Abstract
Background
The impact of cancer on death of elderly kidney transplant recipients has been extensively investigated, but with conflicting results. Unlike their younger counterparts, in elderly kidney transplant recipients cardiovascular and infectious disease may outweigh cancer in causing the patient’s death.
Methods
Using competing risk analysis on a large retrospective cohort of kidney transplant recipients, we estimated the cause-specific cumulative incidence and hazard of death in different age categories and calculated standardized mortality ratios (SMRs) to compare mortality rates with the general population.
Results
Six thousand seven hundred eighty-nine kidney transplant recipients were followed-up for a median of 9 years. Ten years after transplantation, in transplant recipients aged 20–39, 40–59, and 60+, the cumulative incidence of cancer-related death was 0.6 (95% confidence interval [CI]: 0.3–1.0), 2.9 (2.3–3.6) and 5.3% (3.5–7.5), whereas the SMR was 9.1 (5.5–15.0), 2.0 (1.6–2.5), and 0.8 (0.6–1.0), respectively. At variance with young recipients, the hazard and the cumulative incidence of cardiovascular-related death in elderly recipients was well above that of cancer-related death.
Conclusions
Relative to the general population, cancer-related death is increased in young but not in elderly kidney transplant recipients because of the more marked increased incidence of competing cause of death in the latter category.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kidney transplant recipients have a higher incidence of some cancers, but whether this translates into an excess cancer-related mortality in all age categories is still a matter of debate. Some studies have described an overall risk of cancer death after kidney transplantation up to tenfold higher than in the general population [1,2,3], whereas more recent studies have shown that it is increased on average by 2.7 times [4]. In the few studies focussing on the mortality risk in different age categories, a reduced incidence of cancer-related death compared to the general population was reported in older transplant recipients, who may have an increased rate of competing causes of death such as cardiovascular and infectious diseases [5,6,7]. If confirmed, this phenomenon may have relevant practical implications. For instance, active listing for transplantation of elderly candidates with pre-existing malignancies or other conditions at low increased risk for cancer is often postponed because of the fear of cancer recurrence and/or the effects of post-transplant immunosuppression. On the other hand, wait-listing transplant candidates with a prior cardiovascular event has become less debated and is a common practice [8]. However, because the window of opportunity for transplantation in elderly transplant candidates is rather narrow, the practice of delaying wait-listing in the presence of minor risk factors for the development of cancer post-transplantation may no longer be justified if evidence is provided clearly showing that cancer-related mortality is not increased compared to the general population [8,9,10].
In this retrospective follow-up study, using a competitive risk analysis, we estimated cancer-related mortality in adult kidney transplant recipients across different age and gender categories, and assessed how mortality rates compare to age and sex-matched reference populations living in the same geographic area.
Patients and methods
Patients
This was a retrospective cohort study including a large series of consecutive adult kidney transplant recipients transplanted from a deceased donor from 1980 to 2012 at eight Italian transplantation centres. Kidney transplant recipients underwent regular follow-up after transplantation. Induction therapy included anti-interleukin 2 receptor monoclonal antibody (Simulect; Novartis AG, Basel, Switzerland) or antithymocyte immunoglobulins (BioMerieux Italia s.p.a., Bagno a Ripoli, Italy or Genzyme Corp., Cambridge, MA, USA). Acute rejection episodes were treated with pulsed i.v. methylprednisolone (0.5–1.0 g/day for 3 consecutive days), and corticosteroid-resistant acute rejection was treated with antithymocyte immunoglobulins, while antibody-mediated rejection was treated with plasmapheresis and i.v. immunoglobulins most of the times. Long-term maintenance immunosuppressive therapy included a combination of antimetabolites (mycophenolate mofetil, or enteric coated sodium or, rarely, azathioprine), calcineurin inhibitors (cyclosporine or tacrolimus) and methylprednisolone, and with increasing frequency, sirolimus and everolimus [11].
Data recorded for all the kidney transplant recipients were date of birth, sex, date of transplantation, type of immunosuppressive therapy, and the dates of death or of the last follow-up, causes of death or loss to follow-up. The main outcome was death and the cause of death, censored for graft failure.
All patients gave their informed consent to use their data in all centres. The study is exempt of IRB approval because the study is purely observational and not finalized to change treatment. All information entered in the database were anonymized and none of the statistical analyses ever included any identifying process of the patients’ personal information.
Statistical analysis
Stata release 15.1 (StataCorp LLC, College Station, TX, USA) was used for all the analyses. The follow-up time was calculated from transplantation date to death, to dialysis, or to the end of follow-up (June 30th, 2012). Competing risk analysis was carried out in order to calculate the following estimates [12]:
-
(a)
Crude cumulative incidence of multiple causes of death in kidney transplant recipients with functioning graft, in different recipient age and gender categories; for this purpose we used the Stata user program stcompet and stpepemori for non-parametric estimation and testing, respectively [13, 14].
-
(b)
Analysis of historical trends from multiple regression analyses for competing risk. Using the Stata program stcrreg, we tested whether there was an effect modification of historical period [polynomial continuous variate calendar year chosen based on Akaike Information Criterion (AIC)] on the relationship between age, gender and the cumulative incidence of cancer-related death by fitting interaction terms into a semiparametric multiple regression for cause-specific “subhazards” according to the approach of Fine and Gray [15].
-
(c)
Cause-specific hazards of death and their time-change after transplantation. At variance with the model for cumulative incidence functions, the model for hazard functions leads to valid estimates when censoring for other causes of death. Therefore, in order to explore the non-linear time-change of each cause-specific hazard of death after transplantation, we fitted simultaneous proportional- and non-proportional-hazards regression models for cause-specific hazard of death via stratified Cox-proportional hazard regression model, using the Stata user program stpm2 [16]. The stpm2 program fits cumulative hazard regression models which, using restricted cubic splines, allow for fitting separate non-proportional and non-linear hazard functions for each age and gender category; we used AIC to compare models with a different number of knots when using splines to obtain the best fit to observed data [16].
-
(d)
Standardized mortality ratio (SMR). The excess mortality from each cause compared to the general population was estimated calculating the cause specific SMRs, which express how many times the rate of death in kidney transplant recipients is increased compared to the general population. Causes of death were classified according to the Italian version of ICD 10 (https://www.epicentro.it, last access June 30, 2015). SMRs were calculated after matching for age, gender, geographic region of Italy, and period in which the event had occurred, or after stratifying by age and gender. To calculate SMRs, we used the Stata program strate and the default (exact) method for calculating 95% confidence intervals (CI), which is based on a Poisson distribution. P values for the null hypothesis that SMR = 1, for the difference between groups in SMR, and for the test for trend were carried out using the score test statistics. Expected values were computed extracting mortality rates from the National Institute of Statistics (https://www.istat.it, last access June 30, 2015) for non-cancer related deaths, and by the Italian Association of Cancer Registries (AIRTUM—https://www.registri-tumori.it, last access June 30, 2015), for cancer-related deaths.
Results
Baseline characteristics of the cohort
Six thousand seven hundred eighty-nine kidney transplant recipients were enrolled, (4363 men and 2,426 women); median age at transplantation was 46.0 years (range 18.0–68.0), and median follow-up time since transplantation was 9.4 years (range 1.0–24.0). Four hundred and seventy-one of them had received a second transplantation (6.9%). One hundred and seventy (2.5%) presented a history of cancer before transplantation, but no cancer recurrence was observed. Total post-transplantation follow-up was 64,810 person-years. Two hundred and eighty-seven (4.2%) were lost to follow-up and 947 (20.5%) developed chronic graft rejection and returned to dialysis. Further details are presented in Table 1
Causes of death
Eight hundred and fourteen (538 males and 276 females), out of 6,789 kidney transplant recipients (12.0%) died during the observation period. Three hundred and forty-eight died of cardiovascular diseases, 186 of systemic infections, 186 of cancer (135 non-cutaneous tumours, 5 metastatic cutaneous squamous cell carcinomas, 41 post-transplant lymphoproliferative disorders (PTLD), and 5 Kaposi sarcoma), and 94 of other causes (Table 2). One hundred and eighty-six died of de novo cancers. The most common cancers were PTLD, lung cancer, cancer of the native kidney, pancreas carcinoma and colorectal carcinoma (Table 2).
Crude cumulative incidence of multiple causes of death and analysis of historical trends from multiple regression analyses for competing risk
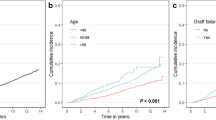
The cumulative incidence of death from each cause in males and females are shown in Fig. 1. Ten years after transplantation, the cumulative incidence of cancer-related death in males and females was 2.7 (95% confidence interval: 2.2–3.2) and 1.6 (1.1–2.2), respectively (Table 3; P = 0.038). On the other hand, as shown in Table 3, the 10-year cumulative incidence of death related to cancer or infection, and other causes was virtually identical between genders. Figure 2 shows the cumulative incidence of death from each cause after stratifying into age categories. Ten years after transplantation, the cumulative incidence of cancer-related death in recipients aged < 20–39 years, 40–59 years, and 60+ years was 0.6 (0.3–1.0), 2.9 (2.3–3.6) and 5.3 (3.5–7.5), respectively (Table 3), well below that of cardiovascular-related death, which was almost + 5% in kidney transplant recipients aged 60+. As shown in Table 3, the 10-year cumulative incidence of death related to cancer or infection also differed between age groups, whereas death from other causes was similar.
Cumulative incidence of death from each cause estimated by the non-parametric method for competing risk in females (left panel) and males (right panel). The difference between genders in the cumulative incidence of cancer-related death was statistically significant (P = 0.038), whereas it was not significant for CVD (P = 0.97), infection (P = 0.91), and other causes (P = 0.20). CVD cardiovascular disease
Cumulative incidence of death from each cause estimated by the non-parametric method for competing risk according to the different age categories. The difference between age categories in the cumulative incidence of cancer-related death, CVD-related death, and infection-related death was statistically significant, whereas it was not significant for death from other causes. CVD cardiovascular disease
Multiple regression analyses for competing risk showed that, unlike what was observed with cardiovascular disease (CVD)-related death (Supplementary Appendix Figure S1) and with infection-related death (not shown), rates of cancer-related death did not consistently increase with the recipient’s age across all historical periods (Supplementary Appendix Figure S2), the increase with recipient’s age being observed more sharply around the mean time frame of the cohort (calendar year 2000) compared to earlier and later periods (interaction term between age category and polynomial calendar year: P < 0.001).
Cause-specific hazards of death and their time-change after transplantation
As already found with cumulative incidence (Table 3), the hazard of cancer-related death was higher in males compared to females (hazard ratio [HR]: 1.50 (95% confidence interval: 1.09–2.09; P = 0.014)), whereas the HR of other causes of death was virtually identical between genders. As expected, under the proportional hazard model, compared to the reference category (age 40–59 years), the relative hazard of death from cancer, cardiovascular disease, and infection was similarly reduced in recipients aged < 40 years (though less so for the hazard of death from “other” causes): HR of cancer-death 0.35 (0.24–0.51), HR of CVD-related death: 0.31 (0.24–0.42), HR of infection-related death: 0.49 (0.34–0.69), HR of death from other causes: HR 0.73 (0.55–0.98). By the same token, compared to the same reference category, the relative hazard of death from cancer, cardiovascular disease, and infection was similarly increased in recipients aged 60+: HR of cancer-death 1.83 (1.24–2.71), HR of CVD-related death: 1.98 (1.50–2.61), HR of infection-related death: 1.70 (1.16–2.50), HR of death from other causes: 1.15 (0.74–1.80). However, as shown by non-proportional hazard models, the time-course of cause-specific hazards of death after transplantation differed greatly according to the age categories, as shown in Fig. 3: Fig. 3, left panel shows the time course of the hazard of a CVD-related death in the three age categories: in younger age categories, the hazard of CVD-death picked early after transplantation, then dropped, and eventually slowly increased over several years, while in recipients aged 60+, the hazard of CVD-death did not drop shortly post-transplantation. On the contrary, the numerical values of the hazard of CVD-death remained high and were numerically greater compared to those of the hazard of cancer-related death throughout (Fig. 3, right panel).
Hazard of CVD-related death (left panel) and cancer-related death (right panel) as estimated by a non-proportional hazard model using restricted cubic splines. The hazard of CVD-death picked early after transplantation, although in recipients aged 60+ it remained high even in years following transplantation and well above the hazard of cancer-related death. The bands represent 95% confidence intervals. Numbers not included in the 95% confidence interval are significantly different at alpha level of 0.05. Therefore, the hazard of CVD in patients aged 60+ (lower panel) was significantly higher than 0.01 in the first years following transplantation, whereas the hazard of cancer-related death (upper panel) was significantly lower than 0.01 in the same age category. CVD cardiovascular disease
Standardized mortality ratio (SMR) for the comparison with the general population
Compared to the general population, mortality was increased for all causes (SMR 4.6 [95% CI 4.3–4.9; P = 0.001]), for cardiovascular diseases (SMR 11.2 [95% CI 10.1–12.4 P = 0.001]), for cancers (SMR 2.2 [95% CI 1.9–2.5; P = 0.001]), for infections (SMR 47.7 [95% CI 43.5–57.6; P = 0.001]); other relevant causes of death were chronic liver disease, (SMR7.6) and suicide (SMR 4.8). More details are reported in Table 2.
Table 4 reports SMR of death from each cause according to gender and age categories. Compared to the general population, and at variance with young recipients, cancer-related death was no higher in recipients aged 60+ in both males and females. In fact, after calculating SMR irrespective of gender categories, SMR was 9.1 (95% CI 5.5–15.0), 2.0 (95% CI 1.6–2.5), and 0.8 (95% CI 0.6–1.0) in transplant recipients aged 20–39, 40–59, and 60+, respectively. The same findings can be inferred from Fig. 4, which shows SMR of death from all causes and of death from cancer, according to gender and age categories. Despite the fact that compared to the general population, overall mortality was increased in all age and gender categories (Fig. 4, left panel), cancer-related death tended to be virtually identical to that in the general population in kidney transplant recipients aged 60+ (Fig. 4, right panel).
Left panel. Standardized mortality ratio (SMR) of death from all causes according to gender and age categories. Horizontal bars represent 95% confidence intervals. Confidence intervals crossing the vertical dotted lines indicate that overall mortality is not different compared to the general population. Compared to the general population, excess mortality was more marked in younger compared to older recipients, and in females compared to males. Right panel. Standardized mortality ratio (SMR) of cancer-related death according to gender and age categories. Horizontal bars represent 95% confidence intervals. Confidence intervals crossing the vertical dotted lines indicate that cancer-related mortality is not different compared to the general population. Compared to the general population, patients aged 60+ did not have increased rates of cancer-related mortality. On the other hand, cancer-related mortality was markedly increased in patients aged 20–39, especially for males
Discussion
Our findings from a large cohort of kidney transplant recipients with a long retrospective follow-up show that cancer-related mortality, which is in absolute terms higher in elderly patients compared to their younger counterparts, is strikingly higher in younger but no higher in older kidney transplant recipients when compared to the general population. Our competing risk analysis provides evidence that in elderly kidney transplant recipients a persistently increased hazard of death from other causes (such as from CVD, and possibly infectious disease) may play a role in reducing the chance of dying of cancer.
There are conflicting reports regarding whether an excess mortality due to cancer exists in patients undergoing solid organ transplantation across all age categories [1,2,3, 17,18,19]. In the study of Farrugia et al. the risk of cancer-related death ranged from 0.5% in kidney transplant recipients younger than 50–6.5% for those aged 70–79. The risk difference between kidney transplant recipients and the general population steadily increased in kidney transplant recipients after the age of 55 [3]. However, in the multicentre ERA-EDTA European study, mortality rate ratio for cancer-related death was 1.7 as compared to the general population, and declined with age due to the increase in infection-related mortality [6]. Moreover, in the US study by Kiberd et al., the calculated SMRs for cancer death were highest in the youngest populations (0–19 and 20–39), not unlike the 1.00 in the 50–59 age, and significantly lower in the older age groups [5]. Au et al. analysed data from the ANZDATA Registry, which includes a population in which the incidence of skin cancers ranks among the highest worldwide, and showed that the SMR of cancer-related death decreased from 11 to less than 2 when comparing kidney transplant recipients aged 20–34 (SMR 11.0; 95% CI 5.5–17.2), to those aged 65 or older (SMR 1.7; 95% CI 1.6–1.9) [4]. Our study confirms and expands the findings from those studies by additionally providing evidence supporting the hypothesis that competing risks of death from other causes (especially cardiovascular mortality) dampen the impact of immunosuppression-induced malignancy in the older transplant population. Kidney transplant recipients younger than 39 have the highest cancer SMRs because this group has longer projected life expectancies (lower competing risks of death) with greater cumulative risks of succumbing to their malignancy, but in older kidney transplant recipients there might be other competing risks of death, especially cardiovascular diseases [4, 5]. However, a possible selection bias may have also occurred, as older patients are more intensively screened for cancer than younger ones. On the other hand, there are possible explanations for the conflicting findings reported by other studies [2, 3, 19, 20]. Published studies are not homogeneous in design, and the different time periods during which they were performed may reflect different types and posologies of immunosuppressive drugs. In some relevant studies, median follow-up time ranged from about 4.4–6.5 years, that is significantly shorter than our follow-up time (9.4 years), and this may explain the occurrence of death due to cardiovascular and infectious causes that occur later in transplantation time [3,4,5, 20,21,22,23,24]. Another possible contribution is represented by the strong decline in cancer mortality in the Italian general population that occurred between 1980 and 2010. This trend is related to the nationwide program of cancer screening that was developed in those years, to continuous and improved control of overweight, tobacco and alcohol consumption, and education to healthy nutrition and lifestyle [25].
Transplant candidates undergo extensive cancer screening. Those affected by recently treated cancer or with a history of cancer are usually withdrawn from the transplantation list for long periods of time (usually 2–5 years) to exclude recurrences [26]. However, being withdrawn from the waiting list for such a long time may be particularly harmful to elderly transplant candidates who may miss the short window of opportunity which is available to them to be eligible for kidney transplantation. Therefore, we contend that given that kidney transplantation does not increase the chance of dying from cancer in elderly candidates as much as it does in younger counterparts, transplant candidate age should be carefully taken into account when assessing the benefit of delaying transplantation in candidates at low-to moderate risk of cancer recurrence. Our findings may support the need for candidates to transplantation with current or previous cancer to undergo an accurate evaluation together with an oncologist, and that waiting times should be considered on a case-by-case basis taking into account the potential for progression or recurrence of the cancer, the age of the patient and the existence of comorbidities. An additional finding of our study is that unlike in the general population, female and males have similar cumulative incidence of death from any cause, suggesting that female kidney transplant recipients lose the survival advantage observed in the general population [7].
Women in the general population present lower mortality rates at any age, and have longer life expectancy from birth due to the lower incidence and a better prognosis of cardiovascular diseases and severe infections [7, 27, 28]. Conversely, in kidney transplant recipients undergoing chronic renal replacement therapy, men and women seem to have similar mortality rates, and limited data exist in the transplantation setting [27,28,29,30]. Women with chronic renal failure present a chronic hypoestrogenic state, hyperprolactinemia, and early onset of menopause [27,28,29,30]. This condition impairs immune response against infectious agents and enhances the risk of cardiovascular diseases [6, 27, 28]. Kidney transplantation restores menstrual cycles and fertility, as observed in the cases of successful pregnancies [28,29,30,31,32], but in our female population median age at transplantation was 46 years, which is near the menopausal age.
The main limitations of our study are the retrospective nature of the study and the lack of data about cancer staging at the time of the diagnosis, and its management. Death in cancer patients may be precipitated by other comorbidities (e.g. sepsis) and determining the exact cause of death may be difficult. In fact, according to Noone et al., cancer-attributable mortality in transplanted patients was above 70% if cause of death was coded as cancer whereas it ranged between 4.2 and 9.4 in patients with other causes of death [33]. The study has also relevant strengths including the long follow-up time, and the standardized immunosuppressive treatment employed across the different centres involved and the regular follow-up of kidney transplant recipients.
Conclusions
In conclusion, the study shows that in kidney transplant recipients, cancer-related mortality in patients aged 60 or older is no higher if compared to the general population, whereas it is strikingly higher in those aged below 40.
References
Vajdic CM, McDonald SP, McCredie MR et al (2006) Cancer incidence before and after kidney transplantation. JAMA 296:2823
Wong G, Chapman JR, Craig JC (2014) Death from cancer: a sobering truth for patients with kidney transplants. Kidney Int 85:1262
Farrugia D, Mahboob S, Cheshire J et al (2014) Malignancy-related mortality following kidney transplantation is common. Kidney Int 85:1395
Au EH, Chapman JR, Craig JC et al (2019) Overall and site-specific cancer mortality in patients on dialysis and after kidney transplant. J Am Soc Nephrol 30:471
Kiberd BA, Rose C, Gill JS (2009) Cancer mortality in kidney transplantation. Am J Transplant 9:1868
Vogelzang JL, van Stralen KJ, Noordzij M et al (2015) Mortality from infections and malignancies in patients treated with renal replacement therapy: data from the ERA-EDTA registry. Nephrol Dial Transplant 30:1028
Hecking M, Bieber BA, Ethier J et al (2014) Sex-specific differences in hemodialysis prevalence and practices and the male-to-female mortality rate: the dialysis outcomes and practice patterns study (DOPPS). PLOS Med 11:e1001750
Watschinger B, Budde K, Crespo M et al (2019) Pre-existing malignancies in renal transplant candidates-time to reconsider waiting times. Nephrol Dial Transplant 34:1292
Lim WH, Au E, Krishnan A, Wong G (2019) Assessment of kidney transplant suitability for patients with prior cancers: is it time for a rethink? Transpl Int 32:1223
Pham PT, Pham PA, Pham PC, Parikh S, Danovitch G (2010) Evaluation of adult kidney transplant candidates. Semin Dial 23:595
Marcen R (2009) Immunosuppressive drugs in kidney transplantation: impact on patient survival, and incidence of cardiovascular disease, malignancy, and infection. Drugs 69:2227
Noordzij M, Leffondré K, van Stralen KJ, Zoccali C, Dekker FW, Jager KJ (2013) When do we need competing risks methods for survival analysis in nephrology? Nephrol Dial Transplant 28:2670
Coviello V, Boggess M (2004) Cumulative incidence estimation in the presence of competing risks. Stata J 4:103
Gaynor JJ, Feuer EJ, Tan CC et al (1993) On the use of cause-specific failure probabilities: Examples from clinical oncology data. J Am Soc Stat Assoc 88:414
Fine JP, Gray RJ (1999) A proportional hazards model for the sub distribution of a competing risk. J Am Stat Assoc 94:496
Royston P, Parmar MKB (2002) Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med 21:2175
Kahwaji J, Bunnapradist S, Hsu JW, Idroos ML, Dudek R (2011) Cause of death with graft function among renal transplant recipients in an integrated healthcare system. Transplantation 91:225
Geissler EK (2015) Post-transplantation malignancies: here today, gone tomorrow? Nat Rev Clin Oncol 12:705
Acuna SA, Fernandes KA, Daly C et al (2016) Cancer mortality among recipients of solid-organ transplantation in Ontario, Canada. JAMA Oncol 2:463
Stoumpos S, Jardine AG, Mark PB (2015) Cardiovascular morbidity and mortality after kidney transplantation. Transplant Int 28:10
Liefeldt L, Budde K (2010) Risk factors for cardiovascular disease in renal transplant recipients and strategies to minimize risk. Transplant Int 23:1191–1204
Devine PA, Courtney AE, Maxwell AP (2019) Cardiovascular risk in renal transplant recipients. J Nephrol 32:389
Snyder JJ, Israni AK, Peng Y, Zhang L, Simon TA, Kasiske BL (2009) Rates of first infection following kidney transplant in the United States. Kidney Int 75:317–326
Au E, Wong G, Chapman JR (2018) Cancer in kidney transplant recipients. Nat Rev Nephrol 14:508
Rosso T, Bertuccio P, la Vecchia C, Negri E, Malvezzi M (2015) Cancer mortality trend analysis in Italy, 1980–2010, and predictions for 2015. Tumori 101:664
EBPG (European Expert Group on Renal Transplantation), European Renal Association (ERA-EDTA), European Society for Organ Transplantation (ESOT) (2000) European best practice guidelines for renal transplantation (part 1). Nephrol Dial Transpl 15:1
George J, Rapsomaniki E, Pujades-Rodriguez M et al (2015) How does cardiovascular disease first present in women and men? Incidence of 12 cardiovascular diseases in a contemporary cohort of 1 937 360 people. Circulation 132:1320
McClelland EE, Smith JM (2011) Gender specific differences in the immune response to infection. Arch Immunol Ther Exp 59:203
Adey DB (2013) Women and kidney transplantation. Adv Chronic Kidney Dis 20:427
Ramesh S, Mann MC, Holroyd-Leduc JM et al (2015) The effect of hormone therapy on all-cause and cardiovascular mortality in women with chronic kidney disease: protocol for a+ systematic review and meta-analysis. Syst Rev 4(44):2
Ahmed SB, Ramesh S (2016) Sex hormones in women with kidney disease. Nephrol Dial Transplant 31:1787
Attini R, Cabiddu G, Montersino B et al (2020) Contraception in chronic kidney disease: a best practice position statement by the Kidney and Pregnancy Group of the Italian Society of Nephrology. J Nephrol. https://doi.org/10.1007/s40620-020-00717-0
Noone AM, Pfeiffer RM, Dorgan JF et al (2019) Cancer-attributable mortality among solid organ transplant recipients in the United States: 1987 through 2014. Cancer 125:2647
Acknowledgements
We gratefully acknowledge Dr. Emanuele Crocetti and Dr. Stefano Ferretti from the AIRTUM, for having provided mortality rates of cancer in the general Italian population, and Dr. Matteo Malvezzi from the Mario Negri Institute of Pharmacological Research of Milano, for having provided mortality rates of squamous cell carcinoma of the skin in the general Italian population.
Funding
None.
Author information
Authors and Affiliations
Contributions
GT, UM, GZ and GG participated in research design, the writing of the paper, in the performance of the research and in data analysis. GT and UM contributed equally. RENM, LN, LB, FN, SS, EG, MC, PM, AP, IC, EM and MR participated in research design, in the performance of the research and revising the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest. No conflict of interest exists in the submission of this manuscript, and the manuscript is approved by all authors for publication. I would like to declare on behalf of my co-authors that the work described was original research, that has not been published previously, and is not under consideration for publication elsewhere, in whole or in part. And all the authors listed have approved the manuscript that is enclosed.
Ethical statement
All subjects were treated with standard care without intervention from this study. All data were obtained via electronic medical records and a database review and were de-identified (the patient’s name was replaced with an identification code, and the patient’s private information was deleted before the analysis) to protect patient privacy.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
40620_2020_847_MOESM1_ESM.pptx
Supplementary file1Figure S1. Sub-hazard ratios (SHRs) of death from each specific cause as predicted by the fitted multiple regression model of Fine and Gray for competing risk. Calendar year was fitted as a polynomial continuous variate. The reference value of SHR (i.e. SHR=1) was represented by age 40-59 and calendar year 2000. Dots do not represent SHRs of groups of actual patients but rather predicted SHR values of hypothetical patients in the selected age category and calendar year: the plot is meant to have a visual appraisal of the SHR estimates from an otherwise complex multiple regression model. Both calendar year and age category were statistically significant (P<0.001). SHR, Sub-hazard ratio (i.e. the ratio between hazards which are calculated including patients who already died from other causes to reflect the hazards the actually the observed cumulative incidences of CVD-related death in the presence of competing causes of death). Figure S2. Sub-hazard ratios (SHRs) of death from each specific cause as predicted by the fitted multiple regression model of Fine and Gray for competing risk. Calendar year was fitted as a polynomial continuous variate. The reference value of SHR (i.e. SHR=1) was represented by age 40-59 and calendar year 2000. Dots do not represent SHRs of groups of actual patients but rather predicted SHR values of hypothetical patients in the selected age category and calendar year: the plot is meant to have a visual appraisal of the SHR estimates from an otherwise complex multiple regression model. Both calendar year and age category were statistically significant (P<0.001); in addition, the interaction term between age category and polynomial calendar year was significant (P<0.001) meaning that the SHR did not consistently increase with age categories in all historical periods. SHR, Sub-hazard ratio (i.e. the ratio between hazards which are calculated including patients who already died from other causes to reflect the hazards the actually the observed cumulative incidences of cancer-related death in the presence of competing causes of death) (PPTX 206 kb)
Rights and permissions
About this article
Cite this article
Tessari, G., Maggiore, U., Zaza, G. et al. Mortality from cancer is not increased in elderly kidney transplant recipients compared to the general population: a competing risk analysis. J Nephrol 33, 1309–1319 (2020). https://doi.org/10.1007/s40620-020-00847-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-020-00847-5