Abstract
The primary concern of this research was to investigate how the contrasting light environments of a native forest (NF) and banana intercrop (BI) affect seed quality of Euterpe edulis. The results from our study also led us to investigate a possible light-induced component of seed dormancy in this species. Mature fruit bunches were collected from both environments and evaluated for seed germination and viability of non-germinated seeds, seed mass, water content and vigor tests (germination rate index and seedling mass). Total phenolics in seed coats and the effects of seed coat extracts on germination of lettuce seeds were also evaluated to investigate a possible cause for the lack of germination of many viable seeds from BI. Results showed that seed vigor did not differ between the environments, but germination of seeds from BI was lower, despite viability of most of the non-germinated ones. Seed coats formed in BI had a greater concentration of phenolics than those that developed in NF. In contrast to seed coat extracts from NF, extracts prepared from BI seeds had a negative, non-osmotic effect on the germination of lettuce seeds. Our results indicate that the more open environment provided by BI does not affect seed vigor, but may add a light-induced component to seed dormancy of the species, which can make seeds produced in more open environments less suitable to generate new plants. Such finding might be particularly useful for the species management in agroforestry systems and for its conservation in the rainforest.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Maternal environmental effects refer to the particular phenomenon in which the external ecological environment of the maternal parent influences the phenotype of its progeny (Donohue 2009). Many aspects of the mother plant environment (e.g., light, photoperiod, temperature, and nutrient and water availabilities) may substantially affect seed quality and dormancy. Seed coat (maternal tissue) and seed provisioning commonly exhibit phenotypic plasticity in response to environmental conditions during seed development. Mechanical and chemical characteristics of the seed coats are important for determining seed dormancy, and both the thickness and color of the seed coats are known to be environmentally responsive in many species (Roach and Wulff 1987; Baskin and Baskin 1998; Donohue 2009). The environment experienced by the mother plant during seed development may also substantially affect nutritious provisions for seeds, as well as hormones, proteins and transcripts, which will affect seed metabolism and gene expression during seed development and even after seed dispersal (Donohue 2009). Seed mass, for example, is an important component of seed quality, as large seeds tend to produce vigorous seedlings (Ambika et al. 2014). Reductions in the availability of light, water and nutrients have all been shown to reduce seed mass and quality, by reducing photosynthesis of the mother plant and thus resource provisions for the seeds (Sawhney and Naylor 1982; Parrish and Bazzaz 1985; Bewley et al. 2013; He et al. 2014).
Here we study the effects of the environment where the mother plant grows on key components of seed quality (seed germination capacity and vigor) of Euterpe edulis Martius (Arecaceae), a native and endemic palm species from the Brazilian Atlantic Rainforest. It is highly relevant for its economic value (both for fruit and palm heart marketing) and for its ecological function (Reis et al. 1993; Fadini et al. 2009). The fruits of this palm tree feed many species of mammals and birds, which act as seed dispersers (Campos et al. 2012), and more recently the fruit pulp has been harvested and commercialized (Favreto et al. 2010; Trevisan et al. 2015). The palm heart extraction leads to the death of the individual, and due to its overharvesting this species is currently listed in the Official List of the Brazilian Threatened Flora Species (Brasil 2008). Euterpe edulis is often cultivated in intercrops, or agroforestry systems, as an alternative to alleviate the illegal extraction, making these agricultural lands ideal places for conservation of this species (Favreto et al. 2010). Although fruits are a sustainable alternative for the species economic exploitation, an uncontrolled exploitation of the food resources can lead to a decrease in the recruitment on new individuals, in addition to a decrease in food supply for natural dispersers. These situations have implications for the conservation and regeneration of E. edulis, and indicate the need for applying efficient methodologies for promoting seed germination and seedling production. The seed quality of this species may play an important role in its conservation because it is one of the main factors determining establishment success (Sulc 1998). A high seed quality ensures a high rate of seedling survival, fast growth and low infection by diseases and infestation by pests (Moestrup and Schmidt 2008).
The seeds of Euterpe edulis are classified as recalcitrant and sensitive to dehydration (Panza et al. 2004, 2007; Cursi and Cicero 2014). At maturity, their embryo is very small, comprising only 0.54% of the seed fresh weight and having a high degree of hydration (85% of water content). The massive endosperm comprises around 99% of the seed fresh weight, has a water content of 48.20% but appears to be in an inactive state (Panza et al. 2004, 2007). Baskin and Baskin (2014) consider the embryos of palm tree seeds to be underdeveloped, which results in morphological seed dormancy (some species, with major delays in germination, would also have morphophysiological dormancy). The underdeveloped embryo then requires an additional amount of time to attain full size after dispersion before germination (root emergence) takes place, and this delay would result in the slow germination of palm seeds in general. Under natural conditions, germination of E. edulis takes from three to six months to complete, but a faster germination can be promoted by small birds that regurgitate depulpped seeds (Leite et al. 2012). Pulp removal also favors seed storage (Martins et al. 2004) and, particularly when performed after water immersion, can increase the physiological performance of the seeds, speeding up germination, which is then completed after 50–90 days (Bovi and Cardoso 1975; Queiroz 1986; Cursi and Cicero 2014).
Variations in light availability and temperature are usually higher in agroforestry systems than in nearby fragments of Atlantic Forest in southern Brazil (Favreto et al. 2010). The initial establishment of E. edulis in environments with higher light incidence than the native forest has been investigated in a few studies (Tsukamoto Filho et al. 2001; Santos 2009; Favreto et al. 2010). However, to date, there is a lack of information on the impact of these higher-light environments on seed quality of E. edulis. Therefore, the main purpose of this study was to investigate how native forest and banana intercrop conditions can affect the germination performance of this palm tree. Based on the expected greater availability of light in most agroforestry systems than in native forests and on the expected greater provision for seeds by the more sun-exposed mother plants present in the former environment, we hypothesized that seeds from mother plants growing in a more open, man-managed environment (agroforestry system) would have a greater quality (as expressed by seed germination and vigor) than those coming from plants growing in a native forest. Our second hypothesis was based on our germination results, which pointed to a lower germination percentage of seeds from the managed environment than from the native forest. We then tested the hypothesis that this lower germination performance was associated to a greater synthesis of phenolic compounds in fruits and seeds.
2 Material and methods
2.1 Study sites and mother plants
Two populations of E. edulis were studied in the northern coast of Rio Grande do Sul, Brazil: one in a native forest (NF), which is a secondary dense ombrophilous forest located in Maquiné (29°49′S and 50°14′W); and a second one in a banana intercrop (BI), 23.46 km away from the NF site, located in a rural property in Itati (29°39′S and 50°12′W). In this site, banana plantations (Musa sp.) are intercropped with E. edulis and Citrus spp., and this is the most common agroforestry type in southern Brazil. We estimated plant density of the each environment by counting all mature trees in a 2 m radius surrounding mother plants: BI (0.21 plants/m2); and NF (0.70 plants/m2).
In each site, we selected five mother plants. We chose reproductive individuals carrying bunches with mature fruits, located at least 10 m away from each other, with heights between 6 and 8 m.
2.2 The mother plant environment
In the summer of 2012 and in the winter of 2013, we collected fifteen soil samples, three around each of the five selected mother plants in each of the two environments. These were made up of about 500 g each and were taken from the upper 20 cm of soil. All chemical soil analyses were made in the Analyses Laboratory of the Soil Department of the Federal University of Rio Grande do Sul. Briefly, clay content was determined by density analysis, pH was measured in water solution (1:1; v/v), P and K were determined by the Mehlich I method, organic matter was obtained by sulfocromic solution oxidation with external heat, Ca and Mg were extracted with KCl 1 mol L−1, and cation exchange capacity (CTC) was estimated at pH 7. Additionally, we determined the gravimetric water content of the soil samples.
Air temperature and relative humidity at each site were recorded at mid-day (11:00–12:30) in the winter and summer, using three dual-channel data loggers with built-in temperature and relative humidity sensors (LOGBOX-AA/DA/RHT Novus, Porto Alegre, Brasil). In the same occasion, the percentage of open canopy was estimated nearby each mother plant with hemispherical photographs. Pictures were taken with a Nikon Coolpix 8700 camera, coupled with a fisheye lens FC-E9 (Raynox DCR-CF 185°-Pro Fisheye circular), 50 cm above the ground. The images were analyzed using the program Gap Light Analyzer 2.0 (Frazer et al. 1999). Additionally, we described the mother plants crown position according to the Dawkin’s method (Dawkins 1958).
In order to summarize the environmental data, we averaged the measurements made for each parameter and mother plant across the two seasons in each environment.
2.3 Plant material
In Oct 2013, we collected one fruit bunch from each of the mother plants. Fruits from each bunch were washed in warm water (40 °C) for 30 min, and manually rubbed until total removal of the pulp. The remaining seeds (seed adhered to the membranous endocarp) from each bunch were then separately stored for 7 days in sealed plastic bags at 5 °C. Despite the recalcitrant nature of the seeds, such storage time and temperature have no negative effects on the physiological performance of the seeds (Martins et al. 2004).
2.4 Seed germination
A germination test was conducted in plastic boxes (gerbox), using vermiculite as substrate (Andrade et al. 1999). Six replicates (boxes) of 25 seeds (five seeds from each mother plant) were used for each environment. Seeds were previously disinfected with a 2% sodium hypochlorite solution for 10 min, rinsed with distilled water and then placed in the boxes containing wet vermiculite. The boxes were kept in a B.O.D. incubation chamber, at a temperature of ~25 °C, receiving ~10 µmol m−2 s−1 of irradiance, during a photoperiod of 8 h. The criteria used for seed germination was plumule emergence, and the counting of normal emerged seedlings was performed weekly, until 14 weeks after sowing. The germination percentage (%G) was calculated according to the following equation %G = (∑ni N−1) × 100, where ∑ni is the total number of germinated seeds in relation to the number of seeds (N).
2.5 Seed vigor
In addition to evaluating seed parameters that are commonly associated to seed vigor (seed mass and moisture), we tested for seed vigor by measuring the germination rate index (GRI) and seedling dry mass. For evaluation of seed mass and moisture, 30 seeds were selected from each of the five bunches (150 seeds from each environment). The fresh (FM) and dry (DM) masses (105 °C for 24 h) were then recorded. The percentage of moisture followed the equation % Moisture (M) = 100 (FM–DM)/DM.
Germination rate index (GRI) was obtained by counting, on a weekly basis, the number of normal emerged seedlings. The equation for the GRI followed the equation proposed by Maguire (1962): GRI = (G1/N1) + (G2/N2) + … + (Gn/Nn), where G1, G2 and Gn are the number of seeds germinated at the first, second and last count, and N 1, N 2 and N n are the number of days after sowing.
After plumule emergence, seedlings were transplanted into 1.0-L plastic bags, filled with medium-sized sand and grown under a light shade cloth until harvest. Seed reserves of E. edulis are totally depleted approximately 6 months after sowing (Venturi and Paulilo 1998). Thus, seedlings were harvested after this period, oven dried at 70 °C until constant weight, and their dry mass (SDM) recorded.
2.6 Seed viability of non-germinated seeds
After the germination trial, non-germinated seeds of each replicate (gerbox) were evaluated for embryo viability using the tetrazolium test (adapted from Lin 1986). Seeds were cut longitudinally in half using a nutcracker, and the embryo was placed face down on a filter paper. The paper was soaked in a 0.5% tetrazolium solution at a pH ~7.0, for 6 h, in the dark, at 30 °C. After soaking, seeds were rinsed with water and examined for staining. The embryos were individually observed with a magnifying stereoscopic and evaluated for color intensity. Viable embryos presented full coloration in pink and red and unviable embryos presented full coloration in milky white/yellowish coloration.
2.7 Extraction and quantification of phenolic compounds
Ten samples of macerated seed coats from each environment had their total phenolics quantified following the methodology described by Fett-Neto et al. (1992). For the extraction, 30 mg of tissue was mixed with 700 µl of HCl 0.1 M and centrifuged at 12,000 rpm. To determine the total phenolics content, we used the Folin-ciocalteau reagent. Calibration curves were obtained from pyrogallol, and absorbance was measured at a wavelength of 750 nm using a spectrophotometer (Spectramax, Molecular Devices).
2.8 Phytotoxicity test of seed coat extracts of E. edulis
A bioassay was conducted using extracts obtained from the seed coat (SC) of E. edulis grown in NF and BI in order to test their phytotoxicity. Seeds from all mother plants in each environment were grated in order to obtain their coats. To obtain the aqueous extract at concentrations of 10 (SC10) and 30% (SC30), 10 and 30 g of macerated seed coat were mixed in 100 mL of distilled water for 24 h. The solution was centrifuged at 12,000 rpm, and the extracts were collected and used in the bioassay. Because the osmotic potential of the extracts can mask the allelopathic effect (Alves et al. 2014), the osmolality of the two extract concentrations was measured with a Wescor Vapor Pressure Osmometer 5520. Then, PEG 6000 solutions, with the same osmolalities of the SC10 and SC30 extracts were prepared (these solutions are referred to as PEG10 and PEG30). Osmolality was converted into osmotic potential (OP) using Van’t Hoff equation (Table 1). Extracts and PEG solutions were used for the analysis of the effects on lettuce (Lactuca sativa L. ‘Grand rapids’) germination and radicle length. Distilled water was the control group (0%).
Seed germination bioassay was performed in Petri dishes containing two sheets of filter paper moistened with the extracts (SC10 and SC30), PEG solutions (PEG10 and PEG30) or distilled water. Four replicates of each of the five treatments received 25 lettuce seeds, and germination counting was performed for five days after sowing. Those seeds that had emerged a 2 mm radicle were considered germinated. Germination percentage (%G), GRI, and radicle length were computed for each dish.
2.9 Data analysis
We adopted a completely randomized design for all evaluations, with five replicates for seed mass and moisture, six replicates for seed germination, GRI, seedling mass and embryo viability and ten replicates for phenolics. All these parameters were analyzed by one-way ANOVA (p < 0.05) to test for the environment effect. To analyze the effect of E. edulis seed coat on lettuce germination, we used four replicates and a two-way ANOVA, where the factors were treatments (Control, SC10, PEG10, SC30, and PEG30) and environments (NF and BI). The means were compared by Tukey’s test (p < 0.05). All analyses were run with the statistical package Statistix 8.0 (Analytical Software).
3 Results
The microclimate parameters measured in this study did not differ between the two environments, and there were very few differences between them regarding soil fertility (higher concentrations of P and Ca in the BI site). Canopy openness, on the other hand, was 2.5 times greater in the BI than in the NF. Also, the Dawkin’s approach revealed more illuminated crowns of the mother plants in BI than in NF environment (Table 2).
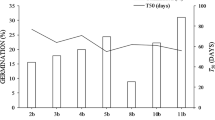
Seed mass and moisture, as well as seedling mass and GRI, did not differ between environments (Table 3). Seeds collected from the two environments also had a similar germination period: germination started 28 days after sowing and stabilized 22 days later (50 days from sowing). However, seeds produced in NF showed a higher final germination percentage than seeds produced in BI (Fig. 1). Most NF non-germinated seeds were non-viable, while most BI non-germinated seeds were viable (Table 3; Fig. 2). The total phenolics concentration in the seed coat of E. edulis was much higher in seeds produced in BI (1.28 ± 0.04 mg g−1) than in seeds produced in NF (0.29 ± 0.04 mg g−1).
The environment, the aqueous solutions, as well as the interaction between these two factors had significant effects on lettuce germination percentage, GRI and radicle length (Table 4). The overall results show that these germination parameters were not negatively affected by both the SC10 extracts and the PEG10 solutions when compared to the control, except for a significant reduction of radicle length when lettuce seeds were exposed to SC10 extract from seeds of BI. On the other hand, seed exposure to PEG30 significantly reduced all germination parameters. SC30 exposure, however, led to a more pronounced effect than PEG30 (germination, GRI and radicle length were all smaller under the effect of SC30 than under the effect of PEG30), but only when extracts where prepared from seeds of BI (Fig. 3a, b, c). Differences between the two sites (not shown in the graphs) were restricted to the effects of SC30 on germination rate and GRI (smaller values in BI than in NF), and on the effects of PÈG10 on radicle length (also smaller values in BI than in NF).
Percentage of germination (a); germination rate index (b); and radicle length (c) of Lactuca sativa seeds in response to aqueous solutions and environments. For each environment, asterisks indicate significant differences from the control, and different small letters indicate significant differences between the SC and the osmotically equivalent PEG extract (p < 0.05). SC10: seed coat aqueous extract at 10% concentration; SC30: seed coat aqueous extract at 30% concentration; PEG10: PEG solution with osmotic potential similar to SC10; PEG30: PEG solution with osmotic potential similar to SC30. In the control treatment, lettuce seeds were exposed to distilled water
4 Discussion
The growing environment of the mother plants did not affect seed vigor, as evidenced by measurements of seed moisture, seed and seedling mass and germination rate. On the other hand, it affected seed germination. These results indicate that our first hypotheses can be partially accepted because only the germination percentage was affected by the environment.
Factors influencing the physiological potential or quality of seeds include germination and vigor. Seed vigor is more closely related to the successful establishment of seedlings than germination capacity because vigor is defined by the combination of characteristics that determine the potential for high performance after sowing (Marcos-Filho 2015). Our results indicated that seeds that developed in mother plants growing in the BI were as vigorous as those developing in mother plants from NF. However, it was quite intriguing the fact that seed germination was significantly lower in BI than in NF.
Seed mass, moisture, age and genetic make-up are important determinants of seed vigor (Ram and Wiesner 1988; Martins et al. 2000). We did not expect seed age and genetic make-up to be important issues for seed quality of E. edulis in our study because we worked with freshly collected seeds, which were stored for seven days in a refrigerator before analyses and tests were run. As for the genetic make-up, a major variability was not expected due to the small distance separating the NF and BI populations of mother plants. On the other hand, seed mass and seed moisture could very well have caused differences in seed vigor due to the contrasting light regimes to which mother plants were submitted in the two sites (we consider light to be the key environmental factor distinguishing the two environments, but we cannot exclude potential effects of soil fertility, which, regarding availability of P and Ca, were also slightly higher in BI than in NF). However, that did not happen, and seed mass and seed moisture were quite similar when comparing the two environments, which may explain why seedling mass and GRI generated similar results for seeds coming from BI and NF. If light availability is the energy source for mass accumulation, why did not it affect seed mass? The mother plants in the BI environment were much more productive ‘fruitwise’ than those in the NF, but instead of producing heavier fruits and seeds, the BI plants produced 47% more fruits per bunch than the NF ones (data not shown). This greater number of fruits (and seeds) per bunch, however, did not compromise the growth and vigor of individual seeds. Brancalion et al. (2011) have also reported significant differences in the number of seeds per bunch but no differences in seed mass when comparing plants of E. edulis grown in three contrasting forest environments. Galetti et al. (2013) have recently reported an adaptive reduction in seed size of E. edulis due to the defaunation caused by human-driven fragmentation of the Atlantic Forest. In this fragmentation scenario, large-gape frugivorous birds, such as toucans, capable of dispersing a broad range of seed sizes, were replaced, in many small fragments, by small-gape ones, such as thrushes, which only disperse small seeds. Taking into account the similarity in average seed mass of both sites, it is possible that they are both under the same selective pressure by the presence of seed dispersers. Even though we have not focused our observations on frugivorous animals, toucans are reported in both the Maquiné region (where NF is located), as well as in the BI, which neighbors the 272-ha ‘Mata Paludosa Biological Reserve’, aimed to preserve remnants of the Atlantic Forest in the state of Rio Grande do Sul. It is thus possible that these large dispersers are still functionally acting in the study sites and that selection for small-size seeds is not operating there. However, a more detailed study on the frequency distribution of seed sizes would be required to better approach this issue.
The question then remains as to why seeds from the BI environment exhibited a germination percentage that was 22% lower than the one from the NF. Euterpe edulis seeds present, at maturation, a high moisture content (Queiroz and Cavalcante 1986; Martins et al. 2009; Cursi and Cicero 2014). Because of their recalcitrant nature, their moisture is often related to their germinability. Studies on seed desiccation and storage have shown that the critical moisture level for the species is ~39%, with lethal levels around 16%, depending on the genetic characteristics of the seed lot (Reis et al. 1999; Panza et al. 2007; Martins et al. 2009). In this study, seed moisture was much above these threshold levels and did not differ between the two sites, so we excluded this factor as responsible for the decrease in germination of BI seeds. Even though seed mass is usually associated to seed vigor, it can also affect germination, and such effect has been reported for E. edulis (Lin 1986; Fleig and Rigo 1998). Again, seed mass did not vary between sites, so it cannot explain our germination results. Another factor that could have reduced germination in seeds developed in BI was the loss of embryo viability. However, the tetrazolium test run on non-germinated seeds revealed that 76% of seeds form BI were viable and could have potentially germinated, in contrast with the fewer non-germinated seeds from NF, where only 28% of them were viable. We are then left with the possibility that the absence of germination in most of the non-germinated seeds from BI after almost three months of evaluation was related to some degree of seed dormancy.
In a recent review on dormancy of palm diaspores, Baskin and Baskin (2014) concluded that most of them have morphophysiological dormancy and those that do not are morphologically dormant. How does E. edulis fall into these categories? Similar to other studies (Bovi and Cardoso 1975; Queiroz 1986; Roberto and Habermann 2010; Cursi and Cicero 2014; Ribeiro et al. 2015), the diaspores of this palm species germinated quite slowly, with stabilization in the number of germinated seeds taking place around 50 days after sowing. Baskin and Baskin (2014) draw a line at 30 days to separate between palm species that have only morphological dormancy due to underdeveloped embryos (germination within 30 days) and those that have both morphological and physiological dormancy (complete germination taking way longer than 30 days). Based on this criterion, E. edulis seems to share morphophysiological dormancy with many other palm species.
While there is substantial information on the underdeveloped nature of the species embryo (Panza et al. 2004; Roberto and Haberman 2010) and on the positive effects of gibberellins, ethylene and scarification on seed germination (these three supporting the physiological component of seed dormancy) (Bovi and Cardoso 1975; Roberto and Habermann 2010; Roberto et al. 2011; Ribeiro et al. 2015), little attention has been given to the physiological status of non-germinated seeds, which, in many experiments, amount to more than 30% (Bovi and Cardoso 1975; Roberto and Habermann 2010, Leite et al. 2012). One important exception is the study by Brancalion et al. (2011), who compared the germination of seed populations from three contrasting forest environments. These authors found that all of the non-germinated seeds were unviable, regardless of the environment from where seeds came from. In our study, we were faced with the fact that only the seed population from the higher light environment had mostly viable seeds among those that failed to germinate after almost 80 days (in order words, they remained dormant). This result suggests that a fraction of the seeds from the BI had an extra dormancy component. Because of the substantial difference in light exposure of the mother plants in the two environments, we looked for possible light-related effects on seed dormancy. Phenolic compounds on the seed coats (which included the stony endocarp) appeared as good candidates, because they are light-regulated (Mole et al. 1988; Kefeli and Kalevitch 2002; Matsuura et al. 2013), are known to delay germination by reducing the seed coat permeability to water and oxygen (Marbach and Mayer 1974; Bewley and Black 1994; Siddiqui and Khan 2010), and are the most abundant secondary compounds in fruits of E. edulis (Tokuhisa et al. 2007; Ribeiro et al. 2011; Bicudo et al. 2014). Differences in phenolics content in the seed coat of E. edulis between the two environments could then help explain why seeds from BI had a lower germination percentage than seeds from NF, leading us to accept our second hypothesis, that the lower germination of seeds developed in BI was associated to an increased concentration of phenolic compounds in their coats.
Even though we cannot say for sure that this lower germination response was indeed caused by a greater accumulation of phenolics, two facts lead us to suggest so: (1) the fruit pulp of E. edulis accumulates large amounts of phenolic compounds (mostly anthocyanins) (Iaderoza et al. 1992; Ribeiro et al. 2011; Bicudo et al. 2014), and seed germination of E. edulis is greatly increased by pulp removal (Bovi and Cardoso 1975; Cursi and Cicero 2014; Leite et al. 2012); such positive effect of pulp removal could be related to the reduced inhibition caused by those compounds; and (2) the phenolic compounds extracted from the seed coats of E. edulis inhibited the germination of lettuce seeds. Similar results were reported by Lima et al. (2011) when using fruit extracts, and by Nazário (2011), when using extracts from recalcitrant seeds of the palm tree Bactris gasipaes.
Despite these indirect evidences supporting an important role of the mother plant light environment on the concentration of phenolic compounds in the seed coats and the negative effects of these compounds on germination percentage, future studies should be conducted in order to answer the following questions: (1). Does a higher incidence of light during seed development result in a greater accumulation of phenolic compounds in the seed coat of E. edulis? In approaching this question, light variations among fruit bunches located in different mother trees as well as among fruits located in the same bunch should be explored. (2). How do the concentration of phenolics in the fruit pulp, the concentration of phenolics in the seed coat and seed germination of E. edulis correlate to one another?
Regardless of the existence of a causal relationship between phenolic compounds and germination, the results from this study also point to the fact that seeds from native forests and agroforestry systems might not be equally suitable to generate new plants. It appears that seeds collected from the more open systems like banana intercrops will attain a lower germination percentage than those collected from the more closed forest environments. Even if the non-germinated, apparently dormant seeds detected in this study eventually germinated, this extra time required to increase the number of germinated seeds would not be desirable.
References
Alves MCS, Medeiros Filho S, Manoel Neto A, Brito RC, Araujo RC (2014) Allelopathic effect of essential oils of medicinal plants in Bidens pilosa L. Rev Bras Plantas Med 16:731–736. doi:10.1590/1983-084X/12_134
Ambika S, Manonmani V, Somasundaram G (2014) Review on effect of seed size on seedling vigour and seed yield. Res J Seed Sci 7:31–38. doi:10.3923/rjss.2014.31.38
Andrade ACS, Loureiro MB, Souza ADO, Ramos FN, Cruz APM (1999) Reavaliação do efeito do substrato e da temperatura na germinação de sementes de palmiteiro (Euterpe edulis Mart.). Rev Árvore 23:279–283
Baskin CC, Baskin JM (1998) Seeds, ecology, biogeography and evolution of dormancy and germination. Academic Press, San Diego
Baskin JM, Baskin CC (2014) What kind of seed dormancy might palms have? Seed Sci Res 24:17–22. doi:10.1017/S0960258513000342
Bewley JD, Black M (1994) Seeds physiology of development and germination. Plenum Press, New York
Bewley JD, Bradford KJ, Hilhorst HWM, Nonogaki H (2013) Seeds: physiology of development, germination and dormancy. Springer, New York
Bicudo MO, Ribani RH, Beta T (2014) Anthocyanins, phenolic acids and antioxidant properties of Juçara fruits (Euterpe edulis M.) along the on-tree ripening process. Plant Food Hum Nutr 69:142–147. doi:10.1007/s11130-014-0406-0
Bovi MLA, Cardoso M (1975) Germinação de sementes de palmiteiro (Euterpe edulis Mart.). Bragantia 34:29–34. doi:10.1590/S0006-87051975000100028
Brancalion PHS, Novembre ADLC, Rodrigues RR (2011) Seed development, yield and quality of two palm species growing in different tropical forest types in SE Brazil: implications for ecological restoration. Seed Sci Technol 39:412–424. doi:10.15258/sst.2011.39.2.13
Brasil (2008) Instrução Normativa N°6. Lista Official das Espécies da Flora Ameaçadas de Extinção. Diário Oficial da União 185:75–83
Campos CT, Steiner J, Zillikens A (2012) Bird and mammal frugivores of Euterpe edulis at Santa Catarina island monitored by camera traps. Stud Neotrop Fauna E 47:105–110. doi:10.1080/01650521.2012.678102
Cursi PR, Cicero SM (2014) Fruit processing and the physiological quality of Euterpe dulis Martius seeds. J Seed Sci 36:134–142. doi:10.1590/2317-1545v32n2847
Dawkins H (1958) The management of natural tropical high-forest with special reference to Uganda. Institute Paper, Oxford
Donohue K (2009) Completing the cycle: maternal effects as the missing link in plant life histories. Phil Trans R Soc B 364:1059–1074. doi:10.1098/rstb.2008.0291
Fadini RF, Fleury M, Donatti CI, Galetti M (2009) Effects of frugivore impoverishment and seed predators on the recruitment of a keystone palm. Acta Oecol 35:188–196. doi:10.1016/j.actao.2008.10.001
Favreto R, Mello RSP, Baptista LRM (2010) Growth of Euterpe edulis Mat. (Arecaceae) under forest and agroforestry in southern Brazil. Agrofor Sys 80:303–313. doi:10.1007/s10457-010-9321-z
Fett-Neto AG, Teixeira SL, Silva EAM, Sant`Anna RS (1992) Biochemical and morphological changes during in vitro rhizogenesis in cuttings of Sequoia sempervirens. J Plant Physiol 140:720–728. doi:10.1016/S0176-1617(11)81029-1
Fleig FD, Rigo SM (1998) Influência do tamanho dos frutos do palmiteiro Euterpe edulis Mart. na germinação das sementes e crescimento das mudas. Ciência Florestal 8:35–41
Frazer GW, Canham CD, Lertzman KP (1999) Gap light analyzer (GLA): imaging software to extract canopy structure and gap light transmission indices from true-colour fisheye photographs, user manual and program documentation, version 2.0. Millbrook, New York
Galetti M, Guevara R, Côrtes MC, Fadini R, Von Matter S, Leite AB, Labeca F, Ribeiro T, Carvalho CS, Collevatti RG, Pires MM, Guimarães PR Jr, Brancalion PH, Ribeiro MC, Jordano P (2013) Functional extinction of birds drives rapid evolutionary changes in seed size. Science 340:1086–1090. doi:10.1126/science.1233774
He H, Vidigal DS, Snoek LB, Schnabel S, Nijveen H, Hilhorst H, Bentsink L (2014) Interaction between parental environment and genotype affects plant and seed performance in Arabidopsis. J Exp Bot 65:6603–6615. doi:10.1093/jxb/eru378
Iaderoza M, Baldini ISD, Draetta SE, Bovi MLA (1992) Anthocyanins from fruits of açaí (Euterpe Oleracea Mart.) and juçara (Euterpe edulis Mart.). Trop Sci 32:41–46
Kefeli VI, Kalevitch MV (2002) Natural growth inhibitors and phytohormones in plant and environment. Kluwer Academic Publishers, Dordrecht
Leite AB, Brancalion PH, Guevara R, Galetti M (2012) Differential seed germination of a keystone palm (Euterpe edulis) dispersed by avian frugivores. J Trop Ecol 28:615–618. doi:10.1017/S0266467412000594
Lima CP, Cunico MM, Trevisan RR, Philippsen AF, Miguel OG, Miguel MD (2011) Efeito alelopático e toxicidade frente à Artemia salina Leach dos extratos do fruto de Euterpe edulis Martius. Acta Bot Bras 25:331–336. doi:10.1590/S0102-33062011000200009
Lin SS (1986) Efeito do tamanho e maturidade sobre a viabilidade, germinação e vigor do fruto de palmiteiro. Rev Bras Sementes 8:57–66. doi:10.17801/0101-3122/rbs.v8n1p57-66
Maguire JD (1962) Speed of germination—aid in selection and evaluation for seedling emergence and vigor. Crop Sci 2:176–177. doi:10.2135/cropsci1962.0011183X000200020033x
Marbach I, Mayer AM (1974) Permeability of seed coats to water as related to drying conditions and metabolism of phenolics. Plant Physiol 54:817–820. doi:10.1104/pp.54.6.817
Marcos-Filho J (2015) Seed vigor testing: an overview of the past, present and future perpective. Scientia Agricola 72:363–374. doi:10.1590/0103-9016-2015-0007
Martins CC, Nakagawa J, Bovi MLA, Stanguerlim H (2000) Influência do peso das sementes de palmito-vermelho (Euterpe espiritosantensis Fernandes) na porcentagem e na velocidade de germinação. Rev Bras Sementes 22:47–53
Martins CC, Bovi MLA, Nakagawa J, Godoi G Jr (2004) Temporary storage of jussara palm seeds: effects of time, temperature and pulp on germination and vigor. Hort Bras. 22:271–276
Martins CC, Bovi MLA, Nakagawa J, Machado CG (2009) Secagem e armazenamento de sementes de juçara. Rev Árvore 33:635–642. doi:10.1590/S0100-67622009000400006
Matsuura HN, Costa F, Yendo AC (2013) Photoelicitation of bioactive secondary metabolites by ultraviolet radiation: mechanisms, strategies, and applications. In: Chandra S, Lata H, Varma A (eds) Biotechnology for medicinal plants. Springer-Verlag, Berlin Heidelberg, pp 171–190
Moestrup S, Schmidt L (2008) A guide to seed quality. Forestry Administration, Cambodia
Mole S, Ross JAM, Waterman PG (1988) Light-induced variation in phenolic levels in foliage of rain-forest plants I. Chemical changes. J Chem Ecol 14:1–21. doi:10.1007/BF01022527
Nazário P (2011) Dormência em sementes de pupunha (Bactris gasipaes KUNTH): uma abordagem anatômica, histoquímica e fisiológica. Dissertation, Instituto Nacional de Pesquisas da Amazônia
Panza V, Láinez V, Maldonado S (2004) Seed structure and histochemistry in the palm Euterpe edulis. Bot J Linn Soc 145:445–453. doi:10.1111/j.1095-8339.2004.00293.x
Panza V, Láinez V, Maldonado S, Maroder HL (2007) Effects of desiccation on Euterpe edulis Martius seeds. Biocell 31:383–390
Parrish JAD, Bazzaz FA (1985) Nutrient content of Abutilon theophrasti seeds and the competitive ability of the resulting plants. Oecologia 65:247–251. doi:10.1007/BF00379224
Queiroz MH (1986) Botão germinativo do palmiteiro como indicador de germinação. Rev Bras Sementes 8:55–59. doi:10.17801/0101-3122/rbs.v8n2p55-59
Queiroz MH, Cavalcante MDT (1986) Efeito do dessecamento das sementes do palmiteiro na germinação e no armazenamento. Rev Bras Sementes 8:121–125. doi:10.17801/0101-3122/rbs.v8n3p121-125
Ram C, Wiesner E (1988) Effect of artificial ageing on physiological and biochemical parameters of seed quality in wheat. Seed Sci Technol 16:579–587
Reis A, Reis MS, Fantini AC (1993) Manejo de Rendimento Sustentado de Euterpe edulis. USP, São Paulo
Reis A, Paulilo MTS, Nazazono EM, Venturi S (1999) Efeito de diferentes níveis de dessecamento na germinação de Euterpe edulis Martius Arecaceae. Insula 28:31–41
Ribeiro LO, Mendes MF, Pereira CSS (2011) Avaliação da composição centesimal, mineral e teor de antocianinas da polpa de Juçaí (Euterpe edulis Martius). Revista Eletrônica Teccen 4:5–16
Ribeiro MS, Steffens CA, Oliveira LM, Araldi CG, Pikart TG, Souza GK (2015) Tratamentos pré-germinativos em sementes de palmiteiro. Br J Forest Res 35:469–473. doi:10.4336/2015.pfb.35.84.663
Roach DA, Wulff RD (1987) Maternal effects in plants. Annu Rev Ecol Syst 18:209–235
Roberto GG, Habermann G (2010) Morphological and physiological responses of the recalcitrant Euterpe edulis seeds to light, temperature and gibberellins. Seed Sci Technol 38:367–378. doi:10.15258/sst.2010.38.2.10
Roberto GG, Coan AI, Habermann G (2011) Water content and GA3-induced embryogenic cell expansion explain Euterpe edulis seed germination, rather than seed reserve mobilisation. Seed Sci Technol 39:559–571. doi:10.15258/sst.2011.39.3.03
Santos MLSS (2009) Estabelecimento e crescimento de mudas de Euterpe edulis em três ambientes florestais. Dissertation, Universidade Estadual de Santa Catarina
Sawhney R, Naylor JM (1982) Dormancy studies in seed of Avena fatua. Influence of drought stress during seed development on duration of seed dormancy. Can J Bot 60:1016–1020. doi:10.1139/b82-127
Siddiqui ZS, Khan MA (2010) The role of seed coat phenols on water uptake and early protein synthesis during germination of dimorphic seeds of Halopyrum mucronatum (L.) Staph. Pak J Bot 42:227–238
Sulc RM (1998) Factors affecting forage stand establishment. Sci Agr 55:110–115. doi:10.1590/S0103-90161998000500020
Tokuhisa D, Dias DCFS, Alvarenga EM, Hilst PC, Demuner AJ (2007) Phenolic compound inhibitors in papaya seeds (Carica papaya L.). Rev Bras Sementes 29:180–188. doi:10.1590/S0101-31222007000300022
Trevisan ACD, Fantini AC, Schmitt-Filho AL, Farley J (2015) Market for Amazonian Açaí (Euterpe oleraceae) stimulates pulp production from Atlantic Forest Juçara berries (Euterpe edulis). Agroecol Sust Food 39:762–781. doi:10.1080/21683565.2015.1025461
Tsukamoto Filho AA, Macedo RLG, Venturini N, Morais AR (2001) Aspectos fisiológicos e silviculturais do palmiteiro (Euterpe edulis Martius) plantado em diferentes tipos de consórcios no município de Lavras, Minas Gerais. Cerne 7:41–53
Venturi S, Paulilo MTS (1998) Esgotamento das reservas na semente de Euterpe edulis Mart. e efeito da nutrição mineral nas plântulas. Acta Bot Bras 12:215–220. doi:10.1590/S0102-33061998000300003
Acknowledgements
We thank the Seed Laboratory of the Agronomy School of the Federal University of Rio Grande do Sul (UFRGS) and the Plant Physiology Laboratory of the Botany Department of the same University for providing space and equipments for various analyses. We acknowledge the Coordination for Improvement of Higher Education Personnel (CAPES/Brazil) and the Brazilian Council for Scientific and Technological Development (CNPq/Brazil) for fellowships granted to the first and second author, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
da Silva Alabarce, F., Dillenburg, L.R. Maternal light environment during seed development can affect seed quality of Euterpe edulis . Theor. Exp. Plant Physiol. 29, 1–11 (2017). https://doi.org/10.1007/s40626-016-0083-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40626-016-0083-5