement and comments on an early version of this paper, and Polly Winsor for encouragement and discussion. We thank two anonymous reviewers for thorough and extremely helpful comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jackson, J.R., Quinn, A. Post-Darwinian fish classifications: theories and methodologies of Günther, Cope, and Gill. HPLS 45, 4 (2023). https://doi.org/10.1007/s40656-022-00556-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40656-022-00556-1