Abstract
Nitrogen (N) fixation in moss-cyanobacteria associations is one of the main sources of ‘new’ N in pristine ecosystems like subarctic and arctic tundra. This fundamental ecosystem process is driven by temperature as well as by moisture. Yet, the effects of temperature and moisture stress on N2 fixation in mosses under controlled conditions have rarely been investigated separately, rendering the interactive effects of the two climatic factors on N2 fixation unknown. Here, we tested the interactive effects of temperature and moisture on N2 fixation in the two most dominant moss species in a temperate heath, subarctic tundra and arctic tundra: Pleurozium schreberi and Tomentypnum nitens. Mosses with different moisture levels (25, 50, 100%) were kept at different temperatures (10, 20, 30 °C) and N2 fixation was measured at different times after exposure to these conditions. T. nitens had the highest nitrogenase activity and this increased with moisture content, while effects were moderate for P. schreberi. Nitrogenase activity increased with temperature in all mosses, and the temperature optimum (Topt) was between 20 °C and 30 °C for all mosses. Quick acclimatization towards higher temperatures occurred. Our results suggest that the contemporary and not the historical climate govern the response of moss-associated N2 fixation to changes in the abiotic environment. Thus, climate change will have substantial impacts on N2 fixation in dominant mosses in temperate, subarctic and arctic habitats.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nitrogen fixation by moss-cyanobacteria associations is a main source of ‘new’ N for ecosystems like boreal forests, and subarctic and arctic tundra (DeLuca et al. 2002; Stewart et al. 2011; Zielke et al. 2005). Several moss species have been found to be colonized by N2 fixing cyanobacteria, Nostoc, Cylindrospermum and Stigonema being the most common cyanobacterial genera (Ininbergs et al. 2011). Moss-associated N2 fixation can contribute 1–3 kg N ha−1 year−1 to total ecosystem N input in pristine, unpolluted areas (Rousk et al. 2015; Rousk and Michelsen 2016), exceeding atmospheric N deposition in these ecosystems (e.g. Gundale et al. 2011). Yet, fixed N2 by moss-associated cyanobacteria is not readily available to the rest of the ecosystem. Release of N from the moss carpet is slow; it can take several months before fixed N2 is transferred to the soil (Rousk et al. 2016).
Moss-associated N2 fixation is strongly affected by abiotic factors. Nitrogen deposition (Gundale et al. 2011; Ackermann et al. 2012; Rousk et al. 2013a), temperature (Smith 1984; Gentili et al. 2005; Rousk and Michelsen 2016) and moss-moisture content (Jackson et al. 2011; Rousk et al. 2014, 2015) are the most important factors controlling N2 fixation activity in mosses. The abiotic factors likely act upon N2 fixation in a hierarchical and interacting way, and moisture has been put forward as the most important driver of N2 fixation (Belnap 2001). However, given that mosses dry out quickly at high temperatures (>25 °C for several hours; e.g. Rousk et al. 2014), temperature and moisture are likely strongly interacting to affect moss and colonizing cyanobacteria.
Although the effects of these abiotic factors on moss-associated N2 fixation have been investigated previously (e.g. Jackson et al. 2011), hardly any experiments have been conducted under controlled conditions that combine the major drivers of N2 fixation—moisture and temperature—in one experiment, despite their strong interconnection. For instance, Gundale et al. (2012a) found that an increase in air temperature of +2 °C combined with less frequent precipitation reduced N2 fixation in feather moss Pleurozium schreberi. However, the effects on N2 fixation were dependent on the sampling date, and the precipitation quantity had no effect. Thus, we still lack a conclusive assessment of the combined effects of temperature and moisture on N2 fixation, especially at higher temperatures that lead to desiccation of the moss-host.
While free-living cyanobacteria can be active and fix N2 at temperatures as low as −1 °C, the minimum temperature for activity can even be lower (−5 °C) when living in associations with lichens (Englund and Meyerson 1974). Soil cyanobacteria and cyanolichens can fix N2 within a large temperature range (−5 and 30 °C, Belnap 2001), and the optimum temperature for N2 fixation in free-living cyanobacteria from the Arctic, from temperate grasslands soils and from subalpine soils is between 20 °C and 30 °C (Jones 1977, Stewart et al. 1977; Coxson and Kershaw 1983). The free-living cyanobacteria Stigonema, collected in Brazil, had a maximum nitrogenase activity at 15–25 °C and declined at 35 °C, with no nitrogenase activity at 0 °C (Isichei 1980). These findings suggest that the response of N2 fixation to increased temperature is not shaped by the historical climate, but rather by the contemporary conditions. A N2 fixer community that is performing best at contemporary climatic conditions that differ from the historic conditions is likely a community that is not driven by the historic climate and visa versa.
The optimum growth temperature for most mosses is around 20 °C (Dilks and Proctor 1975), but the majority of mosses have a heat tolerance of 39–45 °C (Glime 2007) and they can survive high temperatures in a state of desiccation (in dormancy), in which their temperature tolerance is higher (Hearnshaw and Proctor 1982). Cyanobacteria are only active when sufficiently moist, and highest N2 fixation rates in mosses are found at optimal moist conditions (Zielke et al. 2005; Jackson et al. 2011; Rousk et al. 2014, 2015). Similarly, N2 fixation in free-living cyanobacteria increased with moisture content (Rousk et al. 2015). However, the moisture dependence of N2 fixation can differ between habitats, likely as a result of adaptations, and a completely hydrated status may lead to glucose efflux, depleting the energy reserves necessary for N2 fixation (Kershaw 1985). Mosses hosting cyanobacteria are found in a range of ecosystems like subarctic and arctic tundra as well as in temperate habitats. Given the varying climatic conditions these associations are found in, e.g. low precipitation and low temperatures in arctic habitats, with large temperature fluctuations, and a less fluctuating climate in temperate habitats, the long-term exposure to these different climatic regimes could have led to different acclimatisation of N2 fixation to the historic climate (see e.g. Strickland et al. 2015).
Here, we report results from a temperature by moisture experiment in which we exposed two different feather moss species (P. schreberi, Tomentypnum nitens) from three ecosystems (temperate heath, subarctic birch forest, arctic tundra) to different temperatures (10 °C, 20 °C and 30 °C) and moisture levels (25%, 50% and 100% moss water content) to assess their interactive effects on N2 fixation. The differences in the historic climate between the sites—cold and dry in the arctic site vs. milder and more moist in the temperate site, and intermediate conditions at the subarctic site—will likely harbour differently adapted cyanobacterial communities, resulting in different responses of N2 fixation towards changes in temperature and moisture. We selected the moss species that covered the largest fraction of the ground in each ecosystem. Furthermore, we tested if moss-associated N2 fixation can acclimatize to a new temperature and how this acclimatization is altered when exposed to yet another temperature. We hypothesized that (1) N2 fixation in all moss species increases with increasing moss moisture content, and N2 fixation in mosses collected in the Arctic will be higher at lower moisture levels than in moss from the temperate heath and similar to the moss collected in the Subarctic, (2) N2 fixation increases with increasing temperature, and N2 fixation in moss from the temperate heath will peak at higher temperatures than in mosses from the arctic regions, and (3) N2 fixation can acclimatize to new temperatures, and the acclimatization in mosses from the arctic habitats is quicker than in moss from the temperate heath.
2 Material and methods
2.1 Sampling sites
The feather moss P. schreberi was collected in a subarctic birch forest (also referred to as subarctic tundra) close to the Abisko Scientific Research Station, Northern Sweden. The mean annual air temperature in Abisko is 0.2 °C (30-year mean 1986–2015, ANS 2016). Mean annual precipitation is 337 mm (30-year mean 1986–2015, ANS 2016). The same moss species (P. schreberi) was collected 50 km NW of Copenhagen, Denmark, from a temperate heathland. The mean precipitation for the area (1961–1990) is 613 mm distributed on 113 days throughout the year. The mean annual air temperature for the same period is 8 °C [Danish Meteorological Institute 2013 (www.dmi.dk)]. Due to the low cover of P. schreberi at our arctic site, we instead selected the most abundant feather moss found in such settings, T. nitens. Samples of T. nitens were collected from an arctic tundra site on Disko Island, Greenland. This area has a typical low-arctic climate with a mean annual air temperature of −3.0 °C (1992–2012). The mean annual precipitation is 436 mm. Mosses were sampled in February, May and September for the temperate, the subarctic and the arctic sites, in an attempt to sample under similar temperatures despite contrasting environmental conditions. All mosses were kept in climate chambers at 10 °C in light (18 h) and at 2 °C in dark (6 h)—reflecting field conditions at the sampling dates, and also ensuring similar conditions for all mosses one month prior to the start of the experiments.
2.2 Sample preparation
All mosses were gently soaked in ddH2O for 5 min to ensure water saturation (100%) and 3.0 ± 0.1 g of wet moss were put into 50 mL centrifuge tubes. Although we did not check cyanobacterial presence on the mosses before and after the soaking, we assume that the gentle soaking did not affect the cyanobacterial presence. Only alive, green parts of the mosses were used. The mosses from the temperate heath were rinsed thoroughly with ddH2O before water saturation so that any deposited N, which could inhibit N2 fixation (e.g. Rousk et al. 2013a), would be washed away. To reach the moisture levels below 100% moss water content (25% and 50%), mosses were slowly dried with a cold airflow. Moss samples from each site with three moisture levels (25%, 50% and 100% moss water content) were placed in climate chambers with three different temperatures (10 °C, 20 °C and 30 °C). Five replicate samples were used for each treatment from each site, totalling to 45 samples per temperature (5 samples per moisture level per site). Water loss was checked three times per week and adjusted accordingly.
2.3 Temperature by moisture experiment
The temperature by moisture experiment was carried out 1.5 and 2.5 weeks after the mosses had reached the moisture levels we aimed for. The experiment consisted of 45 samples in each temperature (10 °C, 20 °C and 30 °C). Five replicates per temperature and per sampling site were kept at three different moisture levels (25%, 50% and 100% moss water content). We used different incubation times for moss samples exposed to the different temperatures, which was a necessity given the expected dependence of nitrogenase activity on temperatures. Assuming a Q10 of 2.5 for N2 fixation in mosses (see Smith 1984), we incubated the moss samples in the 10 °C, 20 °C and 30 °C for 20 h, 7.5 h and 3 h, respectively.
2.4 The interactive effects of incubation-temperature and -time on acetylene reduction
To test if acetylene reduction (see below) is affected by the incubation time with acetylene, depending on the incubation temperature, we incubated mosses from the 20 °C and 30 °C climate chamber for 20 h and another set of samples from the 30 °C climate chamber for 7.5 h. For these incubations, we used two moisture levels (50% and 100% moss water content) with five replicates from each site, temperature and moisture level. Acetylene reduction in samples from both the temperature by moisture and the incubation experiment was measured after 1.5 and 2.5 weeks kept at the aimed moisture levels.
2.5 Exposure to a higher temperature
To test if N2 fixation in moss-cyanobacteria associations can acclimatize to a higher temperature within a short time frame, we moved the samples from 10 °C and 20 °C to 30 °C (n = 5 per moisture level, per site). For the acetylene reduction assay (ARA), samples were incubated for 3 h and ARA was performed 1 day, 1 week and 2 weeks after the move to the higher temperature.
2.6 Exposure to a lower temperature
Similarly, to test if N2 fixation in moss-cyanobacteria associations can acclimatize to a lower temperature within a short time frame, the samples from the incubation temperature and -time experiment were moved to a lower temperature. One set of samples from the 30 °C treatment were moved to 20 °C and another set of samples was moved to 10 °C. The samples from the incubation temperature and –time experiment from the 20 °C treatment were moved to 10 °C. The incubation times for the samples in the 20 °C climate chamber was 7.5 and 20 h for the samples in 10 °C. Acetylene reduction was measured 1 day, 1 week and 2 weeks after the change to the lower temperature.
2.7 Acetylene reduction assay (ARA)
To estimate N2 fixation, we used the acetylene reduction assay. This assay is a measure of the nitrogenase enzyme activity that catalyzes N2 fixation. In all experiments (see above), 10% of the headspace of the 50 mL tubes was exchanged with acetylene gas. After the incubations, 6 mL air was taken from each sample and injected into 6 mL pre-vacated exetainer (Labco, Ceredigion, UK) and were analysed for ethylene production on a gas chromatograph (SRI 310C, FID, SIR Instruments, California).
2.8 Cyanobacterial counts on moss leaves
Cyanobacterial cells were counted in the samples from the temperature by moisture experiment, using an Olympus fluorescence microscope, with 200× to 400× magnification. Moss leaves from 3 replicate samples (1 sample = one 50 mL tube) from each treatment were counted. From each sample, we randomly picked five shoots, and from each shoot we took 3 fronds (“branches”). The leaves were scraped off the fronds with a needle, and transferred to a microscope-slide. From each frond, we counted 5 randomly selected leaves, totalling 75 leaves per sample.
2.9 Moss-pH
The pH of the mosses was assessed using three samples from each site (3 g wet weight). Moss samples were cut into small pieces and 15 mL ddH2O was added. The samples were shaken for 1 h on a table shaker and centrifuged for 10 min with 4300 rpm. The pH was measured with a pH meter.
2.10 Moss N content
At the end of the experiment, moss samples were dried (70 °C for 24 h), ground and 3–4 mg were packed into tin capsules to determine the total N concentration on an Isoprime isotope ratio mass spectrometer (Isoprime Ltd., Cheadle Hulme, UK) coupled to a CN elemental analyzer (Eurovector, Milan, Italy).
2.11 Statistical analyses
To test the effects of temperature on nitrogenase activity, we performed analyses of Covariance (ANCOVA) for each site and time point separately, with moisture as the covariate. This enabled us to compare the slopes (acetylene reduction in relation to exposure temperature) between the different moisture levels. Acetylene reduction data were log-transformed to fulfil the assumptions of ANCOVA. ANCOVA’s were performed for the data from the temperature by moisture experiment as well as for the change in the exposure experiments. Changes in ARA with incubation times in the incubation temperature and -time experiment as well as changes in Q10 values with time were assessed with linear regression analyses. Differences in moss-pH and moss N content between sites were assessed with ANOVA. All analyses were performed in R. 3.0.3.
3 Results
3.1 Temperature by moisture experiment
Acetylene reduction increased from 10 °C to 20 °C in all mosses 1.5 and 2.5 weeks after exposure to the moisture and temperature levels (Fig. 1a, b) (F > 27; p < 0.0001 for all mosses). The highest AR activity was found in T. nitens (F = 34.17; p < 0.0001), irrespective of temperature and moisture level. While AR activity in P. schreberi from both sites (subarctic and temperate) at 30 °C overlapped with the fitted line for the 10 °C and 20 °C data after 1.5 week of exposure to the moisture and temperature levels, nitrogenase activity acclimatized after 2.5 weeks and the temperate optimum seems to be above 30 °C (Fig. 1d, e). Nitrogenase activity in T. nitens however, did not seem to change over time and 30 °C was above the temperature optimum throughout (Fig. 1c, f). Moisture had a positive effect on acetylene reduction in P. schreberi form the subarctic site (F1.5 wks = 6.06; p1.5 wks = 0.02; F2.5 wks = 8.3; p2.5 wks = 0.006) and T. nitens (F1.5 wks = 37.3; p1.5 wks < 0.0001; F2.5 wks = 90.1; p2.5 wks < 0.0001), but there was no interaction with temperature. Nitrogenase activity in P. schreberi from the Subarctic at 20 °C and 100% moisture content was variable and high, also indicated by the variable numbers of cyanobacteria on the moss leaves (Online Resource 1). Acetylene reduction increased with time in P. schreberi from the subarctic (R2 = 0.14; p = 0.0002) and temperate site (R2 = 0.20; p < 0.0001).
Acetylene reduction (nmol g dw−1 h−1) in P. schreberi from a temperate heath and subarctic tundra, and T. nitens from arctic tundra exposed for 1.5 (a–c) and 2.5 (d–f) weeks to three different moisture levels (open circles, black squares, grey diamonds for the 25%, 50% and 100% moss water content, respectively) within three temperature levels (10 °C, 20 °C, 30 °C). The lines were fitted through the mean acetylene reduction in the 10 °C and 20 °C treatments and thereafter extended to the axes. Data for the 30 °C treatment was plotted separately. Shown are mean ± SE (n = 5). Please note the logarithmic y-axis
The Q10 values ranged from 0.6 to 7.5 in P. schreberi and from 0.9 to 6.7 in T. nitens (Table 1). Nitrogen fixation in these mosses was more sensitive to changes (i.e., higher Q10) in temperature from 10 °C to 20 °C than in a change from 20 °C to 30 °C (Table 1). The Q10 values decreased with time and increased with moisture content (p < 0.006 for all mosses), except for the change from 20 °C to 30 °C in P. schreberi from the temperate site and in T. nitens.
3.2 Incubation-temperature and -time experiment
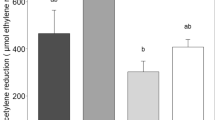
The different incubation times depending on exposure temperature had a large effect on nitrogenase activity (Fig. 2), with lower activity in the longer incubations in all moss species (R2 = 0.79; p < 0.0001).
Acetylene reduction (nmol g dw−1 h−1) in P. schreberi from a temperate heath and subarctic tundra, and T. nitens from arctic tundra incubated for different times with acetylene. Shown are mean values (n = 5) ± SE of acetylene reduction in mosses exposed for 1.5 weeks to two different moisture levels (50%, 100% moss water content) within two temperature levels (20 °C, 30 °C). The samples were incubated for 7.5 (white bars) and 21 h (black bars) at 20 °C and for 3 (grey bars), 7.5 and 21 h at 30 °C. Please note the differences in the y-axes. Different lower case letters indicate significant differences between the incubation times within a moisture level (Tukey’s test)
3.3 Exposure to a higher temperature
The original temperature had an effect on acetylene reduction in all mosses shortly after exposure to the higher temperature (30 °C). Mosses that had been kept at 30 °C throughout had higher nitrogenase activity than mosses previously exposed to 10 °C and 20 °C (F = 5.07; p = 0.02) (Fig. 3). T. nitens had the highest nitrogenase activity, and P. schreberi from the Subarctic had higher activity than the same species from the temperate heath (F = 380.2; p < 0.0001). The highest moisture levels in the subarctic moss resulted in higher nitrogenase activity (F = 6.1; p = 0.02) and acetylene reduction increased with increasing moisture level in T. nitens (F = 37.3; p < 0.0001). However, no effect of moisture on nitrogenase activity was observed in P. schreberi from the temperate heath. Acetylene reduction was lowest shortly (1 day) after the move to 30 °C (F = 8.81; p = 0.0002) (Fig. 3). The data points from P. schreberi kept at 30 °C are above the line extrapolated from the 10 °C and 20 °C data after 1 day and 1 week, indicating that 30 °C is above the temperature optimum for the samples that were previously kept at the lower temperatures (Fig. 3a, b, d, e). However, after 2 weeks exposure to 30 °C, the data points fall on the fitted line, indicating that nitrogenase activity in the samples from 10 °C and 20 °C acclimatized to the new, higher temperature (Fig. 3g, h). The temperature optimum for acetylene reduction in T. nitens seemed to change with time (Fig. 3c, f, i), and after 2 weeks, the data points for the 30 °C samples fitted the extrapolated line from the 10 °C and 20 °C samples at 25% water content. This indicates that the mosses from the colder temperatures acclimatized to 30 °C.
Acetylene reduction (nmol g dw−1 h−1) in P. schreberi from a temperate heath and subarctic tundra, and T. nitens from arctic tundra exposed for 1 day (a–c), 1 week (d–f) and 2 weeks (g–i) to three different moisture levels (open circles, black squares, grey diamonds for the 25%, 50% and 100% moss water content, respectively) within one temperature (30 °C). Samples from the 10 °C and 20 °C treatments were moved into 30 °C. The lines were fitted through the mean acetylene reduction in the 10 °C and 20 °C treatments and thereafter extended to the axes. Data for the 30 °C treatment was plotted separately. Shown are mean ± SE (n = 5). Please note the logarithmic y-axis
3.4 Exposure to a lower temperature
Acetylene reduction in P. schreberi from the temperate heath changed over time, (F = 20.71; p < 0.0001), and was highest in the 20 °C samples (F = 45.4; p < 0.0001) and the effects of temperature was dependent on the time of exposure to the new temperatures (F = 49.89; p < 0.0001), but not on the moisture level of the moss (Online Resource 2a). Similarly, acetylene reduction in P. schreberi from the Subarctic changed over time (F = 4.65; p = 0.01), and was highest in the samples that have been moved from 30 °C to 20 °C (F = 45.6; p < 0.0001) (Online Resource 2b). The effects of temperature were dependent on the time (F = 6.49; p = 0.003) and on the moisture level of the mosses (F = 11.86; p = 0.0009). Nitrogenase activity in T. nitens was lowest shortly after the samples were moved to the new temperatures (F = 8.23; p = 0.0006). Moisture had a positive effect on acetylene reduction (F = 38.71; p < 0.0001), and was highest in the mosses that were moved from 30 °C to 20 °C (F = 362.8; p < 0.0001). Further, moisture and temperature interacted to affect nitrogenase activity (F = 4.57; p = 0.04) (Online Resource 2c).
3.5 Moss-pH
The pH was highest in T. nitens (5.59 ± 0.07), and lower in P. schreberi from subarctic tundra (4.32 ± 0.009) than from the temperate heath (4.76 ± 0.09; F = 94.73; p < 0.0001) (Online Resource 3).
3.6 Moss N content
Pleurozium schreberi from the temperate heath had the highest N tissue concentrations (averaged across all treatments: 0.92 ± 0.04% N) at the end of the experiment, and P. schreberi from the Subarctic (0.65 ± 0.03% N) had higher tissue N content than T. nitens (0.54 ± 0.01% N; F = 86.6, p < 0.00001). Total N content of mosses kept at 30 °C was higher than of mosses kept at 20 °C (F = 3.44, p = 0.035), as well as mosses with 25% moisture compared to mosses with 100% moisture (F = 6.169, p = 0.014) (Online Resource 4).
4 Discussion
4.1 Temperature by moisture experiment
We assessed the combined effects of temperature and moisture on N2 fixation in two moss species, and how the response to the contemporary climatic conditions (i.e. temperature and moisture) is shaped by the climatic history. Our results suggest that the response of N2 fixation in moss-cyanobacteria associations towards changes in temperature and moisture are shaped more strongly by the contemporary climatic conditions than by the historic conditions. Yet, exposure to extreme temperatures (30 °C for the arctic moss) seems to reveal the influence of the historic climate on N2 fixation. Nitrogen fixation was influenced by moisture only in the moss from the Arctic, T. nitens, somewhat contradicting our first hypothesis. Although nitrogenase activity in P. schreberi from the Subarctic seemed to be affected by moisture levels, the effects were mostly driven by the moss samples at 20 °C, 100% moisture, which was very variable in cyanobacterial colonization, and with that, activity. Nonetheless, our study suggests that N2 fixation is controlled in a hierarchical way (see e.g. Belnap 2001), and that the hierarchy differs between moss species and/or ecosystem. Nitrogen fixation in P. schreberi from both the temperate and subarctic site seem to be mostly driven by the temperature the colonized moss is exposed to, whereas activity in T. nitens seemed to be driven by both moisture and temperature. The differences in the response of N2 fixation to the different abiotic factors between the samples could be due to differences between the moss species. T. nitens can hold water two times longer than P. schreberi (Elumeeva et al. 2011), which could explain the much higher cyanobacterial colonization and N2 fixation rates in this moss. Even if we may not be able to ascertain if the differences were driven by the moss species or the ecosystem, our intent to deepen our understanding of the effects of climatic factors on moss-associated N2 fixation at ecosystem level made it necessary to choose the most dominant and abundant species in the respective ecosystem. Nevertheless, our study gives us novel insights into the abiotic controls of moss-associated N2 fixation.
The highest here investigated moisture level did not automatically translate into the highest nitrogenase activity, except for T. nitens, despite that moisture previously has been proposed as a fundamental driver of N2 fixation (Belnap 2001; Rousk et al. 2014, Rousk and Michelsen 2016). However, moist moss is more sensitive to heat stress than dry moss (Glime 2007). When mosses are dry, their activity is low or they are in a dormant state. When mosses are optimally moist, all biological process rates are high, and more damage possible, which in turn can affect the colonizing cyanobacteria. Further, if the photosystem of mosses is damaged too severely, the energy transfer to the colonizing cyanobacteria might be compromised, limiting N2 fixation. However, evidence of carbon (C) transfer from moss to cyanobacteria is still missing.
Temperature had a similar, positive effect on N2 fixation in all mosses, corroborating our second hypothesis only partly. Our experiment suggests that 30 °C is not extreme enough to damage the moss, even when moist. Most bryophytes have an optimum for growth around 20 °C (Dilks and Proctor 1975), Sphagnum mosses being an exception with a growth optimum at 30 – 35 °C (Li and Glime 1990). However, the measured air temperature can be very different from bryophyte temperature and they can cool down via evaporation (Glime 2007), thus growth likely occurs at higher temperatures. Also, cell membrane permeability increases with increasing temperatures (Liu et al. 2003), which could lead to C and nutrient release (e.g. phosphorus; Carleton and Read 1991) at higher temperatures, benefitting the colonizing cyanobacteria.
Although the subarctic site in Northern Sweden receives low amounts of atmospheric N (0–2 kg N ha−1 year−1) (Karlsson et al. 2009; Gundale et al. 2011), N2 fixation was low and comparable to N2 fixation in the same species from the temperate heath, which receives 11 kg N ha−1 year−1 (Ellermann et al. 2011). While this might be below the N deposition threshold of nitrogenase activity in mosses (see e.g. Rousk et al. 2014), the higher N content of the moss from the temperate heath (Online Resource 4) likely reflects the exposure to higher N depositions rates (Harmens et al. 2014).
We observed several different species of cyanobacteria on T. nitens, which could have their activity optimum at different temperatures and moisture levels (see also Gentili et al. 2005), whereas P. schreberi from from the Subarctic as well as from the temperate heath harboured less diverse cyanobacterial communities. This hints to a wider niche breadth of N2 fixation in T. nitens compared to P. schreberi. Future studies using molecular techniques could verify whether this is a general phenomenon.
4.2 Incubation-temperature and -time experiment
The experiment on the different incubation times demonstrates that the incubation time should be adjusted according to the incubation temperature. This is most likely the case for other enzyme assays as well, and not only for the acetylene reduction assay. With higher temperatures, biological process rates increase, and rates will be faster in the first hours and decrease over time. Thus, longer incubations (>20 h) underestimate process rates at higher temperatures. Previous studies assessing temperature effects on nitrogenase activity failed to adjust the incubation times according to temperature, and may potentially have underestimated and thereby, misrepresented N2 fixation rates. Furthermore, increased process rates lead to changes in the incubation conditions. For instance, rates of photosynthesis could increase which could lead to increased O2 concentrations, potentially inhibiting N2 fixation (Staal et al. 2001). Although more energy could be generated via higher photosynthesis rates, promoting N2 fixation, the increased activity will likely level out over time in long incubations, and the average activity will then be lower than it actually was. We assumed a Q10 of 2.5 (Smith 1984) and calculated the incubation times for the different temperatures accordingly, which seemed to be adequate for N2 fixation in mosses from the different ecosystems.
4.3 Exposure to higher and lower temperatures
The Q10 values indicate that N2 fixation in all mosses is more sensitive to a temperature change between 10 °C and 20 °C than between 20 °C and 30 °C. Furthermore, the optimum temperature for N2 fixation in both moss species seems to be between 20 °C and 30 °C and thus, falls within the kinetic optimum for nitrogenase (25 °C, Houlton et al. 2008). Nevertheless, lower temperature optima have been suggested for N2 fixation in mosses (peak activity at 13 °C and 22 °C due to different cyanobacterial colonizers with different optima (Gentili et al. 2005; see also Gundale et al. 2012b). The Q10 values for N2 fixation in P. schreberi from the Subarctic and T. nitens from the Arctic were variable and higher than for N2 fixation in P. schreberi from the temperate heath. This indicates higher sensitivity to a change in temperature in the high latitude ecosystems. Here, mosses are exposed to large temperature fluctuations within a few hours, probably more so than the mosses from the temperate heath. This historical exposure to extreme temperature differences could make the cyanobacterial colonizers more sensitive to a change in temperature. Nevertheless, the temperature optimum for N2 fixation in P. schreberi from both sites seems to increase within a few weeks of exposure to a different temperature, suggesting a capability for acclimatization. This corroborates our third hypothesis only partly since no acclimatization towards high temperatures could be observed in T. nitens. Acclimatization to high temperatures has been observed in bryophytes (e.g. in Polytrichum commune, Sveinbjörnsson and Oechel 1983) and they can increase their heat resistance if exposed to temperatures above their optimum temperature for growth (Antropova 1974). If increased temperatures do not necessarily inhibit moss growth, the cyanobacterial colonizers are also likely to be less affected by an extreme increase in temperatures. However, for T. nitens, 30 °C seems to be above the temperature optimum for N2 fixation. This is not surprising given a mean annual temperature of −3 °C at the arctic site compared to 0.5 °C and 8 °C at the subarctic and temperate site, respectively.
After moving the mosses to a lower temperature, the highest nitrogenase activity was found in the change from 30 °C to 20 °C: activity was higher after the move from 30 °C into 20 °C than activity before the move. This indicates that a temperature closer to 20 °C is more beneficial for N2 fixation in mosses than 30 °C. Although our extended curves suggest a higher temperature optimum of N2 fixation, the maximum temperature is likely to be close to those values (Smith 1984; Gentili et al. 2005). The temperature decrease from 20 °C to 10 °C and from 30 °C to 10 °C resulted in similar nitrogenase activity. This suggests that the historical climatic conditions (temperature) are not as important as the contemporary conditions or that the mosses and colonizing cyanobacteria acclimatize quickly to the new conditions. Nitrogenase activity in mosses that had been moved from 10 °C and 20 °C to 30 °C seemed to acclimatize to the new temperature within a few days. However, activity was higher in those mosses before the move, indicating that the response to an increased temperature is adaptation whereas the response towards a lower temperature is acclimatization.
4.4 Moss-pH
The pH optimum for N2 fixation in mosses is between 5.9 and 6.2 (Smith 1984) and even higher in free-living Nostoc (7 or above) (Granhall 1981). Thus, P. schreberi, with a pH below 5, might not provide the ideal conditions for the cyanobacterial colonizers. Yet, other results show that P. schreberi from boreal forests in Northern Sweden is highly colonized by cyanobacteria (Gundale et al. 2011, Rousk et al. 2013b, 2014), suggesting a habitat effect rather than a moss species effect.
5 Conclusions
Nitrogen fixation has been assumed to be higher in warmer regions than in boreal forests, subarctic and arctic habitats due to temperature limitation in northern ecosystems. Yet, N2 fixation does occur in the boreal biome, even at low temperatures and across a large range of temperatures as well as moisture levels, indicating a wide niche breadth of N2 fixation associated with mosses. Given the here shown ability of N2 fixation to acclimatize to an increase in temperature within a short time period in different moss species, ecosystem N input via moss-associated N2 fixation will increase in a future climate, especially in mosses that are less sensitive to low moisture levels.
References
Ackermann K, Zackrisson O, Rousk J, Jones DL, DeLuca TH (2012) N2 fixation in feather mosses is a sensitive indicator of N deposition in boreal forests. Ecosystems 15:986–998
Antropova TA (1974) Temperature adaptation studies on the cells of some bryophyte species. Tsitologiia 16:38–42
Belnap J (2001) Factors influencing nitrogen fixation and nitrogen release in biological soil crusts. In: Belnap J, Lange OL (ed) Biological soil crusts: structure, function, and management. Ecological studies, vol 150, pp 241–261. Springer, Heidelberg
Carleton TJ, Read DJ (1991) Ectomycorrhizas and nutrient transfer in conifer-feather moss ecosystems. Can J Bot 69:778–785
Coxson DS, Kershaw KA (1983) Rehydration response of nitrogenase activity and carbon fixation in terrestrial Nostoc commune from Stipa-Bouteloua grassland. Can J Bot 61:2658–2668
DeLuca TH, Zackrisson O, Nilsson MC, Sellstedt A (2002) Quantifying nitrogen-fixation in feather moss carpets of boreal forests. Nature 419:917–919
Dilks TJK, Proctor MCF (1975) Comparative experiments on temperature responses of bryophytes: Assimilation, respiration and freezing damage. J Bryol 8:317–336
Ellermann T, Andersen HV, Bossi R et al. (2011) Atmosfærisk deposition. Århus Universitet, pp 18–38, NOVANA
Elumeeva TG, Soudzilovskaia NA, During HJ, Cornelissen JHC (2011) The importance of colony structure versus shoot morphology for the water balance of 22 subarctic bryophyte species. J Veg Sci 22:152–164
Englund B, Meyerson H (1974) In situ measurement of nitrogen fixation at low temperatures. Oikos 25:283–287
Gentili F, Nilsson MC, Zackrisson O, DeLuca TH, Sellstedt A (2005) Physiological and molecular diversity of feather moss associative N2 -fixing cyanobacteria. J Exp Bot 56:3121–3127
Glime JM (2007) Bryophyte Ecology. Volume 1. Physiological Ecology. Ebook sponsored by Michigan Technological University and the International Association of Bryologists. http://www.bryoecol.mtu.edu/. Accessed 01 June 2015
Granhall U (1981) Biological nitrogen fixation in relation to environmental factors and functioning of natural ecosystems. In: Clark FE, Rosswall T (eds) Terrestrial nitrogen cycles, vol 33. Swedish Natural Research Council, Stockholm, pp 131–144
Gundale MJ, DeLuca TH, Nordin A (2011) Bryophytes attenuate anthropogenic nitrogen inputs in boreal forests. Glob Change Biol 17:2743–2753
Gundale MJ, Wardle DA, Nilsson MC (2012a) The effect of altered macroclimate on N-fixation by boreal feather mosses. Biol Lett 8:805–808
Gundale MJ, Nilsson M, Bansal S, Jäderlund A (2012b) The interactive effects of temperature and light on biological nitrogen fixation in boreal forests. New Phytol 194:453–463
Harmens H, Schnyder E, Thöni L, Cooper DM, Mills G, Leblond S et al (2014) Relationship between site-specific nitrogen concentrations in mosses and measured wet bulk atmospheric nitrogen deposition across Europe. Environ Pollut 194:50–59
Hearnshaw GF, Proctor MCF (1982) The effect of temperature on the survival of dry bryophytes. New Phytol 90:221–228
Houlton BZ, Wang YP, Vitousek PM, Field CB (2008) A unifying framework for dinitrogen fixation in the terrestrial biosphere. Nature 454:327–331
Ininbergs K, Bay G, Rasmussen U, Wardle DA, Nilsson MC (2011) Composition and diversity of nifH genes of nitrogen-fixing cyanobacteria associated with boreal forest feather mosses. New Phyt 192:507–517
Isichei AO (1980) Nitrogen fixation by blue-green algae soil cursts in Bigerian svanna. In: Rosswall T (ed) Nitrogen cycling in West African ecosystems. NFR, Stockholm, pp 191–199
Jackson BG, Martin P, Nilsson MC, Wardle DA (2011) Response of feather moss associated N2 fixation and litter decomposition to variations in simulated rainfall intensity and frequency. Oikos 120:570–581
Jones K (1977) Acetylene reduction in the dark by mats of blue-green algae in subtropical grassland. Ann Bot 41:807–812
Karlsson GP, Akselsson C, Hellsten S, Karlsson PE, Malm G (2009) Övervakning av luftföroreningar norra Sverige—mätningar och moddellering. Svenska Miljöinstitut IVL rapport B1851. Lund University
Kershaw KA (1985) Physiological ecology of lichens. Cambridge University Press, London
Li Y, Glime JM (1990) Growth and nutrient ecology of two Sphagnum species. Hikobia 10:445–451
Liu Y, Cao T, Glime JM (2003) The changes of membrane permeability of mosses under high temperature stress. Bryologist 106:53–60
Rousk K, Michelsen A (2016) Ecosystem nitrogen fixation throughout the snow-free period in subarctic tundra: Effects of willow and birch litter addition and warming. Glob Change Biol. doi:10.1111/gcb.13418
Rousk K, Rousk J, Jones DL, Zackrisson O, DeLuca TH (2013a) Feather moss nitrogen acquisition across natural fertility gradients in boreal forests. Soil Biol Biochem 61:86–95
Rousk K, DeLuca TH, Rousk J (2013b) The cyanobacterial role in the resistance of feather mosses to decomposition—toward a new hypothesis. PLoS One. doi:10.1371/journal.pone.0062058
Rousk K, Jones DL, DeLuca TH (2014) The resilience of nitrogen fixation in feather moss (Pleurozium schreberi)-cyanobacteria associations after a drying and rewetting cycle. Plant Soil 374:513–521
Rousk K, Sorensen PL, Lett S, Michelsen A (2015) Across habitat comparison of diazotroph activity in the Subarctic. Microb Ecol 69:778–787
Rousk K, Sorensen PL, Michelsen A (2016) Nitrogen transfer from four nitrogen fixer associations to plants and soils. Ecosystems. doi:10.1007/s10021-016-0018-7
Smith VR (1984) Effects of abiotic factors on acetylene reduction by cyanobacteria epiphytic on moss at a subantarctic island. Appl Environ Microbiol 48:594–600
Staal M, te Lintel-Hekkert S, Harren F, Stal L (2001) Nitrogenase activity in cyanobacteria measured by the acetylene reduction assay: a comparison between batch incubation and on-line monitoring. Environm Microbiol 3:343–351
Stewart WDP, Smapaio MJ, Isichei AO, Sylvester-Bradley R (1977) Nitrogen fixation by soil algae of temperate and tropical soils. In: Döbereiner J, Burris RH, Hollaender A, Franco AA, Neyra CA, Scott DB (eds) Limitations and potential for biological nitrogen fixation in the tropics. Plenum Press, New York, pp 41–63
Stewart KJ, Coxson D, Grogan P (2011) Nitrogen inputs by associative cyanobacteria across a low arctic tundra landscape. Arct Antarct Alp Res 43:267–278
Strickland MS, Keiser AD, Bradford MA (2015) Climate history shapes contemporary leaf litter decomposition. Biogeochem 122:165–174
Sveinbjörnsson B, Oechel WC (1983) The effect of temperature preconditioning on the temperature sensitivity of net CO2 flux in geographically diverse populations of the moss Polytrichum commune. Ecology 64:1100–1108
Zielke M, Solheim B, Spjelkavik S, Olsen RA (2005) Nitrogen fixation in the high arctic: role of vegetation and environmental conditions. Arct Antarct Alp Res 37:372–378
Acknowledgments
Funding was provided by the Danish Council for Independent Research and FP7 Marie Curie Actions “COFUND” (Grant ID: DFF—1325-00025), as well as from the Danish Council for Independent Research “Research Project 1” (Grant ID: DFF—6108-00089), and the Danish National Research Foundation (Center for Permafrost, CENPERM DNRF100). We thank Gosha Sylvester and Maja Holm Wahlgren for assistance with laboratory analyses at the University of Copenhagen, and Abisko Scientific Research Station for logistics and access to climate data.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rousk, K., Pedersen, P.A., Dyrnum, K. et al. The interactive effects of temperature and moisture on nitrogen fixation in two temperate-arctic mosses. Theor. Exp. Plant Physiol. 29, 25–36 (2017). https://doi.org/10.1007/s40626-016-0079-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40626-016-0079-1