Abstract
Background
The ideal long-term maintenance therapy of Lupus Nephritis (LN) is still a matter of debate. The present study was aimed at comparing the efficacy/safety profile of cyclosporine (CsA), mycophenolate mofetil (MMF) and azathioprine (AZA) in long-term maintenance therapy of LN.
Methods
We performed a retrospective study of patients with biopsy-proven active LN. After induction therapy, all patients received maintenance therapy with CsA, MMF or AZA based on medical decision. Primary endpoint was complete renal remission (CRR) after 8 years (defined as proteinuria < 0.5 g/24 h, eGFR > 60 ml/min/1.73 mq); secondary endpoints were: CRR after 1 year, renal and extrarenal flares, progression of chronic kidney disease (CKD stage 3 or above) and side-effects.
Results
Out of 106 patients, 34 received CsA, 36 MMF and 36 AZA. Clinical and histological characteristics at start of induction therapy were comparable among groups. At start of maintenance therapy, CsA patients had significantly higher proteinuria (P = 0.004) or nephrotic syndrome (P = 0.024) and significantly lower CRR (23.5% vs 55.5% on MMF and 41.7% on AZA, P = 0.024). At one year, CRR was similar in the three groups (79.4% on CsA, 63.8% on MMF, 58.3% on AZA, P = 0.2). At 8 years, the primary endpoint was achieved by 79.4% of CsA vs 83.3% of MMF and 77.8% of AZA patients (P = 0.83); 24 h proteinuria, serum creatinine, eGFR were similar. CKD stage 3 or above developed in 8.8% of CsA, in 8.3% of MMF and in 8.3% of AZA patients (P = 0.92). Flares-free survival curves and incidence of side-effects were not different.
Conclusions
This is the first study comparing CsA, MMF and AZA on long-term LN maintenance therapy. All treatments had similar efficacy in achieving and maintaining CRR, despite more severe baseline clinical features in patients treated with CsA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Renal involvement is frequent in Systemic Lupus Erythematosus (SLE) and may greatly influence the course of the disease. Despite current therapeutic strategies, patients with lupus nephritis (LN) have a 10-year cumulative incidence of end-stage-renal disease (ESRD) of 10.1% and 5.9% of death [1]. The treatment of LN consists of two phases: an aggressive initial therapy aimed at induction of remission and a longer period of maintenance treatment [2, 3]. Around 20–50% of LN patients achieve response during the induction phases but renal flares are common during the follow-up [4, 5]. Maintenance treatment is intended to consolidate the response and prevent recurrences using lower and presumably fewer toxic levels of immunosuppressive medications [6].
Azathioprine (AZA), mycophenolate mofetil (MMF) and calcineurin inhibitors (cyclosporine A (CsA) and tacrolimus) have been successfully used as maintenance LN therapy in recent randomized clinical trials [7,8,9,10,11,12]. To the best of our knowledge, no direct comparison of these three drugs has been performed. Only two-by-two comparison studies of MMF vs AZA [8,9,10] and of AZA vs calcineurin inhibitors [11, 12] collected in randomized controlled studies or in retrospective studies are available [13,14,15]. The last Cochrane review reported that disease relapse is increased with AZA compared with MMF but with moderate evidence. No conclusions are available about the efficacy of MMF, CsA, AZA in preventing mortality and ESRD due to the lack of long-term data [16].
In order to fill in these gaps of knowledge, we planned a retrospective, multicenter study enrolling patients with active LN who, after induction therapy, were assigned, based on clinical judgement, to low-dose prednisone plus CsA, MMF or AZA. All patients were followed for at least 8 years after maintenance therapy was started.
The primary outcome measure of this study is complete renal remission at 8 years after the start of maintenance therapy. Secondary outcome measures include complete renal remission at 1 year, number of renal and extrarenal flares, chronic kidney disease (CKD) development, side effects.
Methods
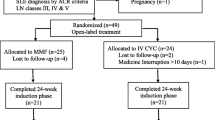
We retrospectively identified patients who had received AZA, MMF or CsA as maintenance therapy for active LN referring to four Italian centers (Nephrological Unit, Fondazione Ca’ Granda IRCCS Ospedale Maggiore Policlinico Milan, Division of Clinical Rheumatology, ASST Gaetano Pini -CTO, Milan, Department of clinical and experimental medicine, Rheumatology Unit, University of Pisa, Division of Rheumatology, University of Padua). The first patient included in the study started induction therapy in May 2000 and the last patient in February 2010. During this time span 165 patients received a diagnosis of LN in the four participating centers and 106 entered this study. Five out of the 59 patients (8.5%) not included in the study developed chronic kidney disease (CKD) within 8 year of observation (3 out of these 5 patients reached ESRD), 3 died (5.1%), and 6 were lost to follow-up (10.2%).
Some of the patients of this study have been included in a previous randomized controlled study that compared AZA to CsA in LN maintenance therapy [11]. Based on the good long-term results achieved in that study we have continued to employee CsA as calcineurin inhibitor in this study.
Eligible patients fulfilled the following inclusion criteria: (1) diagnosis of SLE based on criteria of the American College of Rheumatology (ACR) for the classification of SLE [17, 18]; (2) biopsy-proven lupus nephritis according to the International Society of Nephrology/Renal Pathology Society 2003 (ISN/RPS) classification [19], (3) active lupus nephritis at diagnosis or during a renal flare (4) a follow-up of at least 8 years from the start of maintenance therapy with one of the three drugs in study.
The starting point of the study is the beginning of the maintenance therapy after the induction treatment for active lupus nephritis.
Primary endpoint The primary outcome measure was complete renal remission (CRR) at 8 years from the start of maintenance therapy.
Secondary endpoints (a) CRR at 1 year after the start of maintenance therapy, (b) occurrence of renal and extrarenal flares, (c) CKD development, (d) drug related adverse events.
The study was approved by the Ethics Committee of Fondazione Ca’ Granda IRCCS Ospedale Maggiore Policlinico di Milano, Italy (protocol number 504_2019bis) and of the other participating centers. We acted in the full adherence to the Declaration of Helsinki.
All patients signed an informed consent for the scientific use of their data.
Patients were followed by a dedicated team in each of the participating centers. At each visit laboratory tests included: complete blood count, serum creatinine, estimated glomerular filtration rate (eGFR), glucose, total proteins and albumin, erythrocyte sedimentation rate, C-reactive protein, ANA, ENA, anti-dsDNA, antiphospholipid antibodies, C3, and C4, urinary sediment and 24-h proteinuria. Patients were also periodically subjected to: chest radiography, abdomen ultrasound, bone densitometry and cardiovascular evaluation.
For the study, data had to be recorded at the start of induction therapy, at the start of maintenance therapy, after 1, and 8 years of maintenance therapy at time of flares and at last observation.
Definitions
eGFR in ml/min/1.73 m2: according to the Modification of Diet in Renal Disease (MDRD).
Definitions of renal remission [20].
CRR proteinuria < 0.5 g/24 h, normal or near normal eGFR (within 10% of normal eGFR if previously abnormal)
Partial renal remission (PRR) ≥ 50% reduction in proteinuria to subnephrotic levels, and normal or near-normal eGFR
No Renal remission (NoR) all the other cases.
Renal flares [4].
Nephritic flare a rapid increase in serum creatinine of 30% above baseline associated with an increase in proteinuria, and/or active urine sediment.
Proteinuric flare a rapid increase in proteinuria of at least 2 g/24 h if the previous proteinuria was < 3.5 g/24 h or a doubling if previous proteinuria was > 3.5 g/24 h.
Extra renal flares all extrarenal SLE manifestations requiring increase in immunosuppressive therapy.
Safety assessment: events that require hospitalization, diagnostic investigations and therapeutic modifications.
CKD eGFR < 60 ml/min and inactive urinary sediment, confirmed by at least three determinations during at least 6 months.
Statistical analysis
Descriptive statistics were calculated as median and interquartile ranges, since the distribution of the variables was not normal. For the same reason, the difference of continuous variables between groups was tested with non parametric Wilcoxon test for independent samples. Paired data tests were used to compare the values of clinical parameters at different time points. Chi-square test was used to test correlation of qualitative or dichotomized variables among groups of patients. We performed a logistic regression analysis to evaluate primary outcome (CRR) predictors among the main basal covariates. A Cox survival analysis of SLE flares predictors among the main basal covariates was performed.
Kaplan–Meier estimate was used to draw flares free survival curves, and log-rank test was used to test their difference. The analysis of the data of patients that changed or stopped maintenance therapy was performed based on intention to treat.
Initial and maintenance therapy
Induction therapy Table 1 Briefly: 96.2% of patients received three methylprednisolone pulses (MP) (500–1000 mg each) followed by oral prednisone 0.5–1 mg/kg/day, the other patients received oral prednisone 1 mg/kg day for one month then gradually tapered. Cyclophosphamide (CYC) (monthly pulses 0.5–1 g/m2 or oral 1–2 mg/kg for three months) was given to 73.6% of participants, MMF (target dose 2–3 g/day) or AZA (initial dose of 2 mg/kg per die) to 16% of participants. Rituximab or intravenous immunoglobulins were administered in the remaining patients. In addition, as a concomitant therapy, all patients received hydroxychloroquine and therapy with angiotensin converting enzyme inhibitors and/or angiotensin II receptor blockers therapy throughout the study.
Maintenance therapy
Mycophenolate mofetil: 2 g/day for the first year then reduced to 1.5 g/day.
Cyclosporine: 4 mg/kg per day. After the first month the dose was reduced by 0.5 mg/kg every 2 weeks to a maintenance dose of 2.5–3.0 mg/kg per day for the first year and then 1.5 mg/kg/day.
Azathioprine: 2 mg/kg per day, with reduction to 1.5 mg/kg per day after 2 months. After the first year the mean dosage was 1 mg/kg/day.
Results
We enrolled 106 patients, 101 females, with a median age of 31 years (interquartile ranges (IR) 22.5–37.8) at starting point of the study. Seventy patients (66%) entered this study at histological diagnosis of LN, and 36 during a LN renal flare that occurred in median 54 months (IR 36–69.6) after the diagnosis of LN. The median duration of SLE at enrollment was 6.6 years (IR 1.3–12.5). The renal biopsy showed class III nephritis in 14.2%, class IV in 68.9%, and class V in 16.9%. of patients. At the beginning of induction therapy, 23 patients (22.6%) had eGFR < 60 ml/min and 42 patients (39.6%) had nephrotic syndrome. As maintenance therapy, 34 patients received CsA, and 36 received MMF or AZA based on medical judgeent and with the patients’ approval (Table 1).
At start of initial therapy there were no significant differences in demographic, clinical, histological and therapeutic characteristics of patients assigned to CsA, to AZA or to MMF (Table 1).
At start of maintenance therapy, a significant improvement in renal parameters was obtained in all the three groups. However, in comparison to patients assigned to MMF and to AZA, patients assigned to CsA had significantly higher residual proteinuria (median 0.92 g/24 h in MMF, 0.55 g/24 h in AZA vs 1.4 g/24 h in CsA; P = 0.004), more frequent nephrotic syndrome (2.8% in MMF, 5.2% in AZA vs 20.5% in CsA; P = 0.024) (Table 2), and significantly less frequent CRR (55.5% in MMF, 41.7% in AZA vs 23.5% in CsA P = 0.024). Altogether, at start of maintenance therapy, complete, partial and no response were respectively 23.5%, 55.8%, and 20.6% in CsA, 55.5%, 38.9% and 5.5% in MMF and 41.7%, 50% and 8.3 inAZA group (Fig. 1a).
a Percentage of patients in complete, partial and no renal remission assigned to the three different maintenance drugs, at the start of maintenance therapy. b Percentage of patients in complete, partial and no renal remission assigned to the three different maintenance drugs, after one year of maintenance therapy. c Percentage of patients in complete, partial and no renal remission assigned to the three different maintenance drugs, after 8 years of maintenance therapy. CsA cyclosporine, AZA azathioprine, MMF mycophenolate mofetil
After one year of maintenance therapy, the median proteinuria was ≤ 0.5 g/day in all three groups (P = ns). The percentage of patients in CRR in CsA group (79.4%) was comparable to that of MMF (63.8% P = 0.2) and to that in AZA (58.3% P = 0.1) (Fig. 1b). PRR and NoR were present in 5.9% and in 14.7% respectively in CsA vs 22.2% and 13.9% in MMF and 36.1% and 5.5% in AZA group. The median values of serum creatinine, eGFR, proteinuria and the number of patients with arterial hypertension were not significantly different among the three groups (P = 0.07, P = 0.23, P = 0.44 and P = 0.15 respectively).
Renal and extrarenal flares during maintenance therapy
During the first year of maintenance therapy none of the patients of the three groups developed renal or extrarenal flares.
During the subsequent follow-up, SLE flares occurred in nine patients in CsA group, (26.5%) after a mean of 4.4 ± 2.4 years (range 1.3–7.6 years) from the start of maintenance therapy. Three flares were nephritic flares, five were proteinuric flares and the last was an extrarenal flare. All flares were responsive to therapy.
In MMF group 14 SLE flares (38.8%) occurred in mean 3.9 ± 1.8 years (range 1.16–6.4) after the start of maintenance therapy. Three were nephritic flares, six proteinuric flares and five extrarenal flares. Almost all flares have been treated with benefit, except for only incomplete recovery of proteinuria in one proteinuric flare.
Twelve flares occurred in AZA group (33.3%) after a mean of 5.5 ± 2.8 years (range 1.5–9). Only one nephritic flare occurred in this group, ten were proteinuric flares and one was an extrarenal flare. All flares were treated with success.
The treatment of flares is reported in (Table 3).
The results of Cox regression analysis to evaluate SLE flares predictors among the main baseline covariates are reported in Supplementary Table 1. None of the tested covariates was associated with the development of flares.
The number and the type of SLE flares and the Kaplan Meier flares free survival curves were not significantly different among the three groups (P = 0.54) (Fig. 2).
Renal status at 8 years (Table 2)
No difference at eight years was demonstrated in the primary endpoint of the study among the three groups. The number of patients with CRR was 27 (79.4%) in CsA group vs 30 in MMF group (83.3%) and 28 (77.8%) in AZA group (P = 0.83). PRR and NoR were present in 1.8% and 8.8% of patients in CsA group vs 5.5% and 11.1% in MMF and in 13.9%and 8.3% in AZA group (Fig. 1c). Three patients developed CKD in CsA group (8.8%) in comparison to three in MMF (8.3%) and two in AZA group (8.3%) (P = 0.92). No patient died during the study.
The median values of serum creatinine, eGFR and proteinuria were in normal range in all the three groups. The number of patients with arterial hypertension was similar (P = 0.29).
Logistic regression analysis was performed to identify the predictors of CRR among baseline patient variables. Except for arterial hypertension at baseline (OR 6.486 Confidential intervals 1.754–23.982, P = 0.001) none of the other baseline variables were associated with the occurrence of CRR (Supplementary Table 2). At 8 years, 20 patients in CsA group (58.8), 26 (72%) in MMF group 25 (69.4%) in AZA group, continued treatment with the drug (P = 0.46).
During the study, five successful pregnancies occurred in the CsA group, two in the MMF group and five in the AZA group.
Clinical status at last observation
No patient died. One patient in CRR was lost to follow up after 13.8 years in CsA group, the other 33 were followed for 18.6 (IR 12.2–19.8) years. Six patients (four in CRR and two in CKD) were lost in the AZA group between 12.3 and 24 years, the other 30 patients were followed for 16.6 years (IR 11.8–18.8). All MMF patients were followed for 12.4 years (IR 10.1–14.8). At last observation CRR, PRR and CKD were present in 72.4%, 18.3% and 9.3% of patients in CsA group, 83.3%, 3.3% and 13.4%, in AZA and 75%, 13.9 and 11.1% in MMF group.
Side effects
No differences in number and in severity of side effects was demonstrated among the three drugs as reported in (Table 4).
Discussion
Although earlier diagnosis and refinement of therapeutic approaches have improved the prognosis of LN, the type and duration of maintenance treatment remains a major challenge for clinicians. In 2004 Contreras et al. demonstrated that maintenance therapy with quarterly i.v. CYC was significantly less effective and more toxic than those with MMF and AZA [21]. Following such evidence i.v. CYC was not recommended as maintenance therapy in LN and MMF, AZA and calcineurin inhibitors are used instead. In patients with incomplete response to maintenance therapy, some recent studies suggest that the addition of Belimumab allows the achievement of complete response [22].
This is the first study that compares CsA to AZA and to MMF as maintenance therapy. All patients enrolled had a follow-up of at least 8 years after starting maintenance therapy, the longest follow-up reported until now in particular for MMF. Results are based on every day clinical practice of four Italian Nephrological and Rheumatological tertiary centers. CsA, AZA and MMF proved to be equally effective in consolidating and maintaining the CRR until the end of the study. The primary outcome measure of this study, the CRR at 8 years, was achieved in around 80% of patients in each group. Besides arterial hypertension, no other baseline variables were associated with the occurrence of CRR at logistic regression analysis. This result suggests a rather satisfactory allocation of patients into the three maintenance treatments groups.
The percentage of SLE flares and the SLE flares free survival curves were not different among the three groups. No significant predictors of flares emerged at Cox regression analysis among the basal covariates. This could probably be due to the low number of patients included in the study.
At last observation, despite the potential nephrotoxicity of calcineurin inhibitors [23], CKD and the percentage of arterial hypertension occurred in a comparable number of patients in CsA group than in MMF and in AZA group. No other differences were observed among the three groups in the median value of proteinuria and of eGFR.
To the best of our knowledge, only two-by-two comparison studies of MMF vs AZA [8,9,10] and AZA vs calcineurin inhibitors (CsA and tacrolimus) [11, 12] collected in randomized controlled studies or in retrospective studies are available [13,14,15]. The results of the two first randomized controlled trials comparing AZA and MMF were contrasting. In the maintenance phase of ALMS study, MMF resulted more effective than AZA in preventing treatment failure after 4 years observation [8]. While, in the Maintain study, AZA and MMF were equally effective in preventing renal flares over a 10-year follow-up [9]. These two studies were not comparable for several reasons, including selection of patients, ethnicities differences and selection of endpoints. Two retrospective studies reported equal efficacy of the two drugs [13, 14]. Based on the last Cochrane review on LN therapy, relapses are apparently more frequent in AZA compared with MMF but with moderate certainty evidence [16]. We have not observed significant differences in the percentage of responses and of flares among patients treated with AZA or MMF. Both were equally effective in the long-term in maintaining LN remission.
Not many studies have evaluated the efficacy of CsA in LN patients despite the potential efficacy of this drug in proteinuric forms of LN as demonstrated in animal models. In a mouse model of LN, CsA was effective in reducing proteinuria and preserving renal function through stabilizing podocyte actin cytoskeleton and inhibiting podocyte apoptosis [24]. Low doses of calcineurin inhibitors have been shown to inhibit the function of P-glycoprotein, leading to restoration of intracellular therapeutic levels of glucocorticoids, thus preventing treatment resistance [25]. Following encouraging results in randomized controlled trials, there is growing interest in the role of tacrolimus as potential therapeutic agent in LN induction therapy, particularly in association with MMF [26]. As far maintenance therapy is concerned, only a randomized controlled study comparing tacrolimus with AZA in LN maintenance therapy has been performed in Chinese patients. The two drugs had a similar rate of renal relapses. However, a 6 months follow-up is too short to draw firm conclusions [12]. Based on the satisfactory results at 4 years of our randomized trial in which CsA was compared to AZA in maintenance LN therapy [11] we have used this calcineurin inhibitor in the present study.
The CYCLOFA-LUNE trial compared the efficacy of CsA vs i.v. CYC pulses as induction therapy in patients with proliferative lupus nephritis [27]. After a mean follow-up of 7.7 years, no differences emerged in the incidence of renal insufficiency and ESRD between the two arms. Rihova et al. administered CsA as induction and as maintenance therapy in 31 LN patients. Complete remission was achieved in 93.5% of patients. The relapse rate was 45.2%. After a mean follow-up of 7.1 ± 2.05 years, 67.9% of patients were in remission [15].
CsA maintenance therapy was compared with AZA in one Italian randomized controlled trial that included 69 DPLN patients. The primary endpoint was the prevention of LN flares. After 4 years, the incidence of renal flares was 19% in the CSA group, which was not significantly different from the 24% in AZA group. Of note, the reduction of proteinuria occurred earlier in CsA group and, at the end of the follow-up, 41.7% of patients assigned to CsA had undetectable proteinuria versus 15.1% of those in AZA group (P = 0.045) [11]. Our results confirmed the efficacy of CsA in reduction proteinuria and reinforced the role of CsA in the LN therapy, as other authors have confirmed recently [28, 29]. As a matter of fact, despite no differences in induction therapy, patients in CsA had significantly higher proteinuria and more frequent nephrotic syndrome than those of the other two groups. Therefore, a significantly lower number of CsA patients were in CRR at start of maintenance therapy. After 1 year of maintenance therapy, the situation reversed and CsA patients were slightly more frequent in remission than in the other two groups. It is demonstrated that the normalization in daily proteinuria is a strong predictor of a fairly long-term renal outcome not only in patients with primary glomerular diseases [30] but also in those with LN.
The more rapid reduction of proteinuria with CsA is very relevant in clinical practice, because it could allow to better guide the clinician in the therapeutic choice based on the initial characteristics of the patients such as the proteinuria levels. Despite longer duration of CsA treatment, we have not observed in this group, the most severe potential complications of this drug such as the development/worsening of arterial hypertension and nephrotoxicity. This is probably the result of a regular monitoring of the patients by dedicated teams, of the frequent checking of drug blood levels, and of the progressive reduction of the dosage until its withdrawal in patients who achieve stable remission. Altogether, these results suggest the efficacy and safety of low dose CsA in long-term maintenance treatment of LN. Another interesting point of the study is the high number of patients who had a successful pregnancy in CsA and in AZA group. CsA and AZA having a safety profile can be continued during pregnancy, instead MMF must be withdrawn at least 6 months before conception [31]. This study has many limitations. It is a retrospective study. The number of patients included in the study is low for a proper evaluation of the primary end point. The exclusion of the patients with a shorter follow-up could have biased the results. The assignment to one of the drugs for maintenance therapy was not randomized.
Despite these limitations, this is the first study that compares AZA, CsA, MMF in maintenance therapy of LN. All these three drugs seem to be equally effective in inducing and maintaining the remission of LN during a long follow-up with an acceptable rate of flares and of side effects. The rapid reduction of proteinuria that is obtained with CsA is an added value of this drug which must be valued in clinical practice when choosing LN treatment. However, it is likely that the key for treatment efficacy resides mostly in a strict patient follow-up by a dedicated team.
Data availability
All data are available.
References
Hanly JG, Okeeffe AG, Su L et al (2016) The frequency and outcome of lupus nephritis: results from an international inception cohort study. Rheumatology (Oxford) 55(2):252–262. https://doi.org/10.1093/rheumatology/kev311
Zimmerman R, Radhakrishnan J, Valeri A et al (2001) Advances in the treatment of lupus nephritis. Annu Rev Med 52:63–78. https://doi.org/10.1146/annurev.med.52.1.63
Ponticelli C, Glassock RJ, Moroni G (2010) Induction and maintenance therapy in proliferative lupus nephritis. J Nephrol 23:9–16
Moroni G, Quaglini S, Maccario M et al (1996) “Nephritic flares” are predictors of bad long-term renal outcome in lupus nephritis. Kidney Int 50:2047–2053. https://doi.org/10.1038/ki.1996.528
Dooley MA, Hogan S, Jennette C et al (1997) Cyclophosphamide therapy for lupus nephritis: poor renal survival in black Americans. Glomerular disease collaborative network. Kidney Int 51:1188–1195. https://doi.org/10.1038/ki.1997.162
Mok CC, Wong RWS, Lau CS (1999) Lupus nephritis in southern Chinese patients: clinicopathological findings and long term outcome. Am J Kidney Dis 34:315–323. https://doi.org/10.1016/s0272-6386(99)70361-6
Appel GB, Contreras G, Dooley MA et al (2009) Aspreva Lupus Management Study Group: mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol 20:1103–1112. https://doi.org/10.1681/ASN.2008101028
Dooley MA, Jayne D, Ginzler EM et al (2011) ALMS Group, Mycophenolate versus azathioprine as maintenance therapy for lupus nephritis. N Engl J Med 365:1886–1895. https://doi.org/10.1056/NEJMoa1014460
Houssiau FA, D'Cruz D, Sangle S et al (2010) MAINTAIN Nephritis Trial Group, Azathioprine versus mycophenolate mofetil for long-term immunosuppression in lupus nephritis: results from the MAINTAIN Nephritis Trial. Ann Rheum Dis 69:2083–2089. https://doi.org/10.1136/ard.2010.131995
Tamirou F, D'Cruz D, Sangle S et al (2016) MAINTAIN Nephritis Trial Group, Long-term follow-up of the MAINTAIN Nephritis Trial, comparing azathioprine and mycophenolate mofetil as maintenance therapy of lupus nephritis. Ann Rheum Dis 75:526–531. https://doi.org/10.1136/annrheumdis-2014-206897
Moroni G, Doria A, Mosca M et al (2006) A randomized pilot trial comparing cyclosporine and azathioprine for maintenance therapy in diffuse lupus nephritis over four years. Clin J Am Soc Nephrol 1:925–932. https://doi.org/10.2215/CJN.02271205
Chen W, Liu Q, Chen W et al (2012) Outcomes of maintenance therapy with tacrolimus versus azathioprine for active lupus nephritis: a multicenter randomized clinical trial. Lupus 21:944–952. https://doi.org/10.1177/0961203312442259
Sahin GM, Sahin S, Kiziltas S et al (2008) Mycophenolate mofetil versus azathioprine in the maintenance therapy of lupus nephritis. Ren Fail 30:865–869. https://doi.org/10.1080/08860220802353843
Kaballo BG, Ahmed AE, Nur MM et al (2016) Mycophenolate mofetil versus azathioprine for maintenance treatment of lupus nephritis. Saudi J Kidney Dis Transpl 27:717–725. https://doi.org/10.4103/1319-2442.185233
Rihova Z, Vankova Z, Maixnerova D et al (2007) Treatment of lupus nephritis with cyclosporine—an outcome analysis. Kidney Blood Press Res 30:124–128. https://doi.org/10.1159/000101448
Tunnicliffe DJ, Palmer SC (2018) Immunosuppressive treatment for proliferative lupus nephritis: summary of a cochrane review. AJKD 72:756–757. https://doi.org/10.1053/j.ajkd.2018.07.008
Hochberg MC (1997) Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40:1725. https://doi.org/10.1002/art.1780400928
Aringer M, Costenbader K, Daikh D et al (2019) 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis 78:1151–1159. https://doi.org/10.1002/art.40930
Markowitz GS, D'Agati VD (2007) The ISN/RPS 2003 classification of lupus nephritis: an assessment at 3 years. Kidney Int 71:491–495. https://doi.org/10.1038/sj.ki.5002118
Bertsias GK, Tektonidou M, Amoura Z et al (2012) European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association. Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of adult and paediatric lupus nephritis. Ann Rheum Dis 71:1771–1782. https://doi.org/10.1136/annrheumdis-2012-201940
Contreras G, Pardo V, Leclercq B et al (2004) Sequential therapies for proliferative lupus nephritis. N Engl J Med 350:971–980. https://doi.org/10.1056/NEJMoa031855
Binda V, Trezzi B, Del Papa N et al (2020) Belimumab may decrease flare rate and allow glucocorticoid withdrawal in lupus nephritis (including dialysis and transplanted patient). J Nephrol. https://doi.org/10.1007/s40620-020-00706-3
Moroni G, Doria A, Ponticelli C (2009) Cyclosporine (CsA) in lupus nephritis: assessing the evidence. Nephrol Dial Transplant 24:15–20. https://doi.org/10.1093/ndt/gfn565
Liao R, Liu Q, Zheng Z et al (2015) Tacrolimus protects podocytes from injury in lupus nephritis partly by stabilizing the cytoskeleton and inhibiting podocyte apoptosis. PLoS ONE 10:e0132724. https://doi.org/10.1371/journal.pone.0132724
Suzuki K, Saito K, Tsujimura S et al (2010) Tacrolimus, a calcineurin inhibitor, overcomes treatment unresponsiveness mediated by P-glycoprotein on lymphocytes in refractory rheumatoid arthritis. J Rheumatol 37:512–520. https://doi.org/10.3899/jrheum.090048
Hannah J, Casian A, D’Cruz D (2016) Tacrolimus use in lupus nephritis: a systematic review and meta-analysis. Autoimmun Rev 15:93–101. https://doi.org/10.1016/j.autrev.2015.09.006
Závada J, Sinikka Pesicková S, Rysavá R et al (2014) Extended follow-up of the CYCLOFA-LUNE trial comparing two sequential induction and maintenance treatment regimens for proliferative lupus nephritis based either on cyclophosphamide or on cyclosporine A. Lupus 23:69–74. https://doi.org/10.1177/0961203313511555
Kasitanon N, Boripatkosol P, Louthrenoo W (2018) Response to combination of mycophenolate mofetil, cyclosporin A and corticosteroid treatment in lupus nephritis patients with persistent proteinuria. Int J Rheum Dis 21:200–207. https://doi.org/10.1111/1756-185X.13152
Yang TH, Wu TH, Chang YL et al (2018) Cyclosporine for the treatment of lupus nephritis in patients with systemic lupus erythematosus. Clin Nephrol 89:277–285. https://doi.org/10.5414/CN109325
Heerspink HJL, Greene T, Tighiouart H et al (2019) Chronic Kidney Disease Epidemiology Collaboration. Change in albuminuria as a surrogate endpoint for progression of kidney disease: a meta-analysis of treatment effects in randomised clinical trials. Lancet Diabetes Endocrinol 7:128–139. https://doi.org/10.1016/S2213-8587(18)30314-0
Colla L, Diena D, Rossetti M et al (2018) Immunosuppression in pregnant women with renal disease: review of the latest evidence in the biologics era. J Nephrol 31:361–383
Acknowledgements
We thank professor Claudio Ponticelli for his enlightening suggestions. We thank Roberta Gualtierotti (Department of Medical Biotechnology and Translational Medicine Università degli Studi di Milano) for her help in preparing the figures. Manuscripts based on work presented in the clinical highlights session at EULAR conference 2019 and published as a conference abstract (Number OP0046)
Funding
No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Author information
Authors and Affiliations
Contributions
GM conceived and planned the study and took the lead in writing the manuscript. LMA contributed substantially to data acquisition and interpretation, and to manuscript drafting. GF, EE, FS, IS, LC, MG and CE helped following patients and collecting data. VB, CT, MG, RC, AD, PM, MM critically revised the manuscript and gave their approval to the final version.
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare that they have no conflict of interest.
Ethics approval
The study was approved by the Ethics Committee of Fondazione Ca’ Granda IRCCS Ospedale Maggiore Policlinico di Milano, Italy (Protocol Number 504_2019bis) and of the other participating centers. We acted in the full adherence to the Declaration of Helsinki
Consent to participate
All patients signed an informed consent for the scientific use of their data. Patients were followed by dedicated team in each of the participating centers.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Argolini, L.M., Frontini, G., Elefante, E. et al. Multicentric study comparing cyclosporine, mycophenolate mofetil and azathioprine in the maintenance therapy of lupus nephritis: 8 years follow up. J Nephrol 34, 389–398 (2021). https://doi.org/10.1007/s40620-020-00753-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-020-00753-w