Abstract
Background
Chronic kidney disease (CKD) is commonly associated with psychosocial problems, especially depression, contributing to poor overall outcomes. Depression has not been given adequate priority in the management of CKD patients despite its significant adverse impact on all major outcomes. This systematic review and meta-analysis determined the pooled prevalence of clinical depression in the global CKD population and sub-populations.
Methods
PubMed, African Journals Online (AJOL), and EMBASE were systematically searched to identify published articles with relevant data. The pooled prevalence of clinical depression in the global CKD population was determined using random effects meta-analytic techniques. The study protocol was registered with PROSPERO (CRD42022382708).
Results
Sixty-five articles were included in this review, comprising 80,932 individuals with CKD from 27 countries. The participants' mean age ranged from 11.0 to 76.3 years. Most (70.4%) of the studies had medium methodological quality. The overall pooled prevalence of depression was 26.5% (95% CI 23.1–30.1%). Studies using the Diagnostic Statistical Manual for Mental Diseases (DSM) and International Classification of Disease (ICD) returned a pooled prevalence of 25.5% and 39.6%, respectively, p = 0.03. There was a significant difference in the pooled prevalence across regions; p = 0.002.The prevalence of depression was higher among individuals on chronic hemodialysis compared to pre-dialysis patients (29.9% versus 18.5%; p = 0.01) and among those on hemodialysis compared to peritoneal dialysis (30.6% versus 20.4%; p = 0.04). There was no significant difference between adults and children (26.8% versus 15.9%, p = 0.21). There was an increasing temporal trend in depression prevalence, though this did not achieve statistical significance (p = 0.16).
Conclusion
Depression is common in patients with CKD. The findings of this study highlight the need for clinicians to make efforts to evaluate individuals with CKD for depression, especially those with advanced stages of the disease.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) is a common disease affecting about 700 million people globally [1]. It is a significant cause of mortality worldwide [2, 3]. The burden of CKD is higher in low- and middle-income countries than in high-income countries [3]. It causes a substantial economic, physical, and psychosocial burden for the patient [4, 5]. Psychosocial problems such as depression and anxiety are more commonly encountered in patients with CKD and their caregivers compared to the general population [6, 7]. These problems adversely affect the quality of life and contribute to deterioration in kidney function, progression to an advanced stage of disease, hospitalization, and mortality in individuals with CKD [8, 9]. Depression may contribute to poor adherence to medications, fluid, and dietary prescriptions of individuals with CKD [10, 11]. It also plays a pivotal role in the development of cardiovascular disease in CKD [12]. Psychosocial problems adversely affect the quality of life of individuals with CKD and contribute to overall poor outcomes of CKD [6, 13].
Despite the significant impact of psychosocial problems on the overall outcomes of CKD, they have not been given adequate priority in the management of individuals with CKD. Depression is not routinely screened for and managed in these patients, and mental health professionals were rarely mentioned in their multidisciplinary management [14]. Although there is inertia in the use of medications to manage psychosocial problems such as depression in CKD due to safety concerns by most clinicians, evidence supporting the safety of some medications and associated beneficial effects is now emerging [15]. In addition, non-pharmacological therapy, such as psychosocial interventions, has been established to improve the quality of life and reduce depression and anxiety in individuals with CKD [16]. Routine evaluation for common psychosocial problems, especially depression in this at-risk group, will aid in prompt diagnosis and allow those affected to be managed with consequent improvement in their overall outcomes.
This study, an update of an earlier review [17], assessed the prevalence of clinical depression in the global chronic kidney disease population. In addition, we reported the prevalence of depression in the pediatric CKD population and documented the geographic differences and temporal trends in depression prevalence in the CKD population. The findings of the study will provide helpful information on the burden of depression and also serve as empirical evidence to include its routine evaluation and treatment in the management of CKD, especially in countries where this is not routinely done.
Methods
This systematic review and meta-analysis includes studies that document the prevalence of clinical depression among patients with CKD. The Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guideline [18] was used in reporting this study (Supplementary Table 1). This study was registered with PROSPERO with registration number CRD42022382708.
Literature search
A systematic literature search was done on published articles involving individuals with CKD who had clinically diagnosed depression. We performed a search on PubMed, African Journal Online, and Embase using terms related to depression in chronic kidney disease such as "chronic kidney disease", "CKD", "chronic renal failure", "CRF", "end-stage renal disease, "ESRD", "end-stage kidney failure", "ESKF", "dialysis-dependent chronic kidney disease", "renal failure", " non-dialysis dependent chronic kidney disease", "pre-dialysis chronic kidney disease", "chronic renal insufficiency", "dialysis population" "depression", "depressive symptom", mental health disorder ", psychiatric disorder' in conjunction with all the names of continents. The search strategy, created in April 2023, is detailed in Supplementary Tables 2A and 2B. There was no language limitation. A hand search of the reference list of articles of interest was also undertaken.
Study selection
Inclusion criteria were cross-sectional and prospective studies reporting on clinically diagnosed depression in adult and pediatric populations and pre-dialysis and dialysis populations across all the World Health Organization (WHO) regions. Exclusion criteria were studies that used screening tools to diagnose depression because it has been shown that the latter overestimate depression prevalence [19]; studies that involved kidney transplant patients; abstract papers on depression in CKD without full text; studies that determined clinical diagnosis of depression but did not clearly define the study population in terms of those on peritoneal dialysis (PD), hemodialysis (HD) or who had transplant; and randomized controlled trial studies on depression in CKD. Two investigators (OAA and OOI) independently screened records for eligibility based on titles and abstracts. Full texts of articles deemed potentially eligible were retrieved and screened by the same investigators (OAA and OOI) for final inclusion. All conflicts were resolved by a third investigator (OEY).
Data extraction and management
The following variables were extracted from selected studies: the last name of the first author, year of publication and country and continent in which the study was carried out, sample size of the study, duration of the study, study design, mean age of the study participants, stage of CKD, type of renal replacement therapy, the proportion of subjects with depression, clinical depression diagnostic criteria used [Diagnostic Statistical Manual for Mental Disorders III (DSM III), DSM IV, DSM V, International Classification of Disease-9 (ICD-9), ICD-10], and severity of depression. The data extraction form was developed with input from all the investigators, including Mental Health and Nephrology specialists. The data were extracted by two different investigators, and a third investigator resolved areas of conflict. We categorized study location based on the WHO regions [(African Region, AFR), (Region of the Americas, AMR), (South East Asian Region, SEAR), (European Region, EUR), (Eastern Mediterranean Region, EMR), and (Western Pacific Region, WPR)]. Studies were also categorized based on the year of publication of the study, severity of CKD and dialysis modality.
Methodological quality
The Joanna-Briggs Institute Critical Appraisal Checklist for Studies Reporting Prevalence Data was used to assess the methodological quality of the constituent studies [20]. Studies scored 1 for each of the nine questions with a "yes" response. Studies with scores 0–3 were regarded as poor quality, 4–6 as intermediate or medium quality, and 7–9 as high quality. Two different investigators did the quality assessment, and areas of disagreement were resolved by consensus.
Ethical consideration
Ethical approval was not required. The study protocol was registered with PROSPERO (CRD42022382708).
Statistical analysis
Stata 17.0 (Stata Corp., 2021. Stata Statistical Software: Release 17, College Station, TX) was used for statistical analysis. The pooled prevalence of depression in the global CKD population was determined using meta-analytic techniques. The study-specific estimates derived from the DerSimonian-Laird random effects model [21] were pooled to estimate the prevalence of depression in this population. To minimize the effect of extreme values, the Freeman-Tukey double arcsine transformation [22] was used to stabilize the individual study variances before using the random effects model to obtain the pooled estimates. Publication bias was assessed using the Egger test [23]. We also undertook a subgroup analysis of pooled prevalence by continent, HD versus PD population, pre-dialysis versus dialysis CKD population, pediatric versus adult population with CKD, among studies that used different clinical diagnostic criteria such as DSM III, DSM IV, DSM V, ICD-9 and ICD-10 and among studies that fell into different range of years. Subgroup analysis was performed using the Q-test based on ANOVA. The I2 statistic was used to determine the between-study heterogeneity.
Results
Study selection and characteristics
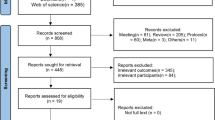
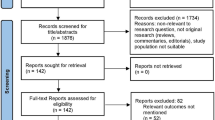
The systematic literature search identified 9240 articles, of which 137 were selected for full-text review after duplicate removal and title and abstract screening. Finally, 65 articles [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88] were eligible and therefore included in this systematic review (Fig. 1), with publication years ranging from 1987 to 2023. Included studies reported on 80,932 CKD and kidney failure patients from 27 countries. There were 249 participants (3 studies) from the African Region [32, 60, 72]; 4,295 (14 studies) from the European Region [33, 35, 36, 38, 41, 45, 58, 63, 68, 70, 77, 80, 82, 84]; 4,160 (19 studies) from the Region of the Americas [24,25,26,27,28, 31, 34, 37, 40, 47, 55, 57, 64, 67, 69, 71, 79, 85, 87]; 679 (7 studies) from the Eastern Mediterranean Region [42, 51, 53, 62, 66, 76, 83]; 846 (7 studies) from the South East Asian Region [30, 49, 52, 61, 73, 75, 81], and 70,703 (15 studies) from the the Western Pacific Region [29, 39, 43, 45, 46, 48, 50, 54, 56, 59, 65, 74, 78, 86, 88]. Figure 2 shows the geographical distribution of the included studies.
The overwhelming majority of the studies (60 studies, 92.3%) used the DSM clinical diagnostic criteria or one of its derivatives, while five studies used the ICD criteria. The sample size of the component studies ranged from 20 [72] to 67,866 [39] patients, with the proportion of women ranging from 0% [27] to 68% [76]. The mean age of the participants ranged from 11.0 years [62] to 76.3 years [79]
Most of the studies had medium methodological quality (70.8%, n = 46) (Table 1); 14 studies (21.5%) were of high quality, including three studies from the Americas [25, 27, 79], one study from the African region [32], two studies from the Western Pacific region [44, 77], three studies from the Eastern Mediterranean region [66, 76, 83], three from the European region [63, 77, 82] and two studies from South East Asia [73, 81]. Tables 1 and 2 summarize the data extracted from the constituent articles and the sub-populations studied. The majority (39, 60.0%) of the articles reported on severe or major depressive illness only; 5 (7.7%) reported on the whole spectrum of depression; 2 (3.1%) reported only on mild or moderate depression, while 15 (23.1%) did not state depression severity.
Prevalence of depression
The overall pooled prevalence of depression was [26.5% 95% CI 23.1–30.1%), N = 65 studies, I2 = 97.2%, p < 0.001 for heterogeneity] irrespective of the clinical depression diagnostic criteria used. Studies using DSM and ICD returned a pooled prevalence of [25.5% (21.3–30.0%), n = 60 studies, I2 = 95.9%, p < 0.001 for DSM] versus [39.6% (95% CI 28.1–51.7%), n = 5 studies, I2 = 98.7%, p < 0.001 for ICD], p = 0.03 for difference between the different diagnostic criteria (Fig. 3). The p-value for the Egger test was 0.83, suggesting no small study effects.
The South East Asian Region had the highest pooled prevalence of 43.2% (32.2–54.5%, I2 = 90.2%, p < 0.001, n = 7 studies) compared to the Region of the Americas 19.9% (16.7–23.3%, n = 19 studies, I2 = 82.2%, p < 0.001), the Western Pacific Region 23.3% (18.8–28.1%, n = 15 studies, I2 = 93.3%), the African Region 27.2% (14.2–42.5%, n = 3 studies, I2 = 78.4%, p = 0.01), and the European Region 25.7% (14.8–38.3%, n = 14 studies, I2 = 97.5%, p < 0.001); p = 0.01 for difference across groups (Supplementary Fig. 1).
There was a significantly higher pooled prevalence of clinical depression among the patients on chronic HD compared to those in the pre-dialytic stages of CKD 29.9% (24.4–35.8%, n = 44 studies, I2 = 95.6%, p < 0.001) versus 18.5% (12.9–24.9%, n = 10 studies, I2 = 93.1%, p < 0.001); p = 0.01 for difference across the groups (Supplementary Fig. 2).
There was no significant difference in the pooled prevalence among adults compared with children (26.8% vs 15.9%; p = 0.21), Supplementary Fig. 3. There was a higher prevalence of depression among the HD than the PD population, 30.6% (25.0–36.6%, n = 43 studies, I2 = 95.5%, p < 0.001) versus 20.4% (13.1–28.7%, n = 3 studies, I2 = 41.7%, p = 0.18); p = 0.047 for difference between the groups, Supplementary Fig. 4. There appeared to be an increasing temporal trend in depression prevalence. However, this increase did not achieve statistical significance (p = 0.16, Supplementary Figs. 5 and 6). The pooled prevalence for the studies published in 2000 and prior was 17.7% (8.9–28.6%) compared to 26.5% (22.2–31.1%) for those published between the years 2001 and 2020 and 34.4% (20.7–49.5%) for those published after 2020. This trend persisted when considering all studies irrespective of the diagnostic criteria used (Supplementary Fig. 5) or when only the DSM criteria were used (Supplementary Fig. 6).
Discussion
This systematic review and meta-analysis determined the pooled prevalence of clinical depression among 80,932 individuals with CKD from 27 countries spread across the globe. The pooled prevalence of clinical depression was 26.5%. The prevalence varied with the CKD population's geographical location, CKD stage, age group of the individuals with CKD, and dialysis modality (HD versus PD).
The pooled prevalence of depression in the CKD population in this study is higher than the prevalence of 8.4% and 6.9% reported in the general adult population in the United States and Norway, respectively [89, 90]. Similarly, the prevalence of depression in the pediatric population with CKD is higher than the 3.2% reported in the general pediatric population in the United States [91]. These findings showed that depression is a common mental health problem in the CKD population compared to the general population. The prevalence of depression in this study is higher than 21.9% and 16.3% reported among individuals with epilepsy and cancer, respectively [92, 93], suggesting that the magnitude of depression in CKD is higher than in some other chronic illnesses.
The pooled prevalence of depression in this study is higher than 21.4% and 22.8% reported in individuals with CKD who were in pre-dialysis and dialytic stages, respectively, in a systematic review and meta-analysis by Palmer et al.[17] conducted a decade ago. It is, however, lower than 62% reported in a systematic review and meta-analysis involving a population with CKD in Iran [94]. The wide difference in the prevalence rates may be due to the method of assessment of depression and the stage of individuals with CKD that were studied. While the diagnosis of depression was made in the present study using standard clinical diagnostic criteria, the study in Iran used a validated depression screening tool for the diagnosis of depression. The Iranian study also involved only patients on maintenance HD, while the present study included individuals with CKD who were both in the pre-dialysis and dialysis stages.
There was no significant difference between the pooled prevalence of depression in the adult and pediatric population. However, this finding should be interpreted with caution as only two studies involving the pediatric population were included in this analysis. In addition, the prevalence of depression from these studies could have been underestimated as depression may have been unrecognized in children who are largely unable to express themselves [95] adequately. Also, the presentation of depression in children may not follow the typically known presentation as commonly seen in the adult population [95]. This underscores the need for physicians to consider these peculiarities in managing the pediatric CKD population.
The pooled prevalence of clinical depression across the various continents ranges between 19.9 and 43.2%, with the South East Asian Region having the highest while the Region of the Americas had the lowest. The African Region had the second-highest pooled prevalence of depression (27.2%). There was a significant difference in the pooled prevalence of depression in CKD across the various continents. The difference in the severity of CKD, age and socioeconomic status of individuals with CKD, available psychosocial support, governmental support, type of available health care financing, level of health care sophistication, type of maintenance HD and race may be partly responsible for the significant variations in prevalence of depression across the continents. The African continent had the lowest number of studies, while the Region of the Americas had the highest number of studies. This underscores the need for more studies on depression in the African continent in order to ascertain the magnitude of the problem in the region.
Among the studies included in this review, DSM diagnostic criteria were more commonly used for the diagnosis of clinical depression. This may be due to the fact that DSM has a better application in research settings than ICD [96]. Although the DSM was primarily designed for use by psychiatrists in the United States, unlike ICD, which is a global disease classification designed for use by all health practitioners, especially in low and middle-income countries, its use has been reported in many other countries [96]. The pooled prevalence of depression in the studies that used ICD criteria was 39.6%, which was significantly higher than 25.5% in the studies that used DSM criteria. This finding is similar to the report of Wittchen et al. [97], which showed a higher depression prevalence of 11.3% with ICD-10 when compared with 4.2% using DSM 4 in the same population. This finding may be because ICD has greater sensitivity in the diagnosis of mild depression than DSM [98].
There was a significantly higher pooled prevalence of depression among individuals with end-stage kidney disease (ESKD) on HD compared to those in the pre-dialysis stage (29.9% versus 18.5%) in this study. The demands of dialysis treatment on patients with ESKD may partly account for this significant difference. Other factors, such as dietary restriction, inflammation, hormonal changes, poor sleep quality, and reduced quality of life associated with dialysis treatment, may also contribute to a higher frequency of depression in patients on HD compared to individuals with pre-dialysis CKD [15, 99]. A qualitative study by Avdal et al. [100] reported that patients with ESKD experienced despair and decreased social support from relatives and caregivers after the commencement of PD or HD. This may effectively contribute to a higher prevalence of depression in them compared to individuals with pre-dialysis CKD. This study also showed that about one in five individuals with pre-dialysis CKD had depression. This underscores the need to screen individuals with CKD, even in the early stages, for depression so that prompt treatment can be instituted. This may consequently improve their overall outcomes.
The pooled prevalence of depression was significantly higher in individuals with ESKD on maintenance HD compared with those on PD (30.6% versus 20.4%) in this study. There are conflicting reports from existing literature on the relationship between depression and mode of dialysis. The finding of this study is, however, supported by Martin et al. [101] but differs from some previous reports that showed a significantly higher prevalence of depression in PD patients compared to those on HD [102, 103]. Although some other studies reported a higher prevalence of depression in HD patients compared with PD patients, the difference was not statistically significant [104, 105].
There was an increasing temporal trend in depression prevalence after the year 2020 (34.4%) compared to 2001–2020 (26.5%) and before 2000 (17.7%). This may be a reflection of the increase in the level of awareness of mental health problems globally with improvement in campaigns geared towards sensitization of the global community. This is supported by a review by Foulke et al. [106], where it was reported that there has been increased recognition and diagnosis of mental health disorders over the years.
The use of clinical methods of assessment of depression is more specific than the use of validated screening tools in the CKD population. Depression screening tools have been reported to over-diagnose depression in individuals with CKD, especially those at advanced stages [17]. The study by Palmer et al. [17] is instructive in this regard, as it showed that the prevalence of depression was higher in the CKD population when depression screening tools were used compared to when clinical diagnostic criteria were deployed. This was also buttressed by Smith et al. [107] in their study, which showed prevalence rates of depression in the same individuals on maintenance HD as 47% and 5% using Beck's depression index and DSM III, respectively. Also, in cases where depression screening tools are being used in the assessment of depression, individuals with advanced CKD may have some uremic symptoms such as poor sleep, anorexia, fatigue, and lack of concentration, which may overlap and be regarded as somatic symptoms of depression [15, 99]. Another limitation of the use of depression screening tools lies in the fact that a higher score is commonly used as a cut-off in advanced CKD compared to the general population [15, 99]. These cut-off values may vary in different studies using the same screening tool, introducing major inconsistencies.
Depression has a significant adverse impact on the overall outcomes of CKD. Hence, it deserves adequate attention by being promptly diagnosed and managed by the clinician. Depression is associated with poor compliance with treatment follow-up, increased suicidal tendency, withdrawal from dialysis, non-adherence to medications, dietary and fluid restrictions and malnutrition-inflammatory-atherosclerosis [10, 11, 108,109,110]. These may account for the increased hospitalization and disease progression, reduced quality of life, and increased mortality in individuals with CKD who have depression [6, 8, 9, 13, 111,112,113]. Prompt diagnosis and management of depression may mitigate these adverse consequences and improve the overall outcome of these patients.
There is clinician inertia in treating depression in CKD with antidepressants because of uncertainty about the pharmacokinetic properties of the medications and safety profile in people with reduced kidney function. This is supported by a report that showed that only about one-third of individuals with CKD diagnosed with depression received treatment [114]. Furthermore, most randomized controlled trials on the efficacy and safety of antidepressants exclude individuals with CKD. Despite the above, there is evidence, though limited, to support the effectiveness of antidepressants in reducing depression and improving QoL in individuals with CKD [115]. This present systematic review showed that clinical depression is common in CKD and lends credence to the need for the inclusion of the CKD population in clinical trials on the efficacy of antidepressant treatment. Management of depression also involves non-pharmacological treatments. There are reports of some randomized controlled trials that showed the efficacy of non-pharmacological treatment of depression, such as cognitive-based therapy and relaxation techniques, in the treatment of depression in CKD [16, 116,117,118]. A multidisciplinary team-based approach that includes mental health professionals will, therefore, be highly valuable in the management of individuals with CKD with depression.
A limitation of this study is that only a few studies in this review determined the prevalence of depression in the early stages of CKD. Secondly, there was limited information on the prevalence of clinically diagnosed depression in the pediatric population, being reported in only two studies. The strength of this systematic review lies in the fact that the pooled prevalence of depression found in the CKD population is a fairly true representation of the magnitude of the disease. This is particularly true because the study included only articles that used clinical interviews, which are more specific than screening tools.
Conclusion
This systematic review and meta-analysis showed that depression is a relatively common mental health disorder in the CKD population. It has brought to the fore the need for clinicians to make deliberate efforts to evaluate individuals with CKD, especially those with advanced stages of the disease for depression. The findings of this review have also given credence to the call to include the CKD population in large, randomized controlled trials on the safety and efficacy of antidepressants because they are potential beneficiaries of the findings of such studies.
Data Availability statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Carney EF (2020) The impact of chronic kidney disease on global health. Nat Rev Nephrol 16(5):251–252
Foreman KJ, Marquez N, Dolgert A et al (2018) Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet 392(10159):2052–2090
GBD Chronic Kidney Disease Collaboration (2020) Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 395(10225):709–733
Wang V, Vilme H, Maciejewski ML et al (2016) The economic burden of chronic kidney disease and end-stage renal disease. Semin Nephrol 36(4):319–330
Fletcher B, Damery S, Aiyegbusi OL et al (2022) Symptom burden and health-related quality of life in chronic kidney disease. PLoS Med 19(4):e1003954
Adejumo OA, Okaka EI, Akinbodewa AA et al (2021) Self-perceived burden on caregivers, anxiety and depression among chronic kidney disease patients in Southern Nigeria. West Afr J Med 38(4):335–341
Adejumo OA, Iyawe IO, Akinbodewa AA et al (2019) Burden, psychological well-being and quality of life of caregivers of end stage renal disease patients. Ghana Med J 53(3):190–196
Tsai YC, Chiu YW, Hung CC et al (2012) Association of symptoms of depression with progression of CKD. Am J Kidney Dis 60(1):54–61
Hedayati SS, Jiang W, O’Connor CM et al (2004) The association between depression and chronic kidney disease and mortality among patients hospitalized with congestive heart failure. Am J Kidney Dis 44(2):207–215
Gebrie MH, Ford J (2019) Depressive symptoms and dietary non-adherence among end stage renal disease patients undergoing hemodialysis therapy: systematic review. BMC Nephrol 20(1):429. https://doi.org/10.1186/s12882-019-1622-5
Safdar N, Baakza H, Kumar H et al (1995) Non-compliance to diet and fluid restrictions in haemodialysis patients. J Pak Med Assoc 45(11):293–295
Aromaa A, Raitasalo R, Reunanen A et al (1994) Depression and cardiovascular diseases. Acta Psychiatr Scand 89:77–82
Khan WA, Ali SK, Prasad S et al (2019) A comparative study of psychosocial determinants and mental well-being in chronic kidney disease patients: a closer look. Ind Psychiatry J 28(1):63–67
Shi Y, Xiong J, Chen Y et al (2018) The effectiveness of multidisciplinary care models for patients with chronic kidney disease: a systematic review and meta-analysis. Int Urol Nephrol 50(2):301–312
Shirazian S, Grant CD, Aina O et al (2016) Depression in chronic kidney disease and end-stage renal disease: similarities and differences in diagnosis, epidemiology, and management. Kidney Int Rep 2(1):94–107
Pascoe MC, Thompson DR, Castle DJ et al (2017) Psychosocial interventions for depressive and anxiety symptoms in individuals with chronic kidney disease: systematic review and meta-analysis. Front Psychol 8:992. https://doi.org/10.3389/fpsyg.2017.00992
Palmer S, Vecchio M, Craig JC et al (2013) Prevalence of depression in chronic kidney disease: systematic review and meta-analysis of observational studies. Kidney Int 84(1):179–191
Page MJ, McKenzie JE, Bossuyt PM, et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev 10(89). https://doi.org/10.1186/s13643-021-01626-4
Levis B, Yan XW, He C et al (2019) Comparison of depression prevalence estimates in meta-analyses based on screening tools and rating scales versus diagnostic interviews: a meta-research review. BMC Med 17(65). https://doi.org/10.1186/s12916-019-1297-6
Joanna Briggs Institute (2017) JBI critical appraisal checklist for studies reporting prevalence data. University of Adelaide, Adelaide
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188
Miller JJ (1978) The inverse of the Freeman-Tukey double arcsine transformation. Am Stat 32(4):138. https://doi.org/10.1080/00031305.1978.10479283
van Enst WA, Ochodo E, Scholten RJ, et al (2014) Investigation of publication bias in meta-analyses of diagnostic test accuracy: a meta-epidemiological study. BMC Med Res Methodol 14(70). https://doi.org/10.1186/1471-2288-14-70
Hedayati SS, Bosworth HB, Kuchibhatla M et al (2006) The predictive value of self-report scales compared with physician diagnosis of depression in hemodialysis patients. Kidney Int 69(9):1662–1668
Hedayati SS, Minhajuddin AT, Toto RD et al (2009) Prevalence of major depressive episode in CKD. Am J Kid Dis 54(3):424–432
Hedayati SS, Minhajuddin AT, Afshar M et al (2010) Association between major depressive episodes in patients with chronic kidney disease and initiation of dialysis, hospitalization, or death. JAMA 303(19):1946–1953
Hedayati SS, Grambow SC, Szczech LA et al (2005) Physician-diagnosed depression as a correlate of hospitalization in patients receiving long-term hemodialysis. Am J Kid Dis 46(4):642–649
Berney-Martinet S, Key F, Bell L et al (2009) Psychological profile of adolescents with a kidney transplant. Pediatr Transplant 13(6):701–710
Bautovich A, Katz I, Loo CK et al (2018) Beck depression inventory as a screening tool for depression in chronic haemodialysis patients. Australas Psychiatry 26(3):281–284
Gupta S, Patil NM, Karishetti M et al (2018) Prevalence and clinical correlates of depression in chronic kidney disease patients in a tertiary care hospital. Indian J Psychiatry 60(4):485–488
Moura Junior JA, Souza CA, Oliveira IR et al (2006) Prevalence of psychiatric disorders in patients in hemodialysis in the state of Bahia. J Bras Psiquiatr 55:178–183
Adesokun OK, Okeafor CU, Stanley PC (2020) Prevalence and correlates of depression among patients with chronic kidney disease. J Egypt Soc Nephrol Trans 20(3):173
Corruble E, Durrbach A, Charpentier B et al (2010) Progressive increase of anxiety and depression in patients waiting for a kidney transplantation. Behav Med 36(1):32–36
Drayer RA, Piraino B, Reynolds CF III et al (2006) Characteristics of depression in hemodialysis patients: symptoms, quality of life and mortality risk. Gen Hosp Psychiatry 28(4):306–312
Cilan H, Sipahioglu MH, Oguzhan N et al (2013) Association between depression, nutritional status, and inflammatory markers in peritoneal dialysis patients. Ren Fail 35(1):17–22
Cilan H, Oguzhan N, Unal A et al (2012) Relationship between depression and proinflammatory cytokine levels in hemodialysis patients. Ren Fail 34(3):275–278
Balogun RA, Turgut F, Balogun SA et al (2011) Screening for depression in elderly hemodialysis patients. Nephron Clin Pract 118(2):72–77
Chilcot J, Wellsted D, Farrington K (2008) Screening for depression while patients dialyze: an evaluation. Nephrol Dial Transplant 23(8):2653–2659
Choi HS, Kim B, Han KD et al (2023) Weight change and risk of depression in patients with diabetic kidney disease: a Nationwide population-based study. Kidney Res Clin Pract 42(1):86–97
Loureiro AC, de Rezende Coelho MC, Coutinho FB et al (2018) The influence of spirituality and religiousn;ess on suicide risk and mental health of patients undergoing hemodialysis. Comp Psychiary 80:39–45
Reckert A, Hinrichs J, Pavenstadt H et al (2013) Prevalence and correlates of anxiety and depression in patients with end stage renal disease. Z Psychosom Med Psycother 59:170–188
Al Zaben F, Khalifa DA, Sehlo MG et al (2014) Depression in patients with chronic kidney disease on dialysis in Saudi Arabia. In Urol Nephrol 46(12):2393–2402
Zhu FX, Zhang XY, Ding XK et al (2017) Protective effect of regular physical activity on major depressive episodes in patients with early stages of chronic kidney disease. Ren Fail 39(1):602–606
Fukunishi I, Kitaoka T, Shirai T et al (2002) Psychiatric disorders among patients undergoing hemodialysis therapy. Nephron 91:344–3347
Soykan A, Boztas H, Kutlay S et al (2004) Depression and is 6-month course in untreated hemodialysis patients: A preliminary prospective follow-up study in Turkey. Int J Behav Med 11(4):243–246
Yeh CY, Chen CK, Hsu HJ et al (2014) Prescription of psychotropic drugs in patients with chronic renal failure on hemodialysis. Ren Fail 36(10):1545–1549
Martiny C, de Oliveira e Silva AC, Neto JP, et al (2011) Factors associated with risk of suicide in patients with hemodialysis. Compr Psychiatry 52(5):465–468
Koo JR, Yoon JW, Kim SG et al (2003) Association of depression with malnutrition in chronic hemodialysis patients. Am J Kid Dis 41(5):1037–1042
Saritha CH, Gautham RC (2012) Anxiety, depression and sleep disturbances among renal disorders in tertiary care center. Int J Res Pharm Sci 12(1):310–314
Dimaano EP, Masendo AB, Oco M et al (2021) Cross- cultural visayan translation and validation of Becks depression inventory scale among ambulatory maintenance hemodialysis at a tertiary training hospital in Southern Mindanao. Philippines Philippines J Int Med 59(2):149–160
Alsuwaida A, Alwahhabi F (2006) The diagnostic utility of self-reporting questionnaire (SRQ) as a screening tool for major depression in hemodialysis patients. Saudi J Kidney Dis Transpl 17(4):503–510
Chandra P, Deo RM, Singh BK (2011) A study of psychiatry co-morbidity in cases of renal failure undergoing hemodialysis. Indian J Public Health Res Dev 2(1):68–70
Donia AF, Zaki NF, Elassy M et al (2015) Study on depression and quality of life among hemodialysis patients: an Egyptian experience. Int Urol Nephrol 47:1855–1862
Chen CK, Tsai YC, Hsu HJ et al (2010) Depression and suicide risk in hemodailysis patients with chronic renal failure. Psychosomatics 51:528–528
Cukor D, Coplan J, Brown C et al (2008) Anxiety disorders in adults treated by hemodialysis: a single-center study. Am J Kid Dis 52(1):128–136
Huang TL, Lee CT (2007) Low serum albumin and high ferritin levels in chronic hemodialysis patients with major depression. Psychiatry Res 152:277–280
Jain N, Carmody T, Minhajuddin AT et al (2016) Prognostic utility of a self-reported depression questionnaire versus clinician-based assessment on renal outcomes. Am J Nephrol 44:234–244
Baykah H, Yargic I (2012) Depression, anxiety disorders, quality of life and stress coping strategies in hemodialysis and continuous ambulatory peritoneal dialysis patients. Bull Clin Psychopharmacol 22(2):167–176
Chan LK, Yu ECS, Li SY (2011) Depression in patients receiving peritoneal dialysis. East Asian Arch Psychiatry 21(3):99–107
Azegbeobor J, Lasebikan VO (2015) Depression and disability in chronic kidney disease in Nigeria: a case control Study. Int Neuropsychiatric Dis J 7(2):1–13
Sumanathissa M, De Silva VA, Hanwella R (2011) Prevalence of major depressive episode among patients with pre-dialysis chronic kidney disease. Intl J Psychiatry Med 41(1):47–56
Bakr A, Amr M, Sarhan A et al (2007) Psychiatric disorders in children with chronic renal failure. Pediatr Nephrol 22:128–131
Preljevic VT, Østhus TB, Os I et al (2013) Anxiety and depressive disorders in dialysis patients: association to health-related quality of life and mortality. Gen Hosp Psychiatry 35(6):619–624
Cruz LN, de Almeida Fleck MP, Polanczyk CA (2010) Depression as a determinant of quality of life in patients with chronic disease: data from Brazil. Soc Psychiatry Psychiatr Epidemiol 45:953–961
Koo JR, Yoon JY, Joo MH et al (2005) Treatment of depression and effect of anti-depression treatment on nutritional status in chronic hemodialysis patients. Am J Med Sci 329(1):1–5
El Filali A, Bentata Y, Ada N et al (2017) Depression and anxiety disorders in chronic hemodialysis patients and their quality of life: A cross-sectional study about 106 cases in the northeast of morocco. Saudi J Kid Dis Transpl 28(2):341–348
Watnick S, Wang PL, Demadura T et al (2005) Validation of 2 depression screening tools in dialysis patients. Am J Kid Dis 46(5):919–924
Kalender B, Ozdemir AC, Koroglu G (2006) Association of depression with markers of nutrition and inflammation in chronic kidney disease and end-stage renal disease. Nephron Clin Pract 102(3–4):115–121
Hinrichsen GA, Lieberman JA, Pollack S et al (1989) Depression in hemodialysis patients. Psychosom 30(3):284–289
Loosman WL, Siegert CE, Korzec A et al (2010) Validity of the Hospital Anxiety and Depression Scale and the Beck Depression Inventory for use in end-stage renal disease patients. British J Clin Psychol 49(4):507–516
de Alencar SB, de Lima FM, Dias LD et al (2019) Depression and quality of life in older adults on hemodialysis. Braz J Psychiatry 42:195–200
Aghanwa HS, Morakinyo O (1997) Psychiatric complications of hemodialysis at a kidney center in Nigeria. J Psychosom Res 42(5):445–451
Gadia P, Awasthi A, Jain S et al (2020) Depression and anxiety in patients of chronic kidney disease undergoing haemodialysis: a study from western Rajasthan. J Fam Med Prim Care 9(8):4282–4286
Choi MJ, Seo JW, Yoon JW et al (2012) The malnutrition-inflammation-depression-arteriosclerosis complex is associated with an increased risk of cardiovascular disease and all-cause death in chronic hemodialysis patients. Nephron Clin Pract 122(1–2):44–52
Niharika N, Yadav NP, Shakur AA et al (2021) Prevalence and clinical correlates of depressive disorder in chronic kidney disease patients in a tertiary care hospital. Eur J Mol Clin Med 8(4):1766–1772
Hamody AR, Kareem AK, Al-Yasri AR et al (2013) Depression in Iraqi hemodialysis patients. Arab J Nephrol Transpl 6(3):169–172
Martens RJ, Kooman JP, Stehouwer CD et al (2018) Albuminuria is associated with a higher prevalence of depression in a population-based cohort study: the Maastricht Study. Nephrol Dial Transpl 33(1):128–138
Wang LJ, Chen CK, Hsu HJ, et al (2014) Depression, 5HTTLPR and BDNF Val66Met polymorphisms, and plasma BDNF levels in hemodialysis patients with chronic renal failure. Neuropsychiatric Dis Treat. 1235–1241
Rodríguez-Angarita CE, Sanabria-Arenas RM, Vargas-Jaramillo JD et al (2016) Cognitive impairment and depression in a population of patients with chronic kidney disease in Colombia: a prevalence study. Can J Kidney Health Dis 3:116. https://doi.org/10.1186/s40697-016-0116-7
Tuna Ö, Balaban ÖD, Mutlu C et al (2021) Depression and cognitive distortions in hemodialysis patients with end stage renal disease: a case-control study. Eur J Psychiatry 35(4):242–250
Agrawaal KK, Chhetri PK, Singh PM et al (2019) Prevalence of depression in patients with chronic kidney disease stage 5 on hemodialysis at a tertiary care center. J Nepal Med Assoc 57(217):172–175
Kokoszka A, Leszczyńska K, Radzio R et al (2016) Prevalence of depressive and anxiety disorders in dialysis patients with chronic kidney disease. Arch Psychiatry Psychother 1:8–13
Macaron G, Fahed M, Matar D et al (2014) Anxiety, depression and suicidal ideation in Lebanese patients undergoing hemodialysis. Community Mental Health J 50:235–238
Kalender B, Dervisoglu E, Sengul E et al (2007) Depression, nutritional status, and serum cytokines in peritoneal dialysis patients: is there a relationship? Perit Dial Int 27(5):593–595
Craven JL, Rodin GM, Johnson L et al (1987) The diagnosis of major depression in renal dialysis patients. Psychosom Med 49:482–492
Chin HJ, Song YR, Lee JJ et al (2008) Moderately decreased renal function negatively affects the health-related quality of life among the elderly Korean population: a population-based study. Nephrol Dial Transpl 23:2810–2817
Abbey SE, Toner BB, Garfinkel PE et al (1990) Self-report symptoms that predict major depression in patients with prominent physical symptoms. Int J Psychiatry Med 3:247–258
Tomita T, Yasui-Furukori N, Sugawara N et al (2016) (2016) Prevalence of major depressive disorder among hemodialysis patients compared with healthy people in Japan using the Structured Clinical Interview for DSM-IV. Neuropsychiatr Dis Treat 12:2503–2508
Major Depression. National Institute of Mental Health (NIMH) (2023) https://www.nimh.nih.gov/health/statistics/major-depression. Accessed 5 Aug 2023
Grønli OK, Bramness JG, Wynn R et al (2022) Depressive symptoms in the general population: the 7th Tromsø study. J Affective Disord Rep 8:1–6
Ghandour RM, Sherman LJ, Vladutiu CJ et al (2019) Prevalence and treatment of depression, anxiety, and conduct problems in US children. J Pediatr 206:256–267
Kim M, Kim YS, Kim DH et al (2018) Major depressive disorder in epilepsy clinics: a meta-analysis. Epilepsy Behav 84:56–69
Mitchell AJ, Chan M, Bhatti H et al (2011) Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interview-based studies. Lancet Oncol 12(2):160–174
Ravaghi H, Behzadifar M, Behzadifar M et al (2017) Prevalence of depression in hemodialysis patients in Iran a systematic review and meta-analysis. Iran J Kidney Dis 11(2):90–98
Carlson GA (2000) The challenge of diagnosing depression in childhood and adolescence. J Affect Disord 61:S3-8
Tyrer P (2014) A comparison of DSM and ICD classifications of mental disorder. Adv Psychiatr Treat 20(4):280–285
Wittchen HU, Höfler M, Meister W (2001) Prevalence and recognition of depressive syndromes in German primary care settings: poorly recognized and treated? Int Clin Psychopharmacol 16(3):121–135
Saito M, Iwata N, Kawakami N et al (2010) Evaluation of the DSM-IV and ICD-10 criteria for depressive disorders in a community population in Japan using item response theory. Int J Methods Psychiatr Res 19(4):211–222
Bautovich A, Katz I, Smith M et al (2014) Depression and chronic kidney disease: a review for clinicians. Aust N Z J Psychiatry 48(6):530–541
Avdal EU, Ayvaz İ, Uran BN et al (2020) Opinions of hemodialysis and peritoneum patients regarding depression and psychological problems which they experience: A qualitative study. Journal of infection and public health. 13(12):1988–1992Martin CR, Tweed AE, Metcalfe MS (2004) A psychometric evaluation of the Hospital Anxiety and Depression Scale in patients diagnosed with end-stage renal disease. Br J Clin Psychol 43:51–64
Martin CR, Tweed AE, Metcalfe MS (2004) A psychometric evaluation of the hospital anxiety and depression scale in patients diagnosed with end-stage renal disease. Br J Clin Psychol 43(1):51–64
AlDukhayel A (2015) Prevalence of depressive symptoms among hemodialysis and peritoneal dialysis patients. Int J Health Sci (Qassim) 9(1):9–16
Turkmen K, Yazici R, Solak Y et al (2012) Health-related qualıty of lıfe, sleep qualıty, and depressıon in peritoneal dialysis and hemodıalysıs patıents. Hemodial Int 16(2):198–206
Eghbali M, Shahqolian N, Nazari F et al (2009) Comparing problems of patients with chronic renal failure undergoing hemodialysis and peritoneal dialysis referring to medical university’s hospitals. Iran J Nurs Midwifery Res 14(1):1–5
Boulware LE, Liu Y, Fink NE et al (2006) Temporal relation among depression symptoms, cardiovascular disease events, and mortality in end-stage renal disease: contribution of reverse causality. Clin J Am Soc Nephrol 1(3):496–504
Foulkes L, Andrews JL (2023) Are mental health awareness efforts contributing to the rise in reported mental health problems? A call to test the prevalence inflation hypothesis. New Ideas Psychol 69:101010
Smith MD, Hong BA, Robson AM (1985) Diagnosis of depression in patients with end-stage renal disease: comparative analysis. Am J Med 79:160–166
Chen CK, Tsai YC, Hsu HJ (2010) Depression and suicide risk in hemodialysis patients with chronic renal failure. Psychosomatics 51(6):528–528
Gregg LP, Carmody T, Le D et al (2019) A systematic review and meta-analysis of depression and protein-energy wasting in kidney disease. Kidney Int Rep 5(3):318–330
Lacson E Jr, Li NC, Guerra-Dean S et al (2012) Depressive symptoms associated with high mortality risk and dialysis withdrawal in incident hemodialysis patients. Nephrol Dial Transpl 27(7):2921–2928
Chilcot J, Davenport A, Wellsted D et al (2011) An association between depressive symptoms and survival in incident dialysis patients. Nephrol Dial Transplant 26(5):1628–1634
Farrokhi F, Abedi N, Beyene J et al (2014) Association between depression and mortality in patients receiving long-term dialysis: a systematic review and meta-analysis. Am J Kidney Dis 63(4):623–635
Palmer SC, Vecchio M, Craig JC et al (2013) Association between depression and death in people with CKD: a meta-analysis of cohort studies. Am J Kidney Dis 62(3):493–505
Lopes AA, Albert JM, Young EW (2004) Screening for depression in hemodialysis patients: associations with diagnosis, treatment, and outcomes in the DOPPS. Kidney Int 66:2047–2053
Wiesinger T, Kremer S, Bschor T et al (2023) Antidepressants and quality of life in patients with major depressive disorder–Systematic review and meta-analysis of double-blind, placebo-controlled RCTs. Acta Psychiatr Scand 147(6):545–560
Ng CZ, Tang SC, Chan M et al (2019) A systematic review and meta-analysis of randomized controlled trials of cognitive behavioral therapy for hemodialysis patients with depression. J Psychosom Res 126:109834
Barello S, Anderson G, Acampora M et al (2023) The effect of psychosocial interventions on depression, anxiety, and quality of life in hemodialysis patients: a systematic review and a meta-analysis. Int Urol Nephrol 55(4):897–912
Hamdi Elzeiny H, Mahmoud Mohamed El-Emary F (2023) Efficacy of Psychological Interventions in Reducing the Prevalence and Intensity of Depression in Patients with Long-Term Hemodialysis. Egyptian Journal of Health Care 14(1):132–143
Acknowledgements
Nil
Funding
This research received no grant from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
OAA, UEE, IRE, FO, and DSO were involved in conceptualizing the study. All authors were involved in the literature search and review, methodology design and data collection. UEE performed the data analysis. OAA, UEE, IRE, FO, DSO, JF, HDP, ASM, JA, and JJN were involved in data interpretation. OAA and UEE were involved in writing the original draft. All authors were involved in the manuscript review and editing. All authors read and approved the final draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
Ethical approval was not required since no new patients were recruited. The study was a meta-analysis based exclusively on already published data.
Research involving human participants and/or animals
Not applicable. The present study is a literature-based meta-analysis.
Informed consent
Not applicable. No new participants were recruited.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Adejumo, O.A., Edeki, I.R., Sunday Oyedepo, D. et al. Global prevalence of depression in chronic kidney disease: a systematic review and meta-analysis. J Nephrol (2024). https://doi.org/10.1007/s40620-024-01998-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40620-024-01998-5