Summary
During the last decade, the field of T cell immunology started to confuse the scientific community. More and more subtypes of T helper cells and their counterparts in the innate immune system are described. We are just at the beginning to understand which specific function the distinct subtypes fulfill. Th22 cells are terminally differentiated and very specialized T helper cells characterized by the secretion of their signature cytokine IL-22 and lack of IL-4, IL-17 and IFN-γ. The main function of Th22 cells is to protect epithelial barrier organs such as skin and lung, but also to modulate inflamed and injured tissue. This review summarizes our current knowledge on Th22 cells and their function in allergic disease.
Cite this as Eyerich K, Eyerich S. Th22 cells in allergic disease. Allergo J Int 2015;24:1–7 DOI: 10.1007/s40629-015-0039-3
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Definition of lymphocytes: a more and more confusing field
Since the eighties, our knowledge on T helper cells increased tremendously. The ongoing description of new T helper (Th) cells and innate lymphocyte populations (ILC), however, further increase the confusion in the field as commonly used classification schemes seem to be antiquated, redundant or insufficient. Therefore, it is reasonable to elaborate on the definition of T helper cells before discussing the function of Th22 cells in allergic disease.
The first classification scheme was suggested by Mosmann and Coffmann at the end of the eighties. Here, two T helper subtypes were distinguished according to their expressed signature cytokines – Th1 cells secreting interferon (IFN)-γ and Th2 secreting interleukin (IL)-4 [1]. Already at this time it was assumed that an additional population of T cells should exist that is able to limit the inflammatory effects of Th1 and Th2 cells in tissue. These cells were originally named T suppressor cells, however, only after description of the transcription factor Fox p 3 and the more attractive naming as regulatory T cells (Treg), these cells were commonly accepted.
Some years after the discovery of IL-17 in 1993 [2], and the description of Th1 and Th2 cells co-secreting IL-17 [3, 4], another T cell subtype specifically secreting IL-17 has been identified. Several studies elucidated that these cells represent an additional lineage in the family of T helper cells and were named according to their signature cytokine as Th17 cells [5]. Being qualified as new lineage goes along with the differentiation of naive T cells into effector T cells in presence of a specific cytokine environment (in case of Th17 cell TGF-β, IL-1β and IL-6) and the expression of a characteristic transcription factor (in case of Th17 cells RORC; ROR: “RAR-related orphan receptor“). IL-22 was described in 2000 [6] and first regarded a Th1 cytokine [5]. However, with growing knowledge on Th17 cells, IL-22 was consequently associated with this T helper subtype.
The phenomenon that some T helper cells secrete more than one signature cytokine is called plasticity. By generation of this concept, the problem in the historical grouping of T helper cells according to the expression of single cytokines was solved. Nowadays, it is widely accepted that Th1, Th2 and Th17 cells exist that co-secrete IL-22. In addition, a subgroup of T helper cells did not express the signature cytokines IFN-γ, IL-4 and IL-17, but IL-22 solely. These cells were described in 2009 for the first time of three research groups and named Th22 cells [7, 8, 9].
Meanwhile, some alternative suggestions for classification of lymphocytes appeared on the scene that take the function (e. g., follicular T helper cells) or chemokine receptor profile into account. This format of this review is not suited to discuss all the existing classification schemes. Therefore, just a summarized personal appreciation: Until now, no concept exists that classifies lymphocytes into subtypes convincingly. Furthermore, our knowledge on lymphocytes from innate immunity (ILC) – lymphocytes without a functional T cell receptor and not belonging to adaptive immunity – is steadily growing. Due to lacking and convincing concepts, also ILC are classified according to their cytokine profile [10]. But we are positive that research in the field will deliver new and reliable concepts. Tab. 1 gives and overview on known T cell subtypes secreting IL-22.
Starting with this pessimistic attitude, it is questionable if Th22 cells can be really defined as separate lineage. Two reasons argue for it: Th22 cells represent the extreme of a spectrum of IL-22 secreting cells and, importantly, Th22 cells are characterized by a combination of cytokines that in their totality have essential function in tissue. This function will be elucidated in more detail in the next section.
Definition and phenotype of Th22 cells
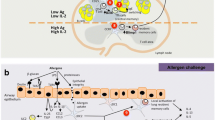
Th22 cells differentiate, like other T helper cells, from naive precursor cells. The specific microenvironment for generation of Th22 is composed of tumornecrosis factor (TNF)-β and IL-6 [8] (Fig. 1). Also skin dendritic cells have been shown to play an essential role in this differentiation process [8, 11]. Once differentiated, the phenotype of Th22 cells remains stable over many weeks in culture and is not skewable into another phenotype [7]. This argues for Th22 cells being terminally differentiated effector cells. The aryl-hydrocarbon-receptor (AHR) was suggested as transcription factor [9], however, it is not exclusively expressed in Th22 cells and does not explain all pattern of the Th22 phenotype. Th22 cells are characterized by expression of the chemokine receptors CCR4 and CCR4 and more characteristic CCR10 [8]. Chemokines that bind to these receptors are strongly expressed in the skin. This explains the abundance of Th22 cells in tissue and their low numbers in circulation. In the skin, Th22 cells are guided into the upper parts of the epidermis due to production of the CCR10 ligand CCL27 by keratinocytes [7, 12].
To evaluate the function of a T helper cell in tissue with a special focus on allergic disease, some more parameters next to migratory capacity or homing have to be defined: the expression of functional surface proteins, secreted mediators and the mechanisms of activation with respect to their antigen-specificity.
Next to the above mentioned chemokine receptors, Th22 cells express all typical markers for T helper cells such as CD3 and CD4 [7]. More characteristic is the high expression of platelet-derived growth factor (PDGF) receptors [7]. The functional relevance of this receptor is not known, yet. Molecules involved in induction of apoptosis such as Fas and TRAIL are weakly expressed in Th22 cells [13] and consequently, the closely related CD8+ T22 cells (Tc22 cells) have a lower capacity to induce apoptosis compared to their IL-22 negative counterparts [14, 15].
As already mentioned, Th22 cells do not secrete signature cytokines of Th2-, Th2-, or Th17 cells. However, Th22 cells co-secrete little amounts of IL-13 and TNF-β [7]. On gene expression level, Th22 have been shown to express a bunch of fibroblast growth factors (FGF) [7]. Until now, it has not been investigated if FGF are secreted in functionally relevant amounts.
IL-22: a many-sided cytokine
The overall phenotype of Th22 cells points to a per se protective role in tissue, in particular the skin. This is represented especially by the function of IL-22 itself (Fig. 2). IL-22 binds to a heterodimeric receptor consisting of the IL-10Rβ and the IL-22RA chain. While the IL-22 receptor (IL-22R) is strongly and abundantly expressed on epithelial cells, immune cells lack IL-22R [16]. Therefore, Th22 cells form an important link between adaptive immunity and barrier organs leading to their functional attribute being “tissue signaling leukocytes” [5]. By binding to its receptor, IL-22 induces proliferation and migration of the target cell and simultaneously inhibits differentiation and reactivity to apoptosis [17, 18, 19]. A combination of these effects builds the basis for efficient wound healing reactions. Indeed, it could have been shown that Th22 cells are important players in wound healing reactions [7, 20]. This per se positive attribute can turn pathologic if regulatory mechanisms are missing. Too much of IL-22 effects can lead to a psoriatic phenotype with over activated metabolism in keratinocytes resulting in acanthosis and hyperkeratosis [21]. In addition, under some circumstances it might be not useful to protect an epithelial cell from apoptosis, especially in case of (pre)-malignancies and intracellular infections. In line with this, it has been reported that Th22 cells have negative effects on gastrointestinal tumors [22, 23]. In tumors, the protective function of IL-22 is additionally reinforced through a direct antagonism with IFN-γ, a known inducer of apoptosis and tumor senescence [14, 24]. Therefore exact regulation of IL-22 is of outermost importance and happens mainly via a soluble antagonist, the IL-22 binding protein (IL-22BP) [25].
Function of IL-22 in dependence of the local microenvironment. Per se IL-22 induces proliferation and migration in epithelial cells and inhibits differentiation and reactivity to apoptosis (middle); these effect are inhibited by IFN-γ (left). Together with TNF-β and IL-17 strong innate immune reactions in epithelial cells are induced (right).
Besides its protective effects on epithelial cells, IL-22 plays an important role in the induction of innate immune responses at barrier organs. A binding of IL-22 to the IL-22R weakly induces the expression of anti-microbial peptides such as S100 proteins and defensins (e.g. HBD-2) [7, 16]. More impressive, however, is the amplifying impact on cytokines that induce innate immunity in epithelial cells. Here, IL-22 clearly synergizes with IL-17 [26] and TNF-α [27] with functional relevance in the defense against pathogens for instance Candida albicans. Patients suffering from the rare disease chronic mucocutaneous candidiasis (CMC) represent a dramatic example. Due to different reasons, CMC patients are not able to express IL-17 and IL-22 leading to severe and persistent infections of skin and mucosa with Candida species [28, 29].
One recommendation at the end of the section. It is not possible to judge on the overall function of Th22 cells without careful consideration of the local microenvironment.
Th22 cells in context of allergic diseases
According to the exclusive expression of the IL-22R in tissue, Th22 cells do interact directly with epithelial cells but do not influence the memory of adaptive immunity in response to allergens. Another essential argument against a direct contribution of Th22 cells to allergic disease is the observation that neither Th17 nor Th22 cells show an allergen reactivity in cell culture models of atopic eczema (AE) or allergic contact dermatitis (ACD) for the relevant allergen or hapten [13, 30]. In fact, both T helper subtypes specifically recognize extracellular microorganisms such as Staphylococci and Candida species or auto-antigens that are presented via CD1 molecules [31, 32, 33]. Fitting to this fact, IL-17 and IL-22 are induced in T cells by enterotoxins of staphylococci [30, 34].
Even if Th22 do not primarily induce immune reactions against allergens or haptens, they are relevant for allergic reactions in the skin in an indirect way. Most patients with AE are colonized permanently with Staphylococcus aureus [35, 36]. The number of IL22 producing cells in AE skin is quite high, also in comparison with other inflammatory skin diseases such as psoriasis. Although many more IL-22 producing Th17 cells are present in psoriasis lesions, the number of IL-22 secreting cells in total is the same in both diseases [37, 38]. This is due to a considerably elevated proportion of Th22 cells in AE [39]. Th22 or Tc22 seem to be of relevance in the chronic state of AE [40]. Actually, for Tc22 a correlation with severity of AE was shown [39]. Therefore, it is likely that Th22 cells act as allergen-independent modulators in inflamed tissue and are involved in remodeling processes during chronification. Similar results have been obtained in models of asthma bronchiale. Here, IL-22 is mediating remodeling in tissue and contributes to transition of disease from active into the chronic state [14, 41]. However, in the early phase of asthmatic inflammation, Th22 seem to have rather positive and tissue-protective effects [14, 42].
Data on a potential role of Th22 cells in allergic Typ-1 reactions such as anaphylaxis, mastocytosis or urticaria do hardly exist. Interestingly, it is speculated about an interaction of IL-22+ cells and mast cells in tissue and that this close proximity of both cell types leads to induction of Th22 cells via mast cells [43]. In conclusion, the demand on motivated allergy researchers in the field of Th22 cells is not covered, yet!
Abbreviations
- ACD:
-
Allergic contact dermatitis
- AE:
-
Atopic eczema
- AHR:
-
Aryl-hydrocarbon-receptor
- CMC:
-
Chronic mucocutaneous candidiasis
- FGF:
-
Fibroblast growth factor
- IFN:
-
Interferon
- IL:
-
Interleukin
- ILC:
-
Innate lymhhoid cell
- Lti:
-
Lymphoid-tissue inducer
- MMP:
-
Matrix metalloproteinase
- NK:
-
Natural killer cell
- PDGF:
-
Platelet-derived growth factor
- ROR:
-
RAR-related orphan receptor
- TCR:
-
T-cell-receptor
- TGF:
-
Transforming growth factor
- Th:
-
T helper cell
- TLR:
-
Toll-like-receptor
- TNF:
-
Tumor necrosis factor
- Treg:
-
Regulatory T cell
Literatur
Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol 1989;7:145–73
Rouvier E, Luciani MF, Mattei MG, Denizot F, Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J Immunol 1993;150:5445–56
Albanesi C, Scarponi C, Cavani A, Federici M, Nasorri F, Girolomoni G. Interleukin-17 is produced by both Th1 and Th2 lymphocytes, and modulates interferon-gammaand interleukin-4-induced activation of human keratinocytes. J Invest Dermatol 2000;115:81–7
Albanesi C, Cavani A, Girolomoni G. IL-17 is produced by nickel-specific T lymphocytes and regulates ICAM-1 expression and chemokine production in human keratinocytes: synergistic or antagonist effects with IFN-gamma and TNF-alpha. J Immunol 1999;162:494–502
Eyerich S, Eyerich K, Cavani A, Schmidt-Weber C. IL-17 and IL-22: siblings, not twins. Trends Immunol 2010;31:354–61
Dumoutier L, Van Roost E, Ameye G, Michaux L, Renauld JC. IL-TIF/IL-22: genomic organization and mapping of the human and mouse genes. Genes Immun 2000;1:488–94
Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest 2009;119:3573–85
Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol 2009;10:857–63
Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol 2009;10:864–71
Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G et al. Innate lymphoid cells - a proposal for uniform nomenclature. Nat Rev Immunol 2013;13:145–9y
Fujita H, Nograles KE, Kikuchi T, Gonzalez J, Carucci JA, Krueger JG. Human Langerhans cells induce distinct IL- 22-producing CD4+ T cells lacking IL-17 production. Proc Natl Acad Sci U S A 2009;106:21795–800
Riis JL, Johansen C, Vestergaard C, Bech R, Kragballe K, Iversen L. Kinetics and differential expression of the skinrelated chemokines CCL27 and CCL17 in psoriasis, atopic dermatitis and allergic contact dermatitis. Exp Dermatol 2011;20:789–94
Pennino D, Eyerich K, Scarponi C, Carbone T, Eyerich S, Nasorri F et al. IL-17 amplifies human contact hypersensitivity by licensing hapten nonspecific Th1 cells to kill autologous keratinocytes. J Immunol 2010;184:4880–8
Pennino D, Bhavsar PK, Effner R, Avitabile S, Venn P, Quaranta M et al. IL-22 suppresses IFN-gamma-mediated lung inflammation in asthmatic patients. J Allergy Clin Immunol 2013;131:562–70
Zhang S, Fujita H, Mitsui H, Yanofsky VR, Fuentes-Duculan J, Pettersen JS et al. Increased Tc22 and Treg/CD8 ratio contribute to aggressive growth of transplant associated squamous cell carcinoma. PLoS One 2013;8:e62154
Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity 2004;21:241–54
Nograles KE, Zaba LC, Guttman-Yassky E, Fuentes-Duculan J, Suarez-Farinas M, Cardinale I et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol 2008;159:1092–102
Wolk K, Witte E, Wallace E, Docke WD, Kunz S, Asadullah K et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol 2006;36:1309–23
Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol 2005;174: 3695–702
Rendon JL, Choudhry MA. Th17 cells: critical mediators of host responses to burn injury and sepsis. J Leukoc Biol 2012;92:529–38
Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 2007;445:648–51
Zhuang Y, Peng LS, Zhao YL, Shi Y, Mao XH, Guo G et al. Increased intratumoral IL-22-producing CD4(+) T cells and Th22 cells correlate with gastric cancer progression and predict poor patient survival. Cancer Immunol Immunother 2012;61:1965–75
Xu X, Tang Y, Guo S, Zhang Y, Tian Y, Ni B et al. Increased intratumoral interleukin 22 levels and frequencies of interleukin 22-producing CD4+ T cells correlate with pancreatic cancer progression. Pancreas 2014;43:470–7
Braumuller H, Wieder T, Brenner E, Assmann S, Hahn M, Alkhaled M et al. T-helper-1-cell cytokines drive cancer into senescence. Nature 2013;494:361–5
Xu W, Presnell SR, Parrish-Novak J, Kindsvogel W, Jaspers S, Chen Z et al. A soluble class II cytokine receptor, IL- 22RA2, is a naturally occurring IL-22 antagonist. Proc Natl Acad Sci U S A 2001;98:9511–6
Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med 2006;203:2271–9
Eyerich S, Wagener J, Wenzel V, Scarponi C, Pennino D, Albanesi C et al. IL-22 and TNF-alpha represent a key cytokine combination for epidermal integrity during infection with Candida albicans. Eur J Immunol 2011;41:1894–901
Eyerich K, Eyerich S, Hiller J, Behrendt H, Traidl-Hoffmann C. Chronic mucocutaneous candidiasis, from bench to bedside. Eur J Dermatol 2010;20:260–5
Eyerich K, Foerster S, Rombold S, Seidl HP, Behrendt H, Hofmann H et al. Patients with chronic mucocutaneous candidiasis exhibit reduced production of Th17-associated cytokines IL-17 and IL-22. J Invest Dermatol 2008;128:2640–5
Eyerich K, Pennino D, Scarponi C, Foerster S, Nasorri F, Behrendt H et al. IL-17 in atopic eczema: linking allergen- specific adaptive and microbial-triggered innate immune response. J Allergy Clin Immunol 2009;123: 59–66.e4
Geiger R, Duhen T, Lanzavecchia A, Sallusto F. Human naive and memory CD4+ T cell repertoires specific for naturally processed antigens analyzed using libraries of amplified T cells. J Exp Med 2009;206:1525–34
Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, Gattorno M et al. Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL- 1beta. Nature 2012;484:514–8
Jong A de, Pena-Cruz V, Cheng TY, Clark RA, Van Rhijn I, Moody DB. CD1a-autoreactive T cells are a normal component of the human alphabeta T cell repertoire. Nat Immunol 2010;11:1102–9
Niebuhr M, Scharonow H, Gathmann M, Mamerow D, Werfel T. Staphylococcal exotoxins are strong inducers of IL-22: a potential role in atopic dermatitis. J Allergy Clin Immunol 2010;126:1176–83.e4
Eyerich K, Novak N. Immunology of atopic eczema: overcoming the Th1/Th2 paradigm. Allergy 2013;68:974–82
Bieber T. Atopic dermatitis. N Engl J Med 2008;358:1483–94
Eyerich S, Onken AT, Weidinger S, Franke A, Nasorri F, Pennino D et al. Mutual antagonism of T cells causing psoriasis and atopic eczema. N Engl J Med 2011;365:231–8
Quaranta M, Knapp B, Garzorz N, Mattii M, Pullabhatla V, Pennino D et al. Intra-individual genome expression analysis reveals a specific molecular signature of psoriasis and eczema. Sci Transl Med 2014;6:244ra90
Nograles KE, Zaba LC, Shemer A, Fuentes-Duculan J, Cardinale I, Kikuchi T et al. IL-22-producing „T22“ T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J Allergy Clin Immunol 2009;123:1244–52.e2
Gittler JK, Shemer A, Suarez-Farinas M, Fuentes-Duculan J, Gulewicz KJ, Wang CQ et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol 2012;130:1344–54
Johnson JR, Nishioka M, Chakir J, Risse PA, Almaghlouth I, Bazarbashi AN et al. IL-22 contributes to TGF-beta1-mediated epithelial-mesenchymal transition in asthmatic bronchial epithelial cells. Respir Res 2013;14:118
Nakagome K, Imamura M, Kawahata K, Harada H, Okunishi K, Matsumoto T et al. High expression of IL-22 suppresses antigen-induced immune responses and eosinophilic airway inflammation via an IL-10-associated mechanism. J Immunol 2011;187:5077–89
Gaudenzio N, Laurent C, Valitutti S, Espinosa E. Human mast cells drive memory CD4+ T cells toward an inflammatory IL-22+ phenotype. J Allergy Clin Immunol 2013;131:1400–7.e11
Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol 2007;8:639–46
Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factorbeta and induction of the nuclear receptor RORgammat. Nat Immunol 2008;9:641–9
Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol 2007;8:942–9
Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M et al. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature 2008;454:350–2
Aggarwal S, Ghilardi N, Xie MH, Sauvage FJ de, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem 2003;278:1910–4
Cosmi L, De Palma R, Santarlasci V, Maggi L, Capone M, Frosali F et al. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J Exp Med 2008;205:1903–16
Kleinschek MA, Boniface K, Sadekova S, Grein J, Murphy EE, Turner SP et al. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J Exp Med 2009;206:525–34
Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest 2009;119:3573–85
Shin HC, Benbernou N, Esnault S, Guenounou M. Expression of IL-17 in human memory CD45RO+ T lymphocytes and its regulation by protein kinase A pathway. Cytokine 1999;11:257–66
Liu SJ, Tsai JP, Shen CR, Sher YP, Hsieh CL, Yeh YC et al. Induction of a distinct CD8 Tnc17 subset by transforming growth factor-beta and interleukin-6. J Leukoc Biol 2007;82:354–60
Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med 2006;203:1685–91
Wu HY, Quintana FJ, Weiner HL. Nasal anti-CD3 antibody ameliorates lupus by inducing an IL-10-secreting CD4+ CD25- LAP+ regulatory T cell and is associated with down-regulation of IL-17+ CD4+ ICOS+ CXCR5+ follicular helper T cells. J Immunol 2008;181:6038–50
Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho IC, Sharpe AH et al. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat Immunol 2009;10:167–75
Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD et al. Bcl6 mediates the development of T follicular helper cells. Science 2009;325:1001–5
Cupedo T, Crellin NK, Papazian N, Rombouts EJ, Weijer K, Grogan JL et al. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat Immunol 2009;10:66–74
Rachitskaya AV, Hansen AM, Horai R, Li Z, Villasmil R, Luger D et al. Cutting edge: NKT cells constitutively express IL-23 receptor and RORgammat and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J Immunol 2008;180:5167–71
Peng MY, Wang ZH, Yao CY, Jiang LN, Jin QL, Wang J et al. Interleukin 17-producing gamma delta T cells increased in patients with active pulmonary tuberculosis. Cell Mol Immunol 2008;5:203–8
Ribot JC, Barros A de, Pang DJ, Neves JF, Peperzak V, Roberts SJ et al. CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nat Immunol 2009;10:427–36
6Shibata K, Yamada H, Nakamura R, Sun X, Itsumi M, Yoshikai Y. Identification of CD25+ gamma delta T cells as fetal thymus-derived naturally occurring IL-17 producers. J Immunol 2008;181:5940–7
Walker JA, Barlow JL, McKenzie AN. Innate lymphoid cells - how did we miss them? Nat Rev Immunol 2013;13:75–87
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Eyerich, K., Eyerich, S. Th22 cells in allergic disease. Allergo J Int 24, 1–7 (2015). https://doi.org/10.1007/s40629-015-0039-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40629-015-0039-3