Abstract
Background
Magnesium deficiency is common in patients with chronic kidney diseases (CKD) due to restricted magnesium intake and impaired magnesium reabsorption. Based on pathophysiological risk factors influencing kidney magnesium reabsorption, a magnesium depletion score (MDS) was developed. Using MDS as a novel indicator for assessing body magnesium status, we hypothesized that it was associated with clinical prognosis.
Methods
We conducted a prospective population-based cohort study using data from the National Health and Nutrition Examination Survey 1999–2014 to explore the impact of MDS on the clinical outcomes of CKD patients. Propensity score-matched analyses were conducted to increase comparability. The primary outcome was all-cause mortality, and the secondary outcomes were cardiovascular-cause and cancer-cause mortality.
Results
After propensity score matching, 3294 CKD patients were divided into 2 groups: MDS ≤ 2 (N = 1647), and MDS > 2 (N = 1647). During a median follow-up of 75 months, Kaplan–Meier analyses showed that MDS > 2 was associated with worse 5- and 10-year overall survival (78.5% vs 73.4%; 53.1% vs 43.1%, P < 0.001). After adjusting for confounding variables, MDS was found to be an independent risk factor for all-cause mortality (HR:1.34, 95% CI 1.20–1.50, P < 0.001). MDS > 2 was also associated with higher cardiovascular-cause mortality (16.2% VS 11.6%, P = 0.005). Multivariate competing risk analysis revealed that MDS > 2 was an independent risk factor (HR: 1.33, 95% CI 1.06–1.66, P = 0.012). Subgroup analyses reported that MDS > 2 increased all-cause mortality and cardiovascular-cause mortality only in patients with inadequate magnesium intake (P < 0.001, P < 0.001) but not in those with adequate intake (P = 0.068, P = 0.920).
Conclusions
A magnesium depletion score > 2 was independently associated with higher long-term cardiovascular-cause and all-cause mortality in CKD patients.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The global burden of chronic kidney disease (CKD) is estimated at approximately 10%, resulting in 1.2 million deaths annually [1]. Cardiovascular diseases are reported as the leading cause of death in CKD patients, accounting for up to 40% of all deaths. While common risk factors, including overweight, hypertension, hyperlipidemia, and diabetes, have been identified as associated with cardiovascular complications and higher mortality, non-traditional risk factors can also increase risk [2,3,4]. In particular, mineral disorders contribute to the progression and worse prognosis of CKD [5, 6].
Magnesium, the second most abundant ion in the intracellular space of the human body, is an essential cofactor for more than 300 enzymatic reactions [7]. Due to impaired kidney function, magnesium intake or supplementation is restricted in CKD patients, which increases the risk of magnesium deficiency. In a national cross-sectional study of 5126 CKD patients, hypomagnesemia was the most common electrolyte abnormality (14.7%), with a similar prevalence across CKD stages 1–5 [8]. The magnesium tolerance test (MTT) is used as the standardized measure of body magnesium status [9, 10]. However, widespread application of the magnesium tolerance test in research and clinical practice is difficult and impractical because it requires 24-h urine collection, followed by an intravenous magnesium infusion for 4 h, and then by a second 24-h urine collection. Instead, current measurement methods include evaluating daily magnesium intake, serum magnesium levels, and urine magnesium levels in the clinical practice. However, total body magnesium is mainly stored in bone and muscle, with only 0.3% of magnesium being present in serum. Previous epidemiological studies showed less consistent associations between current magnesium measurements and health outcomes. One meta-analysis of more than 400,000 adults reported a 14% increased risk of cardiovascular-cause death in patients with magnesium deficiency, while no statistical difference was reported in another meta-analysis that included 6 prospective studies involving over 200,000 participants [11, 12].
Notably, the kidneys play a critical role in maintaining magnesium homeostasis, as over 80% of serum magnesium is reabsorbed by the kidneys [13]. Previous studies have reported pathophysiological factors that diminish renal magnesium reabsorption capacity, including alcohol consumption, diuretic use, proton pump inhibitor (PPI) use, and kidney diseases [14, 15]. Recently, Fan et al. developed the magnesium depletion score (MDS) [16], a composite score combining these risk factors as an indicator of the status of kidney magnesium resorption. Using the magnesium tolerance test, the authors found that MDS can better predict total body magnesium compared with serum magnesium levels, urine magnesium levels, and dietary magnesium intake. Therefore, MDS may serve as a promising indicator for evaluating magnesium deficiency. The pathophysiological factors associated with low magnesium reabsorption capacity are common in CKD patients. Diuretics are often used to treat edema and hypertension, and PPIs to protect gastric mucosa, for example after using high-dose glucocorticoids. In the present study, we explored the association between MDS and clinical outcomes and the possibility of using MDS to guide the administration of magnesium supplements in CKD patients.
Methods
Data Source and Study Population
All data were extracted from the National Health and Nutrition Examination Survey (NHANES), which is a nationally representative survey designed to assess health and nutrition of the non-institutionalized US population [17]. The NHANES data are released every two years and managed by the National Center for Health Statistics under the purview of the Centers for Disease Control and Prevention. Data from eight continuous cycles, 1999–2014 NHANES, were used in this study. Mortality information was obtained from the National Death Index as previously reported.
The inclusion criteria were as follows: (1) age ≥ 18 years; (2) the presence of CKD; (3) enough data to calculate MDS. However, those without mortality information were excluded. As previously reported [19], CKD was defined by impaired estimated glomerular filtration rate (eGFR) and/or albuminuria (urinary albumin-to-creatinine ratio > 30 mg/g). CKD was graded as follows: Stage 1, eGFR ≥ 90 mL/min/1.73 m2 with albuminuria; stage 2, eGFR of 60–89 mL/min/1.73 m2 and albuminuria; stage 3, eGFR of 30 to 59 mL/min/1.73 m2; stage 4, eGFR of 15–29 mL/min/1.73 m2; and stage 5, eGFR < 15 mL/min/1.73 m2.
Magnesium Depletion Score
Based on the previous publication by Fan et al. [16], magnesium depletion score was calculated by including the following 4 risk factors: (1) current diuretic use (1 point), (2) current PPI use (1 point), (3) kidney function: 60 mL/min/1.73 m2 ≤ eGFR < 90 mL/(min/1.73 m2) 1 point; eGFR < 60 mL/(min · 1.73 m2) 2 points, and (4) heavy alcohol consumption (1 point).
Variables and Study Outcomes
Based on previous publications and clinical experience, we considered age, sex, body mass index (BMI), race, income to poverty ratio, education levels, total daily magnesium intake, smoking, chronic diseases (diabetes, and hypertension), and laboratory tests (eGFR, serum total calcium and phosphorus) as potential confounders of the relationship between MDS and long-term prognosis. Inadequate magnesium intake was defined as intake < 350 mg/day for men > 30 years, < 330 mg/day for men < 30 years, < 265 for women > 30 years, and < 255 for women < 30 years [20]. Clinical outcomes included all-cause mortality, cardiovascular-cause mortality, and cancer-cause mortality, which was diagnosed based on the 10th Revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) codes. Cardiovascular-specific deaths included codes I00–I09 (acute rheumatic fever and chronic rheumatic heart diseases), I11 (hypertensive heart disease), I13 (hypertensive heart and renal disease), I20–I25 (ischemic heart disease), I26–I51 (other heart diseases), and I60–I69 (cerebrovascular diseases). Cancer-specific deaths were based on codes C00–C97.
Multiple Imputation of Missing Data
Missing data were as follows: education (n = 99/4322; 2.3%), income to poverty ratio (n = 298/4322, 6.9%), BMI (n = 134/4322; 3.1%), calcium (n = 3/4322, 0.0%), phosphorus (n = 1/4322, 0.0%), and smoking (n = 49/4322, 1.1%). As recommended in the NHANES analytic guidelines, missing values were imputed by multiple imputation with chained equations, in which data were assumed to be missing at random. Normally distributed continuous variables were modeled using linear regression, non-normally distributed continuous variables were modeled using predictive mean matching, and binary variables were modeled using logistic regression.
Propensity-Score Matching
We conducted propensity-score matching (PSM) analyses to increase comparability between groups. First, all patients were divided into two groups: MDS ≤ 2 and MDS > 2 based on the previous publication by Fan et al. Second, covariant factors were selected to estimate the propensity scores by logistic regression analysis on the following factors: age, sex, body mass index, race, income to poverty ratio, education levels, smoking, chronic diseases (diabetes, and hypertension), and laboratory tests (serum total calcium and phosphorus). Then patients were matched 1:1 between groups using the “nearest” method. This matching procedure was done using Package “MatchIt” of R software (Version: 4.1.2).
Statistical Analyses
Descriptive analyses were performed before and after PSM. Continuous variables are expressed as mean (standard deviation) or median (range) for normally or non normally distributed variables. Categorical variables are presented as numbers and proportions. Continuous and categorical demographic variables were compared using analysis of variance (ANOVA) and Chi-square tests, respectively.
Kaplan–Meier survival analyses were performed using log-rank tests to compare all-cause mortality between the MDS > 2 and MDS ≤ 2 groups. To estimate cardiovascular-specific and cancer-specific mortality, we conducted competing risk analyses using sub-distribution hazard models with Fine and Gray tests. Subgroup survival analyses were conducted based on dietary magnesium intake (adequate, inadequate).
Further, we conducted Cox proportional hazards analyses to explore the independent effects of MDS, each item of MDS (diuretic use, PPI use, drinking, eGFR), and dietary magnesium intake on all-cause mortality. Model 1 was a crude model with no adjusted covariates. Model 2 adjusted for sociodemographic variables (age, sex, race, education, income to poverty ratio, BMI, smoking). Model 3 built on model 2 and additionally adjusted for diabetes and hypertension. Model 4 was the fully adjusted model, which was based on model 3 and further adjusted for calcium and phosphorus. Likewise, by using sub-distribution hazard models, competing risk analyses were conducted to determine the impact of MDS, each item of calculating MDS, and dietary magnesium intake on cancer-specific and cardiovascular-specific death.
To address potential confounding bias, we conducted several subgroup analyses based on age (< 65, ≥ 65 years), sex (male, female), dietary magnesium intake (adequate, inadequate), diabetes (Yes, No), and hypertension (Yes, No). All statistical analyses were conducted by R software (Version 4.1.2).
Results
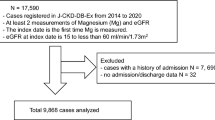
Patient selection is shown in Fig. 1. Overall, 4322 subjects were selected from among 82,091 individuals. Baseline characteristics are shown in Table 1. Before PSM, patients in the MDS > 2 group were older, had higher BMI and had more hypertension and eGFR < 60 ml/min/1.73 m2. After PSM, baseline characteristics of the two groups were matched with regard to age, BMI, hypertension and eGFR.
Survival Analyses of MDS and All-cause, Cardiovascular-Specific, Cancer-Specific Mortality
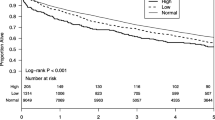
Kaplan–Meier survival analyses with log-rank tests were performed before and after PSM, as shown in Fig. 2. After PSM, there were 600 deaths in the MDS ≤ 2 group with a median follow up of 77 months (range: 13–181 months) and 700 deaths in the MDS > 2 group with a median follow up of 74 months (range 11–179 months). Kaplan–Meier analyses indicated that MDS > 2 was associated with poorer 5- and 10-year overall survival (5-year: 78.5% vs 73.4%; 10-year: 53.1% vs 43.1%, P < 0.001, Fig. 2B). Subgroup analyses were conducted based on magnesium intake, observing that MDS was associated with all-cause mortality in patients with inadequate levels of magnesium (P < 0.001, Fig. 2D), but not in patients with adequate levels of magnesium (P = 0.140, Fig. 2F).
Sub-distribution hazard analyses with Fine and Gray tests were conducted to estimate cardiovascular-specific and cancer-specific mortality. Among 3294 CKD patients, there were 125 cardiovascular-specific deaths in the MDS ≤ 2 group and 169 deaths in the MDS > 2 group, suggesting that MDS > 2 was associated with higher cumulative cardiovascular-specific mortality (16.2% vs 11.6%, P < 0.001, Fig. 3B). Subgroup analyses revealed that MDS > 2 caused higher cardiovascular-specific mortality in individuals with inadequate magnesium intake (P = 0.021), but not in those with adequate intake (P = 0.591). Among 3294 CKD patients, there were 98 and 104 cancer-specific deaths in the MDS ≤ 2 and > 2 groups, respectively, showing that MDS was not associated with cancer-cause mortality (P = 0.609) (Fig. 3H).
The effect of magnesium depletion score on cardiovascular-specific (A, B) and subgroup analysis by inadequate magnesium intake (C, D) and adequate magnesium intake (E, F) before and after propensity-score matching. The effect of magnesium depletion score on cancer-specific mortality (G, H) before and after propensity-score matching
Multivariable Analyses
We conducted multivariable COX proportional regression analyses to determine the independent effect of MDS, each term of MDS, and dietary magnesium intake on all-cause mortality (Table 2). In the fully adjusted model (model 4), MDS was associated with higher all-cause mortality (HR: 1.34, 95% CI 1.20–1.50, P < 0.001). We further conducted competing risk analyses to determine the impact on cardiovascular-specific and cancer-specific mortality. Similarly, MDS was independently associated with higher cardiovascular-specific mortality (HR: 1.33, 95% CI 1.06–1.66, P = 0.012) but not cancer-specific mortality (HR: 1.17, 95% CI 0.88–1.55, P = 0.290) in the fully adjusted models.
Subgroup Analyses
We conducted subgroup analyses to address potential confounding bias. After fully adjusting, the impact of MDS on all-cause and cardiovascular-specific mortality was similar in subgroups based on age, sex, diabetes, and hypertension (Fig. 4). In the subgroup analysis of dietary magnesium intake, MDS was associated with all-cause and cardiovascular-specific mortality only in those with inadequate magnesium intake.
Discussion
To the best of our knowledge, this is the first study to evaluate the association between renal magnesium reabsorption status and long-term all-cause and cardiovascular-cause mortality in patients with CKD. This large population-based cohort study reports a close association between high MDS and worse prognosis in CKD patients. Notably, this inverse association was found only in patients with inadequate magnesium intake but not in patients with adequate intake. These findings provide a novel indicator evaluating magnesium in individuals with CKD, and may contribute to clinical trials exploring the value of magnesium supplementation.
In the MDS development study [16], Fan et al. enrolled 77 individuals to compare the capacity of serum magnesium levels, urine magnesium levels, total magnesium intake and MDS in predicting total body magnesium by using the magnesium tolerance test as the reference measure. They found that MDS had a higher area under the receiver operating characteristic (ROC) curve (AUC) (AUC: 0.68, 95% CI 0.53–0.83) compared with serum magnesium levels (AUC:0.53, 95% CI 0.31–0.74), urine magnesium levels (AUC: 0.49, 95% CI 0.23–0.74), and total magnesium intake (AUC: 0.52, 95% CI 0.33–0.72). When combined with MDS, age and sex, the AUC reached 0.77 (95% CI 0.60–0.95). Further, in the validation cohort study including 10,049 individuals, the authors found that MDS > 2 had a 1.29-fold risk of all-cause mortality (P = 0.059) and a 3.13-fold risk of cardiovascular-cause mortality (P = 0.024) compared to MDS at 0.
Previous epidemiological studies have reported that magnesium deficiency is common in CKD patients. Numerous studies have explored the relationship between magnesium deficiency and the prognosis of CKD patients, evaluated by dietary magnesium intake, serum magnesium level or urine magnesium levels. However, the associations were not consistent. Using the Cleveland Clinic CKD registry, Azem et al. identified 10,568 CKD patients with eGFR between 15 and 59 ml/min/1.73 m2 [21]. During a median follow-up of 3.7 years, the authors showed a U-shaped relationship between serum magnesium and all-cause mortality, finding that hypomagnesemia (HR:1.14, 95% CI 1.04–1.24) was associated with an increased risk after adjusting the covariates. However, in another prospective population-based cohort study including 3179 CKD patients, Yuan et al. [22]. reported that 24 h urinary magnesium concentration was not associated with death risk.
Considering the conflicting data, there was no consensus regarding the need for magnesium supplementation in CKD patients. The randomized placebo-controlled double-blinded clinical trial by Bressendorff et al. [23] investigated the safety and efficacy of oral magnesium supplementation in 34 subjects with CKD stages 3 and 4. Although no serious adverse events related to the study medication were reported, no benefits were reported either. Similarly to the findings by Fan et al. [16], in our CKD patients, we observed that MDS was independently associated with cardiovascular-cause and all-cause mortality. Notably, this association was significant only in patients with inadequate magnesium intake. These results suggest that supplementing magnesium based on dietary magnesium intake was not the best strategy because more than 80% of serum magnesium can be reabsorbed by the kidneys. By using MDS, we identified those CKD patients with likely magnesium reabsorption dysfunction. Hence, based upon score, it seems reasonable to administer magnesium supplements when CKD patients simultaneously have inadequate intake and reabsorption dysfunction. Our study provides a novel measure for evaluating magnesium status and identifing CKD patients at higher risk of magnesium deficiency.
Several mechanisms may explain the benefit of magnesium supplements in CKD patients with magnesium deficiency: magnesium has both anti-atherosclerotic and anti-calcification effects through its anti-inflammatory and antioxidant properties [24]. Magnesium status has an important impact on the cardiovascular system. Magnesium deficiency is closely related to insulin resistance and metabolic syndrome [25]. Insulin resistance is an early metabolic alteration in CKD patients and becomes almost universal at stages 4–5 CKD. In two studies involving Japanese patients with moderate-to-severe CKD [26, 27], the prevalence of insulin resistance was 30% and 44%. Magnesium supplementation can ameliorate chronic inflammation. In rat models with induced CKD, magnesium supplementation alleviated inflammation, TNF-α, IL-1β and IL-6 [28].
Our study has the merits of population-based analyses, large sample size, long follow-up, application of propensity score matches and adjustments for a wide range of potential confounders. However, because of lack of availability of serum magnesium levels, we cannot compare the capacity of MDS and serum magnesium levels in predicting the prognosis in CKD patients. Furthermore, despite the comprehensive adjustment for confounders, we cannot exclude the possibility of residual confounding by dietary-related or other variables.
Conclusions
High magnesium depletion score was associated with higher long-term cardiovascular-cause and all-cause mortality. This finding provides a novel indicator for evaluating magnesium in individuals with CKD, and may contribute to clinical trials to explore the value of magnesium supplementation.
References
GBD Chronic Kidney Disease Collaboration (2020) Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 395(10225):709–733. https://doi.org/10.1016/S0140-6736(20)30045-3
Webster AC, Nagler EV, Morton RL, Masson P (2017) Chronic kidney disease. Lancet 389(10075):1238–1252. https://doi.org/10.1016/S0140-6736(16)32064-5
Bello AK, Alrukhaimi M, Ashuntantang GE, Basnet S, Rotter RC, Douthat WG, Kazancioglu R, Köttgen A, Nangaku M, Powe NR, White SL, Wheeler DC, Moe O (2017) Complications of chronic kidney disease: current state, knowledge gaps, and strategy for action. Kidney Int Suppl (2011) 7(2):122–129. https://doi.org/10.1016/j.kisu.2017.07.007
Luyckx VA, Tuttle KR, Garcia-Garcia G, Gharbi MB, Heerspink HJL, Johnson DW, Liu ZH, Massy ZA, Moe O, Nelson RG, Sola L, Wheeler DC, White SL (2017) Reducing major risk factors for chronic kidney disease. Kidney Int Suppl (2011). 7(2):71–87. https://doi.org/10.1016/j.kisu.2017.07.003
Covic A, Vervloet M, Massy ZA, Torres PU, Goldsmith D, Brandenburg V, Mazzaferro S, Evenepoel P, Bover J, Apetrii M, Cozzolino M (2018) Bone and mineral disorders in chronic kidney disease: implications for cardiovascular health and ageing in the general population. Lancet Diabetes Endocrinol 6(4):319–331. https://doi.org/10.1016/S2213-8587(17)30310-8
Floege J, Kronenberg F, Froissart M (2011) Mortality in chronic kidney disease and mineral metabolism. JAMA 306(2):159. https://doi.org/10.1001/jama.2011.948 (author reply 159-60)
Massy ZA, Drüeke TB (2015) Magnesium and cardiovascular complications of chronic kidney disease. Nat Rev Nephrol 11(7):432–442. https://doi.org/10.1038/nrneph.2015.74
Oka T, Hamano T, Sakaguchi Y, Yamaguchi S, Kubota K, Senda M, Yonemoto S, Shimada K, Matsumoto A, Hashimoto N, Mori D, Monden C, Takahashi A, Obi Y, Yamamoto R, Takabatake Y, Kaimori JY, Moriyama T, Horio M, Matsui I, Isaka Y (2019) Proteinuria-associated renal magnesium wasting leads to hypomagnesemia: a common electrolyte abnormality in chronic kidney disease. Nephrol Dial Transplant 34(7):1154–1162. https://doi.org/10.1093/ndt/gfy119
Arnaud MJ (2008) Update on the assessment of magnesium status. Br J Nutr 99(Suppl 3):S24-36. https://doi.org/10.1017/S000711450800682X
Tong GM, Rude RK (2005) Magnesium deficiency in critical illness. J Intensive Care Med 20(1):3–17. https://doi.org/10.1177/0885066604271539
Fang X, Liang C, Li M, Montgomery S, Fall K, Aaseth J, Cao Y (2016) Dose-response relationship between dietary magnesium intake and cardiovascular mortality: a systematic review and dose-based meta-regression analysis of prospective studies. J Trace Elem Med Biol 38:64–73. https://doi.org/10.1016/j.jtemb.2016.03.014
Xu T, Sun Y, Xu T, Zhang Y (2013) Magnesium intake and cardiovascular disease mortality: a meta-analysis of prospective cohort studies. Int J Cardiol 167(6):3044–3047. https://doi.org/10.1016/j.ijcard.2012.11.090
Ellison DH, Maeoka Y, McCormick JA (2021) Molecular mechanisms of renal magnesium reabsorption. J Am Soc Nephrol 32(9):2125–2136. https://doi.org/10.1681/ASN.2021010042
Blaine J, Chonchol M, Levi M (2015) Renal control of calcium, phosphate, and magnesium homeostasis. Clin J Am Soc Nephrol. 10(7):1257–1272. https://doi.org/10.2215/CJN.09750913 (Epub 2014 Oct 6. Erratum in: Clin J Am Soc Nephrol. 2015 Oct 7;10(10):1886-7)
William JH, Danziger J (2016) Magnesium deficiency and proton-pump inhibitor use: a clinical review. J Clin Pharmacol 56(6):660–668
Fan L, Zhu X, Rosanoff A, Costello RB, Yu C, Ness R, Seidner DL, Murff HJ, Roumie CL, Shrubsole MJ, Dai Q (2021) magnesium depletion score (MDS) predicts risk of systemic inflammation and cardiovascular mortality among US adults. J Nutr. 151(8):2226–2235. https://doi.org/10.1093/jn/nxab138
Ahluwalia N, Dwyer J, Terry A, Moshfegh A, Johnson C (2016) Update on NHANES dietary data: focus on collection, release, analytical considerations, and uses to inform public policy. Adv Nutr 7(1):121–134. https://doi.org/10.3945/an.115.009258
Pfeiffer CM, Lacher DA, Schleicher RL, Johnson CL, Yetley EA (2017) Challenges and lessons learned in generating and interpreting NHANES nutritional biomarker data. Adv Nutr 8(2):290–307. https://doi.org/10.3945/an.116.014076
Gor D, Gerber BS, Walton SM et al (2020May) Antidiabetic drug use trends in patients with type 2 diabetes mellitus and chronic kidney disease: a cross-sectional analysis of the National Health and Nutrition Examination Survey. J Diabetes 12(5):385–395
Institute of Medicine (1997) Dietary reference intakes: calcium, phosphorous, magnesium, vitamin D, and flouride. National Academy Press, Washington, DC
Azem R, Daou R, Bassil E, Anvari EM, Taliercio JJ, Arrigain S, Schold JD, Vachharajani T, Nally J, Na Khoul GN (2020) Serum magnesium, mortality and disease progression in chronic kidney disease. BMC Nephrol 21(1):49. https://doi.org/10.1186/s12882-020-1713-3
Yuan Q, Xie Y, Peng Z, Wang J, Zhou Q, Xiao X, Wang W, Huang L, Tang W, Li X, Zhang L, Wang F, Zhao MH, Tao L, He K, Wanggou S, Xu H, C-STRIDE study group (2021) Urinary magnesium predicts risk of cardiovascular disease in Chronic Kidney Disease stage 1–4 patients. Clin Nutr. 40(4):2394–2400. https://doi.org/10.1016/j.clnu.2020.10.036
Bressendorff I, Hansen D, Schou M, Silver B, Pasch A, Bouchelouche P, Pedersen L, Rasmussen LM, Brandi L (2016) Oral magnesium supplementation in chronic kidney disease stages 3 and 4: efficacy, safety, and effect on serum calcification propensity-a prospective randomized double-blinded placebo-controlled clinical trial. Kidney Int Rep 2(3):380–389. https://doi.org/10.1016/j.ekir.2016.12.008
Diaz-Tocados JM, Peralta-Ramirez A, Rodríguez-Ortiz ME, Raya AI, Lopez I, Pineda C, Herencia C et al (2017) Dietary magnesium supplementation prevents and reverses vascular and soft tissue calcifications in uremic rats. Kidney Int 92:1084–1099
Sarrafzadegan N, Khosravi-Boroujeni H, Lotfizadeh M, Pourmogaddas A, Salehi-Abargouei A (2016) Magnesium status and the metabolic syndrome: a systematic review and meta-analysis. Nutrition 32(4):409–417. https://doi.org/10.1016/j.nut.2015.09.014
Artunc F, Schleicher E, Weigert C, Fritsche A, Stefan N, Häring HU (2016) The impact of insulin resistance on the kidney and vasculature. Nat Rev Nephrol 12(12):721–737
Spoto B, Pisano A, Zoccali C (2016) Insulin resistance in chronic kidney disease: a systematic review. Am J Physiol Renal Physiol 311(6):F1087–F1108
López-Baltanás R, Encarnación Rodríguez-Ortiz M, Canalejo A, Díaz-Tocados JM, Herencia C, Leiva-Cepas F, Torres-Peña JD, Ortíz-Morales A, Muñoz-Castañeda JR, Rodríguez M, Almadén Y (2021) Magnesium supplementation reduces inflammation in rats with induced chronic kidney disease. Eur J Clin Invest 51(8):e13561. https://doi.org/10.1111/eci.13561
Acknowledgements
None.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This work was supported by grants from 1.3.5 Project for Disciplines of Excellence-Clinical Research Incubation Project, West China Hospital, Sichuan University (2021HXFH007). The funders had no role in study design, data collection or analysis, preparation of the manuscript, or the decision to publish.
Conflict of interest disclosures (for all authors)
None.
Ethics Approval Statement
This study involved secondary data analysis of a nationally representative publicly available dataset. The study we conducted was exempt from institutional review for this reason.
Data availability
All data are publicly available at [https://wwwn.cdc.gov/nchs/nhanes/Default.aspx].
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yin, S., Zhou, Z., Lin, T. et al. Magnesium Depletion Score is Associated with Long-Term Mortality in Chronic Kidney Diseases: A Prospective Population-Based Cohort Study. J Nephrol 36, 755–765 (2023). https://doi.org/10.1007/s40620-022-01489-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-022-01489-5