Abstract
Objectives
Class IV lupus nephritis (LN) is one of the most frequent and severe types of involvement in pediatric systemic lupus erythematosus. Gold standard treatment consists of intravenous (i.v.) Cyclophosphamide (CYC) associated with corticosteroids. Recent studies in adults have shown similar efficacy of oral Mycophenolate Mofetil (MMF) with fewer adverse events. Our aim was to compare the efficacy and tolerance of CYC and MMF as induction therapy in children with class IV LN.
Methods
We conducted a retrospective study of children diagnosed with class IV LN who started oral MMF or i.v. CYC treatment at Necker Enfants Malades Hospital (Paris, France).
Results
The study included 33 patients, 17 treated with oral MMF (51%) and 16 with i.v. CYC (48%). The characteristics at treatment induction did not significantly differ between the two groups except for the neurological involvement, that was only present in the CYC group. Complete remission was obtained in 9/17 (53%) children treated with MMF versus 10/16 (71%) treated with CYC (p = 0.46). Relapse was observed in 59% of patients receiving MMF versus 50% receiving CYC (p = 0.87), after a median of 3.4 years and 4.7 years after the beginning of treatment, respectively (p = 0.41). During the 6.5 years of follow-up, we observed no significant difference regarding the number of treatment-related adverse events between the two groups (p = 0.48).
Conclusion
We report similar efficacy and tolerance of MMF or CYC as induction therapy of class IV LN in children. However, the long-term adverse events such as infertility could not be systematically evaluated in this retrospective pediatric study. Overall, however, considering the long-term safety profile reported in the literature, we suggest that MMF may be used as first-line induction therapy in LN.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pediatric-onset systemic lupus erythematosus (SLE) is a rare chronic systemic disease. The incidence is 0.3 to 0.9 per 100,000 children-years worldwide [1] and the initial organ damage is generally more severe than in the adult presentation [2]. Lupus nephritis (LN) occurs in 50–75% of children with pediatric onset SLE and develops within two years of diagnosis in more than 90% of patients [3]. Although survival rates of children have greatly improved, morbidity remains high. LN is an important cause of chronic kidney disease and can lead to kidney failure. Therefore, early efficient and safe treatment is essential to improve the prognosis. It consists of induction therapy to rapidly control kidney inflammation, improve kidney function and decrease proteinuria, followed by maintenance therapy to prevent kidney failure by limiting the incidence of kidney flares.
Austin et al. introduced the National Institutes of Health (NIH) protocol in the 1980s for adult LN with a combination of corticosteroids and cyclophosphamide (CYC), which drastically reduced the risk of kidney failure at 5 years [4]. This treatment consists of 6 monthly pulses of intravenous (i.v.) CYC at a dose of 1000 mg/m2 with or without methylprednisolone pulses, followed by daily oral corticosteroids. Severe short and long-term CYC-related toxic effects include gastrointestinal disturbances, infections, bone marrow suppression, hemorrhagic cystitis, or gonadal dysfunction. In 2002, the Euro-Lupus Nephritis Trial [5] was introduced as a less toxic alternative to the NIH protocol since it reported similar outcomes with reduced doses of the molecule. This protocol consists of the administration of 6 fortnightly i.v. CYC mini pulses at a fixed dose of 500 mg in association with 3 daily pulses of 750 mg of intravenous methylprednisolone followed by oral glucocorticoid therapy at an initial dosage of 0.5 mg/kg/day (or equivalent) for 4 weeks. Looking for an even less toxic alternative to CYC mini-pulses, Mycophenolate mofetil (MMF) was considered [6]. The main toxicities of this drug are hematological manifestations such as leukopenia and gastrointestinal disturbances. In 2005, Ginzler et al. showed that induction therapy of LN by MMF and i.v. CYC achieved a similar remission rate at 3 months [7]. In 2017, a meta-analysis of randomized trials concluded that compared to i.v. CYC, the most effective therapies for inducing remission were MMF, calcineurin inhibitors, or their combination while conferring similar or lower toxicity [8]. Nowadays, the most recent 2019 update of the Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommends the use of MMF or low-dose i.v. CYC combined with glucocorticoids as induction therapy in adults with systemic lupus erythematosus. Guidelines for the management of pediatric LN are scarce and pediatric recommendations are mostly extrapolated from adult studies [9]. In 2017, the Single Hub and Access point for pediatric Rheumatology in Europe (SHARE) recommendations [1] were published based on data from the literature for pediatric onset SLE and suggested similar recommendations. Pediatric studies are mostly retrospective, such as the work of Lau et al. in 2008 involving 13 children [10].
In this work, we aimed to compare the efficacy, safety, and tolerance of i.v. CYC and oral MMF as induction treatment of newly diagnosed pediatric class IV LN in a single center retrospective pediatric cohort.
Methods
Study design
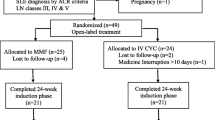
This retrospective single-center study includes patients with a first episode of class IV LN with pediatric onset SLE followed at Necker Enfants Malades Hospital in Paris, France, from December 2004 to August 2020. The inclusion criteria were similar to those used by Ginzler et al. [7]: (i) at least 4 American College of Rheumatology (ACR) criteria, (ii) newly-diagnosed class IV glomerulonephritis according to the International Society of Nephrology/Renal Pathology Society (ISN/RPS) classification, (iii) at least one sign of active disease among: Acute Kidney Injury (AKI) with estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73m2 (Schwartz formula) or increase in serum creatinine by > 50% for ≤ 3 months, proteinuria > 50 mg/mmol of creatinine, microscopic hematuria, (iv) first flare with kidney involvement, (v) age < 18 years old at diagnosis of LN and (vi) a follow-up of at least 6 months after lupus nephritis diagnosis. Exclusion criteria were treatment of class IV nephropathy by immunosuppressive drugs other than MMF or CYC.
Using the local database Dr Warehouse [11], we searched for all pediatric cases of LN treated at our center. We used the 1997 ACR classification criteria for SLE [12]. The treatment protocol consisted of induction therapy either by 6 fortnightly i.v. CYC pulses at a fixed dose of 500 mg/1.73 m2 or by MMF (1200 mg/m2/day divided in 2 doses, adapted to an area under the curve between 40 and 60 mg.h/L), in association with 3 daily pulses of 500 mg/m2 of intravenous methylprednisolone followed by oral glucocorticoid therapy at an initial dosage ranging from 0.5 to 1 mg/kg/day according to the physician’s decision. After induction treatment with MMF or CYC, maintenance treatment with MMF was prescribed in both groups at the same doses. Treatment adhesion was assessed by the physician’s comments noted in the file.
Kidney biopsies were fixed in formalin, acetic acid and alcohol solution (FAA) and paraffin embedded. Three µm sections were stained by Masson trichrome, hematoxylin and eosin, PAS and Jones silver stain. Immunofluorescence was performed on frozen sections with antibodies directed against heavy and light chains, and C3 and C1q antibodies (DAKO, Glostrup, Denmark). The kidney biopsy slides (32/33, 97%) were reassessed by two kidney pathologists blinded to the clinical data and group treatment (JPDVH, RB) and were scored according to the latest ISN/RPS classification [13]. Class IV LN was defined accordingly as an active or inactive, segmental or global, endo- or extra-capillary glomerulonephritis involving ≥ 50% of glomeruli, with or without mesangial alterations, with diffuse immunoglobulin and complement subendothelial deposits [13]. Kidney disease activity and chronicity were also reassessed using the 2018 modified NIH scoring system, ranking activity on a 0–24 scale and on a 0–12 scale for chronicity.
We collected the following data: demographic characteristics (sex, ethnic group, age at diagnosis), familial history of lupus (familial or monogenic form), characteristics of kidney involvement (eGFR according to the Schwartz formula, serum albumin, proteinuria, hematuria, leukocyturia), clinical symptoms of lupus (cutaneous, articular, neurologic, digestive, pulmonary, cardiac, ophthalmologic involvement), biological data (blood count, Anti-Nuclear Antibodies [ANA], anti-Ro/SS-A, anti-ribonucleoprotein [RNP], anti-Sm, anti-phospholipid antibodies), kidney histological lesions, clinical and biological evolution at 6/12/36 months, relapses, other treatments received (molecules and doses), therapeutic compliance that was explicitly mentioned in follow-up reports and adverse events. Severe adverse events (SAE) were the ones requiring patient hospitalization.
The study was approved by the ethical committee of Necker-Enfants Malades Hospital (APHP general register N° 2020 0805180729).
Study endpoints
The primary outcome was complete renal remission rate at 6 months defined by a urinary protein over creatinine ratio < 30 mg/mmol with an eGFR > 60 ml/min/1.73 m2 using the Schwartz formula. The secondary outcomes were (i) complete renal remission rate at 1 and 3 years, (ii) partial renal remission rate defined by a urinary protein over creatinine ratio between 30 and 50 mg/mmol and normal eGFR at 6 months, 1 and 3 years, (iii) relapse-free survival rate, (iv) lupus activity markers (dsDNA titers, complement, serum albumin) at 6 months, 1 and 3 years and (v) treatment-related complications.
Statistical analysis
Quantitative values are expressed as the median (interquartile range, IQR), and qualitative values are presented as numbers (percentages), unless otherwise specified. Univariate analysis was performed using the Fisher exact test for qualitative variables and the Wilcoxon test for quantitative variables. All tests were two-sided, and a p value < 0.05 was considered significant. Because of alpha inflation due to multiple comparisons, findings should be interpreted as exploratory. Analyses were performed using the R version 4.0.3 (packages survival, tidyr, dplyr, ggplot2).
Results
Thirty-three patients with pediatric class IV LN were included between December 2004 and August 2020. Seventeen patients were treated with MMF and 16 with CYC (Table 1). The characteristics at induction of treatment did not significantly differ in both groups except for the neurological involvement (headaches, confusion and hallucinations), that were only present in the CYC group (6/16 vs. 0/17, p = 0.007), because CYC is the LN reference treatment. On the other hand, there was no significant difference in the activity and chronicity scores in kidney histological damage. Furthermore, there was no significant difference in the years of introduction of MMF or CYC (p = 0.22).
Moreover, patients from both groups received similar associated treatments: hydroxychloroquine, Angiotensin Converting Enzyme Inhibitors (ACEis) or Angiotensin II Receptor Blockers (ARBs); the choice was made according to the physician’s decision. All patients received MMF as maintenance therapy. Overall compliance was good, estimated at 12 patients out of 17 for the MMF cohort and 11 patients out of 16 for the CYC (p = 0.91).
Study endpoints
Complete remission was obtained in 9/17 (53%) patients in the MMF group and 11/16 (71%) patients in the CYC group (p = 0.46) at 6 months, 9/16 (56%) vs. 10/16 (77%) (p = 0.43) at 1 year and 9/12 (75%) vs. 8/11 (79%) (p = 1) at 3 years. These results are presented in Fig. 1. Partial remission was obtained in 1/17 (6%) patients in the MMF group and 0/16 (0%) patients in the CYC group (p = 0.92) at 6 months, 1/17 (6%) vs 1/16 (6%) (p = 0.97) at 1 year and 1/12 (8%) vs 1/11 (9%) (p = 0.95) at 3 years. There was no significant difference between the 2 groups regarding median proteinuria and median serum albumin; proportion of patients whose complement (CH50, C3, C4) normalized was not statistically different between both groups at each follow-up time-point (Table 2).
Remission according to treatment at 6 months, 1 year and 3 years. A Percentage of complete remission in each group (MMF versus CYC); at 6 months: p = 0.46, at 1 year: p = 0.43, at 3 years: p = 1. B Progressive decrease of corticosteroids in each group (MMF versus CYC); to inclusion: p = 0.39, at 6 months: p = 0.15, at 1 year: p = 0.35, at 3 years: p = 0.65. sem standard error of the mean, ns non significant
Thirty-two of the 33 children received intravenous corticosteroids in parallel with induction therapy. This treatment was followed by oral corticosteroids at a dosage of 60 mg/day and was then progressively tapered according to the physician’s decision. The decrease in the dose of oral corticosteroids after induction therapy was similar between the two groups at 6 months, 1 year and 3 years (Fig. 1).
Subgroup analyses were performed to compare study endpoints between both groups excluding patients with neurological involvement, and showed similar results.
Events during follow-up
Median follow-up was 6.5 years and did not differ between the two groups (p = 0.22). All the results are presented in Table 3. During follow-up, 10/17 patients (59%) from the MMF group presented a relapse or absence of remission of their nephropathy versus 8/16 (50%) in the CYC group (p = 0.87). In the MMF group, 47% of patients failed to achieve remission at 6 months: 50% of them continued MMF and achieved remission (75% at 12 months and 25% at 36 months) while 50% of them changed treatment (50% received Eurolupus and 50% another protocol). In the CYC group, amongst 29% of patients who did not achieve remission, 40% remained treated with their first protocol (CYC followed by MMF) and achieved remission at 12 months and 60% changed maintenance treatment to Rituximab or higher doses of MMF.
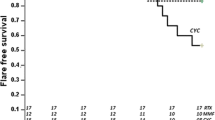
Relapses occurred at a median time of 3.4 years in the MMF group and of 4.7 years in the CYC group (p = 0.41). No patient died in this series. There was no significant difference in time to relapse between the two groups (p = 0.46) as shown in Fig. 2.
Adverse events
The most frequent adverse events were infections, among which six were severe: one septic shock and two cases of pneumonia in the CYC group; one of miliary tuberculosis, one of pneumonia and one of herpes hepatitis in the MMF group. The other infections were herpes zoster, varicella, warts, folliculitis with the same distribution in both groups except for varicella (none in the CYC group). In the MMF group, two children developed transitory lymphopenia (both at 800/mm3 at 1 year and 3 years after starting MMF) and two others had digestive symptoms (abdominal pain, diarrhea) leading to a dosage reduction in one of them. One patient in the CYC group got pregnant but experienced a miscarriage. Gonadal toxicity was not evaluated. Considering all complications, there was no significant difference in their occurrence between both groups (p = 0.48).
Discussion
Treatment of pediatric LN is not standardized, and no international recommendation favors a specific induction therapy. Nowadays the choice of pediatric treatments is inspired by data reported in adult patients. Even though CYC has been considered for many years as the gold standard treatment for class IV LN, its efficacy is still insufficient and its toxicity important. It may be hazardous to extrapolate children’s treatments from adults’ guidelines. It is important to take into consideration pediatric specificities such as growth, pubertal development, disease acceptance, therapeutic compliance and adverse events. Since Ginzler’s work was published in the New England Journal of Medicine in 2005 [7], MMF emerged as a good alternative with similar efficacy and lower toxicity. Many studies in the adult population confirmed this observation. Groot and colleagues, in the 2017 European Pediatric recommendations proposed low dose intravenous CYC and oral MMF as first-line treatment of proliferative LN [1]. In the present study, we sought to compare efficacy, safety and tolerance of i.v. CYC and oral MMF in the induction of remission of 33 children with LN. We did not observe any significant difference in remission rates at 6 months, 1 year and 3 years when comparing induction therapy by i.v. CYC and oral MMF.
In 2014, Tian et al. [14] conducted a prospective study to examine the effect of induction therapy by MMF, compared to the use of other immunosuppressive drugs (CYC and Azathioprine), on the long-term outcome of a pediatric-onset proliferative LN cohort. Their primary endpoint was the eGFR and they showed that MMF was more effective (statistically significant 6% improvement of kidney function) than the two other therapies in improving and maintaining long-term kidney function in these patients. However, this study did not include evaluation of proteinuria nor any other disease activity marker. According to the MAINTAIN analysis and to the Euro-Lupus Nephritis Trial [5], proteinuria at 12 months of induction therapy seems to be the best predictive marker of long-term kidney outcome. Thus, our primary endpoint was the complete remission rate (UPCR < 30 mg/mmol associated with normal eGFR) and the secondary endpoint was partial remission (UPCR 30-50 mg/mmol) at 6, 12 and 36 months of follow up. We observed complete and partial remission rates at 6 months respectively in 53% and 6% in the MMF group and 71% and 0% in the CYC groups. This figure is higher than the remission rates reported in adult cohorts. For instance, in 2009, Appel et al. [15] reported the outcome of 370 adult patients with class III, IV and V LN. For classes III and IV, 56.4% of patients treated with MMF achieved remission (proteinuria ≤ 0.5 g/day) at 6 months versus 53.9% in the CYC group. In Ginzler’s work [7], complete remission was seen in 29% of patients treated with MMF and partial remission in 37% of them, versus 10% and 40% in the CYC group. Similar rates were found in other studies, with variable definitions of complete (normalization of eGFR and proteinuria < 0.5 g/day or /L) and partial remission (improvement of 50% of kidney parameters) that were less stringent than the ones used herein, and in general in the pediatric practice. As an example of a pediatric study, in the study by Suhlrie et al. on 79 children with a proliferative form of LN in 2019, remission was considered as complete if proteinuria was < 20 mg/mmol with a normal eGFR, and partial if proteinuria was below the nephrotic range. At 12 months, complete and partial remissions were achieved in 38% and 41% of children. Sixty-five percent of the children were treated with CYC, 27% with MMF and 8% with anti-calcineurins but the detailed remission rate according to the treatments given was not specified. These remission rates are very low but the efficacy criteria were quite strict. We therefore chose a threshold of 30 mg/mmol of proteinuria/creatinuria ratio, which seemed a reasonable, albeit demanding goal in this young population at risk of unfavorable kidney outcome.
In the present study, the efficacy and safety of MMF and CYC did not significantly differ. At 1 year, complete renal remission was observed in 56% and 77% of patients in the MMF group and the CYC group, respectively (p = 0.46). Our results are consistent with those found by Smith et al. [16]. In their study of a Caucasian pediatric population with class III and IV LN, the median time to remission was similar between the MMF and CYC groups (p = 0.17). However, in the study by Appel and coworkers, subgroup analyses highlighted a significant difference in efficacy depending on the patient’s ethnicity. Indeed, MMF was superior to CYC in the treatment of Black African patients (60.4% versus 38.5%, p = 0.033) and Hispanic patients (60.9% versus 38.8%, p = 0.011). We could not confirm this result in our study because of the low number of patients from these ethnic backgrounds.
Treatment with CYC did not show more frequent or severe adverse events than treatment with MMF. We did not observe significantly more infections, hematologic toxicity or gastrointestinal intolerance in either group. Fertility was difficult to evaluate in this retrospective pediatric study; amongst all patients only one pregnancy was reported in the CYC group, that resulted in a miscarriage. In a prospective study in 2017,Tamirou et al. showed that the Euro-Lupus regimen of low-dose i.v. CYC does not impact the ovarian reserve of patients with SLE [17]. These results concerned adult patients and no study exists on the evolution of the ovarian reserve if low-dose i.v. CYC is given at pre-pubertal or pubertal stages. An evaluation of gonadal toxicity should be made by measuring anti Mullerian hormone dosages over time. The lack of difference in adverse events between the two treatments could be explained on one hand by better tolerance of chemotherapy by children, and on the other hand by a loss to follow-up of the cohort after transition to the adult world. To come to conclusions on long-term adverse events, it would be optimal to carry out a prospective follow-up from diagnosis in childhood until adulthood.
The major limitation of our study is related to its retrospective design which results in missing data. Adherence to treatment is difficult to assess and is left entirely to the practitioner’s observation, which is subjective. Since compliance is a major point in the effectiveness of a treatment, particularly in the comparison of oral treatment at home and i.v. treatment administered in the hospital, it would be necessary to evaluate it in future studies by questionnaires or drug dosages. In addition, the 6 children with neurologic involvement received CYC, and thus no conclusion can be drawn regarding the efficacy of MMF in lupus with neurologic involvement. Another important limitation of the study is its small size.
Conclusion
In summary, our study provides additional data to the currently limited literature on the efficacy and safety of CYC and MMF as induction treatment of class IV LN in children. Efficacy and toxicity did not significantly differ between these two induction treatments in pediatric-onset class IV LN. A randomized controlled trial would help to confirm these results. Overall, we suggest that MMF may be used as first-line induction therapy in LN without neurological involvement, and if good adhesion to treatment is anticipated. Additional data are needed to evaluate the efficacy of MMF in cases of neurological symptoms. Finally, failure to achieve complete renal remission at 12 months in 1/3–1/5 of patients emphasizes the need for new therapeutic approaches.
Abbreviations
- ACR:
-
American College of Rheumatology
- CYC:
-
Cyclophosphamide
- eGFR:
-
Estimated glomerular filtration rate
- ISN/RPS:
-
International Society of Nephrology/Renal Pathology Society
- i.v.:
-
Intravenous
- LN:
-
Lupus nephritis
- MMF:
-
Mycophenolate mofetil
- NIH:
-
National Institutes of Health
- SAE:
-
Severe adverse events
- SHARE:
-
Single Hub and Access point for pediatric Rheumatology in Europe
- SLE:
-
Systemic lupus erythematosus
References
Groot N, de Graeff N, Marks SD, Brogan P, Avcin T, Bader-Meunier B et al (2017) European evidence-based recommendations for the diagnosis and treatment of childhood-onset lupus nephritis: the SHARE initiative. Ann Rheum Dis 76(12):1965–1973
Watson L, Leone V, Pilkington C, Tullus K, Rangaraj S, McDonagh JE et al (2012) Disease activity, severity, and damage in the UK Juvenile-Onset Systemic Lupus Erythematosus Cohort. Arthritis Rheum 64(7):2356–2365
Pinheiro SVB, Dias RF, Fabiano RCG, Araujo SA, Silva ACS (2019) Pediatric lupus nephritis. J Bras Nefrol 41(2):252–265
Therapy of lupus nephritis. Controlled trial of prednisone and cytotoxic drugs. - PubMed - NCBI. [cité 24 déc 2019]. Disponible sur: https://www.ncbi.nlm.nih.gov/pubmed/3511372
Houssiau FA, Vasconcelos C, D’Cruz D, Sebastiani GD, Garrido EER, Danieli MG et al (2002) Immunosuppressive therapy in lupus nephritis: the Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum 46(8):2121–2131
Kingdon EJ, McLean AG, Psimenou E, Davenport A, Powis SH, Sweny P et al (2001) The safety and efficacy of MMF in lupus nephritis: a pilot study. Lupus 10(9):606–611
Ginzler E, Dooley M, Aranow C, Kim MY, Buyon J, Merrill J et al (2005) Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N Engl J Med 353:2219–2228
Palmer SC, Tunnicliffe DJ, Singh-Grewal D, Mavridis D, Tonelli M, Johnson DW et al (2017) Induction and maintenance immunosuppression treatment of proliferative lupus nephritis: a network meta-analysis of randomized trials. Am J Kidney Dis sept 70(3):324–336
Mina R, von Scheven E, Ardoin SP, Eberhard BA, Punaro M, Ilowite N et al (2012) Consensus treatment plans for induction therapy of newly diagnosed proliferative lupus nephritis in juvenile systemic lupus erythematosus. Arthritis Care Res 64(3):375–383
Lau KK, Ault BH, Jones DP, Butani L (2008) Induction therapy for pediatric focal proliferative lupus nephritis: cyclophosphamide versus mycophenolate Mofetil. J Pediatr Health Care 22(5):282–288
Garcelon N, Neuraz A, Salomon R, Faour H, Benoit V, Delapalme A et al (2018) A clinician friendly data warehouse oriented toward narrative reports: Dr. Warehouse. J Biomed Inform avr 80:52–63
Hochberg MC (1997) Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40(9):1725
Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB et al (2004) The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int 65(2):521–530
Tian SY, Feldman BM, Beyene J, Brown PE, Uleryk EM, Silverman ED (2014) Immunosuppressive therapies for the induction treatment of proliferative lupus nephritis: a systematic review and network metaanalysis. J Rheumatol 41(10):1998–2007
Appel GB, Contreras G, Dooley MA, Ginzler EM, Isenberg D, Jayne D et al (2009) Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol JASN 20(5):1103–1112
Smith EMD, Al-Abadi E, Armon K, Bailey K, Ciurtin C, Davidson J et al (2019) Outcomes following mycophenolate mofetil versus cyclophosphamide induction treatment for proliferative juvenile-onset lupus nephritis. Lupus. https://doi.org/10.1177/0961203319836712?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub++0pubmed
Tamirou F, Husson SN, Gruson D, Debiève F, Lauwerys BR, Houssiau FA (2017) Brief report: The Euro-Lupus low-dose intravenous cyclophosphamide regimen does not impact the ovarian reserve, as measured by serum levels of anti-Müllerian Hormone. Arthritis Rheumatol Hoboken NJ 69(6):1267–1271
Funding
None.
Author information
Authors and Affiliations
Contributions
OB and BBM designed the study. LAE, MC, OB and BBM designed and created the clinical database. QR performed the statistical analysis. LAE, MC, OB and BMM analyzed and interpreted the data and drafted the article. All authors were involved in data collections, review and approval of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to disclose. The authors have no financial relationship relevant to this article to disclose.
Ethical statement
The study was approved by the ethical committee of Necker-Enfants Malades hospital (APHP general register Number 2020 0805180729).
Clinical trial registration
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chbihi, M., Eveillard, LA., Riller, Q. et al. Induction therapy for pediatric onset class IV lupus nephritis: Mycophenolate Mofetil versus Cyclophosphamide. J Nephrol 36, 829–839 (2023). https://doi.org/10.1007/s40620-022-01438-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-022-01438-2