Abstract
Diabetic kidney disease (DKD) is the leading cause of end-stage kidney disease (ESKD) in the Western world. Better control of glycemia and blood pressure, including renin-angiotensin system blockade (RASB), appear to have slowed DKD progression rate but have been unable to substantially decrease the annual incidence of new cases of DKD related ESKD. Thus, new treatment targets are needed. Higher levels of serum uric acid (SUA) have been associated with increased risk and progression of DKD in persons with types 1 (T1D) and 2 (T2D) diabetes and of chronic kidney disease (CKD) in general. This review presents the epidemiological, clinical, and clinical trial evidence regarding the hypothesis that SUA reduction could slow progression of DKD and/or CKD in general.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite improvements in glycemia [1] and blood pressure control and the use of renin angiotensin system blocking (RASB) drugs [2] the number of persons in the US who develop diabetes-related end-stage kidney disease (ESKD) each year continues to rise [3], in parallel with the worldwide epidemic of diabetes [4]. In fact, we have yet to experience the full impact of the recent marked increases in the number of people with obesity and diabetes, who may take decades to reach ESKD [4]. Importantly, the benefits of RASB on progression of glomerular filtration rate loss (GFR) loss in patients with diabetic kidney disease (DKD) has only been seen in patients with estimated GFRs < 60 mL/min/1.73 m2 and the delay in reaching ESKD averaged less than 2 years [2]. Thus, new treatments are needed. Herein we examine the clinical evidence linking higher levels of serum uric acid (SUA) with the risk and progression of DKD and review trials that have attempted to test whether SUA reduction can slow or arrest GFR decline in this condition.
Serum uric acid and DKD risk
A number of epidemiological studies have reported an association between SUA levels and risk of DKD. The first was an inception cohort study that was carried out at the Steno Diabetes Center [5]. The study enrolled all newly diagnosed type 1 diabetic (T1D) patients seen at this center between September 1979 and August 1984. The patients all had measurements of SUA within the first 3 years after T1D onset i.e., before any abnormalities in albuminuria or GFR5. Follow-up was for a median of 18.1 years. During this time, macroalbuminuria developed in 25% of the subjects in the highest quartile of SUA values (> 4.2 mg/dL) vs. only 10% in the lower three quartiles (p = 0.006). These findings were independent of baseline body mass index (BMI), HbA1c, albumin excretion rate, serum creatinine, serum cholesterol, and mean arterial blood pressure (BP). Adjusted for these variables, the HR was 2.93 [1.25–6.86] per 1.7 mg/dL (100 µmol/L) increase in SUA; P 0.013) [5]. However, baseline SUA did not predict the risk of microalbuminuria or GFR loss.
In another study of normoalbuminuric T1D patients, conducted in the US, Jalal et al. [6] asked whether baseline SUA was a risk factor for micro- or macroalbuminuria over the subsequent 6-years; SUA at baseline was higher in the 25 subjects progressing to micro- or macroalbuminuria vs. the 299 remaining normoalbuminuric (p = 0.02). After adjustments for age, sex, T1D duration, treatment with renin-angiotensin system blockers (RASB), BMI, BP, HbA1c, serum creatinine, cystatin C, and lipid levels, higher baseline SUA increased the odds of new onset of increased albuminuria by 1.8 fold (p = 0.005) [6]. Consistent with these findings, a cross-sectional study of 20,464 adult patients with T1D from Italy, 11,162 of whom had SUA measurements, found that each 1 mg/dL increase of SUA was associated with an increased prevalence of DKD (OR = 1.56; 95% CI 1.49–1.63; P < 0.001), low eGFR (< 60 mL/min/1.73 M2, OR = 2.31; 95% CI 2.17–2.47; P < 0.001), and increased albumin excretion rate (OR = 1.30; 95% CI 1.25–1.36; P < 0.001) [7].
An association between SUA and DKD risk has also been reported among individuals with T2D. In another study from Italy, Zoppini et al. followed 1,449 T2D patients with eGFR > 60 mL/min/1.73 m2 and no overt proteinuria. Hyperuricemia in this study was defined as SUA ≥ 7.0 mg/dL in men and ≥ 6.5 mg/dL in women. After 5-years of follow-up, the cumulative incidence of new onset CKD (eGFR < 60 mL/min/1.73 m2) was higher in patients with (29.5%) vs. without hyperuricemia (11.4%, P = 0.001) [8]. Hyperuricemia was also associated with increased CKD risk after adjusting for age, sex, BMI, smoking, T2D duration, BP, BP treatment, insulin therapy, HbA1c, eGFR, and albuminuria, (OR 2.10 [1.16–3.76], P = 0.01), corresponding to a 21% increase in CKD risk for each 1-SD increase in SUA [8].
Serum uric acid and DKD progression
Increase SUA levels have been also implicated in the progression of DKD among patients who already have developed this complication. A group of 355 T1D patients from the T1D Joslin Kidney Study with high normoalbuminuria or microalbuminuria and baseline eGFR > 60 mL/min/1.73 m2 was followed for 6 years for the study outcome of rapid GFR decline defined as GFR loss > 3.3%/yr, this selected because this rate was above the 95th %tile of normal GFR loss with increasing age. The study found a progressively increasing risk of rapid eGFR decline with SUA levels increasing from < 3.0 to > 6.0 mg/dL (p = 0.0002) [9]. Patients with rapid GFR decline had lower eGFR, higher albumin excretion rate, and a higher prevalence of microalbuminuria. They also had longer T1D duration and were older than those with slower decline, but were not significantly different for glycemia, BMI or lipid levels [9]. The odds ratio for rapid GFR decline, 1.5 (95% CI 1.3–1.9) per 1 mg/dL increment in baseline SUA, remained statistically significant after adjustment for baseline albumin excretion rate, sex, and HbA1C and eGFR [9].
In a large two year follow-up study of 2518 patients with T2D, Hayashino et al. considered the endpoints of transition from normoalbuminuria to micro- or macroalbuminuria and progression from microalbuminuria to macroalbuminuria [10]. Using a Cox proportional hazards model, they evaluated the association between baseline SUA quartiles (means of 3.6, 4.9, 5.8, and 7.3 mg/dL for the 1st to 4th quartiles, respectively) and these outcomes after adjustment for potential confounders. They observed a U-shaped risk curve for progression from micro- to macroalbuminuria. As compared to the 2nd quartile, hazards ratios for this outcome were 2.17 [95% CI 1.15–4.08; p = 0.016] for the 1st quartile, 3.04 (95% CI 1.67–5.53; P < 0.001) for the 3rd, and 3.56 (95% CI 1.83–6.93; P < 0.0011) for the 4th quartile. The association of higher SUA levels with DKD progression might have been expected, but the increased hazard for lowest SUA quartile was surprising and remains unexplained. Also, this study found no significant associations of SUA with progression from normo-to microalbuminuria or with eGFR change [10].
In another study, Chang et al. followed 2367 patients with T2D for a mean of 4.6 years. They categorized their outcomes by CKD stage as stable (47.9%), progressing (20.6%), or regressing (31.5%) [11]. The progression group had the highest (6.9 ± 1.8 mg/dL), and the regression group the lowest (5.4 ± 1.5 mg/dL) baseline SUA levels. By multivariate Cox regression analyses SUA > 6.3 mg/dL was an independent risk factor associated with progression in CKD stage. [HR 1.54 (95% CI 1.42–1.68)] [11].
SUA reduction and DKD progression
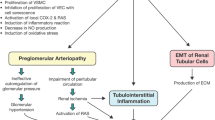
In pre-clinical studies, uric acid was shown to induce alterations of the nitric oxide (NO) pathway, to induce pro-inflammatory cytokines, and to activate the renin-angiotensin system, these findings suggesting possible causal links between SUA and kidney disease [12, 13]. This was also supported by reports of mild UA elevation causing renal disease in animal models [14]. Two small and relatively brief randomized, placebo-controlled, proof-of-concept studies from Hong-Kong [15] and Spain [16] (including 37% and 25% of subjects with diabetes, respectively) suggested that allopurinol-induced decreases in SUA reduced the rate of GFR loss in humans. Consistent with these findings, a post hoc analysis of a 3-year randomized placebo-controlled trial of losartan in persons with T2D and CKD (RENAAL) found that participants experiencing a SUA reduction in the first 6 months after randomization to losartan had reduced risk of serum creatinine doubling or ESKD compared with patients where SUA did not decrease [17]. Also, a large 4-year randomized placebo controlled primary cardiovascular disease (CVD) trial with the sodium-glucose co-transporter 2 (SGLT2) inhibitor empagliflozin demonstrated substantial reductions in CVD endpoints [18]. A post hoc analysis also found preservation of eGFR and reduced renal events (doubling of serum creatinine and ESKD) in empagliflozin treated patients in this study [19]. The SUA lowering effect of SGLT2 inhibitors was among several hypothesized mechanisms for this potential renoprotective effect of empagliflozin [19]; however, SUA reduction was not mentioned in the report of a primary renal outcome trial which demonstrated similar renal benefits for canagliflozin, another SGLT-2 inhibitor [20].
Although these studies and a thorough review of the role of SUA in renal disease [21] were suggestive of a potential benefit of SUA reduction on renal disease progression, they were not sufficient to recommend widespread addition of SUA reduction to the standard of care for patients with diabetes at increased risk of DKD. Nonetheless, in aggregate, these studies provided the conceptual basis for large clinical trials to determine whether lowering SUA in established DKD specifically, or in CKD in general, could slow or prevent further GFR loss. Two such trials have been undertaken [22, 23]. One, the Preventing Early Renal Function Loss in Type 1 diabetes (PERL), was a double-blind, placebo-controlled, parallel group, randomized clinical trial conducted at 16 centers in the USA, Canada and Denmark with NIH and JDRF support. PERL enrolled 530 persons with T1D, eGFR 45–100 mL/min/1.73 m2, microalbuminuria to moderate macroalbuminuria OR significant GFR loss (> 3 mL/min/1.73 m2/year) in the previous 3-5 yrs, and SUA ≥ 4.5 mg/dL [24]. The primary outcome was measured GFR [iohexol plasma disappearance (iGFR)] adjusted for the baseline iGFR after 3 years of allopurinol or placebo administration and 2 months of drug washout. Secondary outcomes included eGFR trajectories based on serum creatinine and or cystatin C, albumin excretion rate and CVD events. Participants were randomized in a 1:1 ratio to oral allopurinol (200–400 mg/day, adjusted for eGFR) or to placebo. PERL also followed guideline recommendations for RASB in persons with DKD [25]. At baseline, the mean age was 50.7 years in the allopurinol and 51.8 years in the placebo group; the iGFR values were 68.7 and 67.3 mL/min/1.73 m2, respectively. Mean SUA decreased in the allopurinol group from 6.1 at baseline to 3.9 mg/dL during treatment whereas it remained at 6.1 mg/dL in the placebo group. Despite this sustained, 36% reduction in SUA, the iGFR decreased at similar rates in the allopurinol and placebo groups, leading to virtually identical mean baseline-adjusted iGFR at the end of the 2-month washout in the two treatment groups (61.2 mL/min/1.73 m2 in both, p = 0.99) [22]. Results were similarly neutral in secondary analyses limited to participants with average drug exposure > 80% over the 3-year treatment and in pre-specified subgroup analyses based on SUA levels (≤ 6.0 vs. > 6.0 mg/dL), iGFR values (≤ 60 vs. > 60 mL/min/1.73 m2), and other clinical characteristics. Also, no clinically meaningful effects were observed on secondary outcomes including iGFR at the end of treatment, iGFR and eGFR slopes, progression to ESKD or serum creatinine doubling, and time to CVD events. Urinary albumin excretion rates at the end of the washout period and at the end of treatment were significantly higher [40% (95% CI 0–80) and 30% (95% CI 0–60) respectively], in patients treated with allopurinol vs. those treated with placebo. Thus, in PERL, SUA reduction with allopurinol had no beneficial effect on the rate of GFR decline or other kidney outcomes in patients with type 1 diabetes, early-to-moderate diabetic kidney disease, and high-normal SUA.
The other trial (controlled trial of slowing of Kidney Disease progression From the Inhibition of Xanthine oxidase [CKD-FIX]) randomized 369 patients to either allopurinol (n = 185) or placebo (n = 184) [23]. Eligible patients were adults with stage 3 or 4 CKD and high risk of progression based on ACR ≥ 265 mg/g or eGFR decline rate ≥ 3.0 mL/min/1.73 m2 in the preceding 12 months. CKD was considered as caused by DKD in 75 (41.2%) and 90 (49.7%) of the allopurinol and placebo groups, respectively. Overall baseline age, 62.4 ± 12.7, eGFR, 31.7 ± 12.0 mL/min/1.73 m2, and SUA levels, 8.2 ± 1.8 mg/dL, were nearly identical in the two groups. Allopurinol, initially 100 mg/day, was escalated, as tolerated, to 300 mg/day. The primary outcome was change in eGFR at 104 weeks. One-hundred and thirty-two (73%) allopurinol and 144 (80%) placebo group patients completed the trial. Mean SUA levels remained constant in the placebo group but decreased in the allopurinol group to 5.1 mg/dL [95% CI 4.8–5.3] at 12 weeks and remained at 5.3 mg/dL [95% CI 5.1–5.6], a ~ 35% reduction, up to 104 weeks. eGFR declined by 3.33 mL/min/1.73 m2/year [95% CI 4 × 11 to 2 × 55] in the allopurinol and 3.23 mL/min/1.73 m2/year [95% CI 3 × 98 to 2 × 47] in the placebo group (P = 0.85). There were no group differences in the secondary composite endpoints of a 30% or a 40% eGFR decline plus ESKD, or death from any cause. Baseline ACR [716.9 (244.3, 1857)], [median (range)], was nearly identical in the two groups and changed little over the 104 weeks in both groups, thus not confirming the adverse influence of allopurinol on urinary albumin excretion observed in the PERL trial. In summary, the CKD-FIX Study in patients, a majority with non-diabetic kidney disease and with older age, lower GFR and higher SUA levels at baseline than in the PERL study, but with a similar relative SUA level reduction during the trial, also failed to demonstrate any benefit of allopurinol on renal functional decline.
Conclusions
Several observational studies have pointed to SUA as a risk factor for DKD development and/or progression, and small proof-of-concept clinical trials have suggested that SUA reduction with allopurinol could have beneficial effects in preventing or slowing GFR decline. However, two recent pivotal trials, properly designed, powered, and executed, have failed to show statistically benefit of allopurinol on kidney outcomes [22, 23]. A likely explanation for the discrepancy between these trials and previous observational studies is that the predictive effect of SUA on kidney function loss may be indirect and attributable to the association of SUA with other traits that are causally related to DKD, such as, for instance, insulin-resistance and the metabolic syndrome [26]. This hypothesis is also supported by two recent Mendelian randomization studies in large cohorts [27, 28], one of which involved T1D subjects [27], which could not demonstrate a causal relationship between SUA and eGFR or CKD. Thus, current evidence does not support a therapeutic role of SUA reduction in the progression of renal disease.
References
de Boer IH, Sun W, Cleary PA, Lachin JM, Molitch ME, Steffes MW, Zinman B (2011) Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med 365:2366–2376. https://doi.org/10.1056/NEJMoa1111732
Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I (2001) Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345:851–860. https://doi.org/10.1056/NEJMoa011303
Afkarian M, Zelnick LR, Hall YN, Heagerty PJ, Tuttle K, Weiss NS, de Boer IH (2016) Clinical manifestations of kidney disease among US adults with diabetes, 1988-2014. JAMA 316:602–610. https://doi.org/10.1001/jama.2016.10924
Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B (2018) IDF diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 138:271–281. https://doi.org/10.1016/j.diabres.2018.02.023
Hovind P, Rossing P, Tarnow L, Johnson RJ, Parving HH (2009) Serum uric acid as a predictor for development of diabetic nephropathy in type 1 diabetes: an inception cohort study. Diabetes 58:1668–1671
Jalal DI, Rivard CJ, Johnson RJ, Maahs DM, McFann K, Rewers M, Snell-Bergeon JK (2010) Serum uric acid levels predict the development of albuminuria over 6 years in patients with type 1 diabetes: findings from the coronary artery calcification in type 1 diabetes study. Nephrol Dial Transpl 25:1865–1869
Pacilli A, Viazzi F, Fioretto P, Giorda C, Ceriello A, Genovese S, Russo G, Guida P, Pontremoli R, De Cosmo S (2017) Group AM-AS: epidemiology of diabetic kidney disease in adult patients with type 1 diabetes in Italy: the AMD-Annals initiative. Diabetes Metab Res Rev. https://doi.org/10.1002/dmrr.2873
Zoppini G, Targher G, Chonchol M, Ortalda V, Abaterusso C, Pichiri I, Negri C, Bonora E (2012) Serum uric acid levels and incident chronic kidney disease in patients with type 2 diabetes and preserved kidney function. Diabetes Care 35:99–104. https://doi.org/10.2337/dc11-1346
Ficociello LH, Rosolowsky ET, Niewczas MA, Maselli NJ, Weinberg JM, Aschengrau A, Eckfeldt JH, Stanton RC, Galecki AT, Doria A, Warram JH, Krolewski AS (2010) High-normal serum uric acid increases risk of early progressive renal function loss in type 1 diabetes: results of a 6-year follow-up. Diabetes Care 33:1337–1343
Hayashino Y, Okamura S, Tsujii S, Ishii H (2016) Association of serum uric acid levels with the risk of development or progression of albuminuria among Japanese patients with type 2 diabetes: a prospective cohort study [Diabetes Distress and Care Registry at Tenri (DDCRT 10)]. Acta Diabetol 53:599–607. https://doi.org/10.1007/s00592-015-0825-x
Chang YH, Lei CC, Lin KC, Chang DM, Hsieh CH, Lee YJ (2016) Serum uric acid level as an indicator for CKD regression and progression in patients with type 2 diabetes mellitus-a 4.6-year cohort study. Diabetes Metab Res Rev 32:557–564. https://doi.org/10.1002/dmrr.2768
Johnson RJ, Segal MS, Srinivas T, Ejaz A, Mu W, Roncal C, Sanchez-Lozada LG, Gersch M, Rodriguez-Iturbe B, Kang DH, Acosta JH (2005) Essential hypertension, progressive renal disease, and uric acid: a pathogenetic link? J Am Soc Nephrol 16:1909–1919
Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL, Lan HY, Kivlighn S, Johnson RJ (2001) Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension 38:1101–1106
Mazzali M, Kanellis J, Han L, Feng L, Xia YY, Chen Q, Kang DH, Gordon KL, Watanabe S, Nakagawa T, Lan HY, Johnson RJ (2002) Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am J Physiol Renal Physiol 282:F991–F997
Siu YP, Leung KT, Tong MK, Kwan TH (2006) Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis 47:51–59
Goicoechea M, de Vinuesa SG, Verdalles U, Ruiz-Caro C, Ampuero J, Rincon A, Arroyo D, Luno J (2010) Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol 5:1388–1393
Miao Y, Ottenbros SA, Laverman GD, Brenner BM, Cooper ME, Parving HH, Grobbee DE, Shahinfar S, de Zeeuw D, Lambers Heerspink HJ (2011) Effect of a reduction in uric acid on renal outcomes during Losartan treatment: a post hoc analysis of the reduction of endpoints in non-insulin-dependent diabetes mellitus with the angiotensin II Antagonist Losartan Trial. Hypertension 58:2–7
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE (2015) Investigators E-RO: empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 373:2117–2128. https://doi.org/10.1056/NEJMoa1504720
Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B (2016) Investigators E-RO: empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 375:323–334. https://doi.org/10.1056/NEJMoa1515920
Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW, Investigators CT (2019) Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 380:2295–2306. https://doi.org/10.1056/NEJMoa1811744
Sato Y, Feig DI, Stack AG, Kang DH, Lanaspa MA, Ejaz AA, Sanchez-Lozada LG, Kuwabara M, Borghi C, Johnson RJ (2019) The case for uric acid-lowering treatment in patients with hyperuricaemia and CKD. Nat Rev Nephrol 15:767–775. https://doi.org/10.1038/s41581-019-0174-z
Doria A, Galecki AT, Spino C, Pop-Busui R, Cherney DZ, Lingvay I, Parsa A, Rossing P, Sigal RJ, Afkarian M, Aronson R, Caramori ML, Crandall JP, de Boer IH, Elliott TG, Goldfine AB, Haw JS, Hirsch IB, Karger AB, Maahs DM, McGill JB, Molitch ME, Perkins BA, Polsky S, Pragnell M, Robiner WN, Rosas SE, Senior P, Tuttle KR, Umpierrez GE, Wallia A, Weinstock RS, Wu C, Mauer M (2020) PERL Study Group: serum urate lowering with allopurinol and kidney function in type 1 diabetes. N Engl J Med 382:2493–2503. https://doi.org/10.1056/NEJMoa1916624
Badve S, Pascoe E, Tiku A, Boudville N, Brown FG, Cass A, Clarke P, Dalbeth N, Day RO, de Zoysa J, Douglas B, Faull R, Harris D, Hawley C, Jones GR, Kanellis J, Palmer S, Perkovic V, Rangan G, Reidlinger D, Robison L, Walker RJ, Walters G, Johnson DW (2020) CKD-FIX Study Investigators: effects of allopurinol on the progression of chronic kidney disease. N Engl J Med 382:2504–2513. https://doi.org/10.1056/NEJMoa1915833
Afkarian M, Polsky S, Parsa A, Aronson R, Caramori ML, Cherney DZ, Crandall JP, de Boer IH, Elliott TG, Galecki AT, Goldfine AB, Haw JS, Hirsch IB, Karger AB, Lingvay I, Maahs DM, McGill JB, Molitch ME, Perkins BA, Pop-Busui R, Pragnell M, Rosas SE, Rossing P, Senior P, Sigal RJ, Spino C, Tuttle KR, Umpierrez GE, Wallia A, Weinstock RS, Wu C, Mauer M, Doria A (2019) PERL Study Group: preventing early renal loss in diabetes (PERL) Study: a randomized double-blinded trial of allopurinol—rationale, design, and baseline data. Diabetes Care 42:1454–1463. https://doi.org/10.2337/dc19-0342
National Kidney F (2012) KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis 60:850–886. https://doi.org/10.1053/j.ajkd.2012.07.005
Choi HK, Ford ES (2007) Prevalence of the metabolic syndrome in individuals with hyperuricemia. Am J Med 120:442–447. https://doi.org/10.1016/j.amjmed.2006.06.040
Ahola AJ, Sandholm N, Forsblom C, Harjutsalo V, Dahlstrom E, Groop PH, FinnDiane Study G (2017) The serum uric acid concentration is not causally linked to diabetic nephropathy in type 1 diabetes. Kidney Int 91:1178–1185. https://doi.org/10.1016/j.kint.2016.11.025
Jordan DM, Choi HK, Verbanck M, Topless R, Won HH, Nadkarni G, Merriman TR, Do R (2019) No causal effects of serum urate levels on the risk of chronic kidney disease: a Mendelian randomization study. PLoS Med 16:e1002725. https://doi.org/10.1371/journal.pmed.1002725
Acknowledgements
The authors thank the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and Juvenile Diabetes Research Foundation (JDRF) for their support of the Preventing Early Renal Loss in Diabetes (PERL) clinical trial.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical statement
The PERL trial was conducted in accordance with the principles of the Declaration of Helsinki. The protocol was approved by the ethical committees of all institutions participating in the study, and all participants provided written informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mauer, M., Doria, A. Uric acid and risk of diabetic kidney disease. J Nephrol 33, 995–999 (2020). https://doi.org/10.1007/s40620-020-00796-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-020-00796-z