Abstract
Hypophosphatemia is a common but often underestimated electrolyte derangement among intensive care unit (ICU) patients. Low phosphate levels can lead to cellular dysfunction with potentially relevant clinical manifestations (e.g., muscle weakness, respiratory failure, lethargy, confusion, arrhythmias). In critically ill patients with severe acute kidney injury (AKI) renal replacement therapies (RRTs) represent a well-known risk factor for hypophosphatemia, especially if the most intensive and prolonged modalities of RRT, such as continuous RRT or prolonged intermittent RRT, are used. Currently, no evidence-based specific guidelines are available for the treatment of hypophosphatemia in the critically ill; however, considering the potentially negative impact of hypophosphatemia on morbidity and mortality, strategies aimed at reducing its incidence and severity should be timely implemented in the ICUs. In the clinical setting of critically ill patients on RRT, the most appropriate strategy could be to anticipate the onset of RRT-related hypophosphatemia by implementing the use of phosphate-containing solutions for RRT through specifically designed protocols. The present review is aimed at summarizing the most relevant evidence concerning epidemiology, prognostic impact, prevention and treatment of hypophosphatemia in critically ill patients with AKI on RRT, with a specific focus on RRT-induced hypophosphatemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypophosphatemia is a common electrolyte disorder among critically ill patients, but it is frequently underestimated in the intensive care units (ICUs) [1, 2]. Critical illness exposes patients to an increased risk of developing hypophosphatemia [3, 4] throughout several mechanisms involved in serum phosphate level regulation; indeed, many causal factors are involved, such as volume expansion, drugs, acid–base derangements, refeeding syndrome, sepsis [3, 5]. In patients with severe acute kidney injury (AKI) renal replacement therapies (RRTs) may represent a further risk factor for hypophosphatemia, especially if the most intensive and prolonged modalities of RRT are used [6,7,8,9,10,11,12,13].
Hypophosphatemia may have a negative impact on patient’ outcomes, with an increase in morbidity and mortality [10, 13,14,15,16]. Indeed, low phosphate levels can lead to cellular dysfunction with possibly relevant clinical manifestations involving different organs and/or systems (e.g., muscle weakness, respiratory failure, lethargy, confusion, arrhythmias) [3, 16]. Therefore, strategies aimed at reducing the incidence and the severity of hypophosphatemia should be timely implemented in the ICUs. However, no evidence-based specific guidelines are currently available for the management of hypophosphatemia in this clinical setting [17]. In any case, many protocols for phosphate supplementation by means of intravenous administration of phosphate solutions have been reported; however, this approach, if adopted without a strict protocol and in absence of a scheduled monitoring, carries the risk of further electrolyte abnormalities (hyperphosphatemia, hyperkalemia, hypocalcemia, hypomagnesemia) [5, 18]. In the specific case of critically ill patients on RRT, a rational approach could be the prevention of RRT-related hypophosphatemia through the adoption of phosphate-containing RRT solutions. In the last few years, this strategy has been reported as safe and effective in preventing this form of hypophosphatemia [6, 9, 11, 19,20,21,22,23,24,25]; thus, the implementation of protocols including phosphate-containing solutions may represent an effective option for RRT optimization in critically ill patients with AKI.
The purpose of the present review is to summarize the most relevant evidence concerning hypophosphatemia in critically ill patients with AKI on RRT in terms of epidemiology, prognostic impact, prevention and treatment, with a specific focus on RRT-induced hypophosphatemia.
Methods
An extensive review of the English language literature was performed to identify all relevant articles describing the epidemiology, pathogenesis, preventive measures, and outcome of hypophosphatemia in critically ill patients. To this end, we searched PubMed, EMBASE™, CINHAL, Web of Science and Cochrane databases. The search terms were used as follows: ((“Hypophosphatemia”[mh] OR “Hypophosphatemia”[tiab] OR “hypophosphatemia”[tiab] OR “critically ill”[mh] OR “critically ill”[tiab]) AND (“renal replacement therapies”[mh] OR “renal replacement therapies”[tiab] OR “renal replacement therapy”[tiab] OR “continuous renal replacement therapies”[tiab] OR “continuous renal replacement therapy”[tiab] OR “continuous veno-venous hemodiafiltration”[tiab] OR “continuous veno-venous hemofiltration”[tiab] OR “continuous veno-venous hemodialysis”[tiab])) NOT (animal[mh] NOT human[mh]). Medical subject heading terms were used to enhance electronic searches. Additional studies of interest were identified by manual search of references, and at least two reviewers independently evaluated each article for eligibility. The search was last updated on May 31, 2019.
Hypophosphatemia in critically ill patients
Definition and epidemiology
Hypophosphatemia is defined as a serum phosphate level < 0.81 mmol/l, and it is usually categorized, according to serum phosphate level, as mild (< 0.81 mmol/l), moderate (< 0.61 mmol/l), or severe (< 0.32 mmol/l) [11]. Even despite the presence of hypophosphatemia can be suggestive of phosphorus depletion, there is no close correlation between extra- and intracellular phosphorus [26, 27].
Hypophosphatemia is frequently observed in hospitalized patients with a prevalence up to 80% in the ICU setting [1, 2, 5, 13, 28, 29]. Some patient subgroups are considered at particularly high risk; for example, hypophosphatemia has been observed in 60–80% of septic patients [5, 30], and an extremely high prevalence of hypophosphatemia has been also documented in trauma patients, especially in the presence of burn wounds and head trauma [5, 31, 32]. Moreover, surgical patients are more prone to develop hypophosphatemia in the immediate post-operative phase, mostly following cardiac and hepatic surgery [5, 33,34,35]. Lastly, hypophosphatemia represents one of the most common electrolyte derangements related to RRT, especially when the most intensive and prolonged modalities, such as continuous RRT (CRRT) or Prolonged intermittent RRT (PIRRT)—also known as Sustained low-efficiency dialysis (SLED)—are adopted [2, 12, 15, 28]. Indeed, it can occur in up to 80% of adult and pediatric patients undergoing CRRT with standard dialysis/replacement solutions, especially if high dialysis dose is delivered [7,8,9,10,11, 13, 14, 36, 37] (Table 1). Although with a frequence lower than that observed in CRRT, hypophosphatemia related to the use of phosphate-free dialysis solutions is also reported in roughly 20–30% of patients undergoing PIRRT [38, 39] (Table 1).
Etiology
Phosphate balance is maintained through a complex interaction between phosphate uptake and phosphate excretion; in critically ill patients the mechanisms regulating this interaction are frequently disrupted, leading to an increased risk for hypophosphatemia. Three main mechanisms may induce hypophosphatemia: inadequate intake and/or decreased intestinal absorption, redistribution, and loss of phosphate (Fig. 1). As for the first mechanism, inadequate phosphorus intake alone rarely causes clinically relevant hypophosphatemia due to protective mechanisms such as increased renal tubular reabsorption of phosphate. However, a prolonged inadequate intake of phosphorus is not unusual in ICU patients and may contribute to induce phosphate depletion, especially if associated with conditions characterized by a reduced gut phosphate absorption (e.g., vitamin D deficiency, prolonged treatment with aluminum/magnesium antacids) [3, 5]. Internal redistribution of phosphate, due to the reallocation of phosphate to the intracellular compartment in the presence of pre-existent depletion of total body phosphorus stores, represents the most common mechanism of hypophosphatemia in ICU patients. Different conditions associated to a stimulation of glycolysis are able to increase intracellular phosphorylation processes, potentially leading to extracellular inorganic phosphate depletion. For example, an intracellular shift of phosphate may occur during alkalosis, of either respiratory or metabolic origin; indeed, the increase of intracellular pH which characterize these conditions is able to stimulate glycolysis and to induce hypophosphatemia by this mechanism [3]. Moreover, hypophosphatemia observed in the refeeding syndrome, that appears in severely malnourished patients when artificial nutrition is started, is mediated by intracellular redistribution of phosphate; in this clinical context, the high glucose load stimulates insulin release and accelerates carbohydrate metabolism, thus increasing intracellular phosphate requirements and its transport into the cells, so unmasking severe intracellular phosphate depletion [3, 40, 41]. Similarly, increased cellular uptake of phosphate may occur during beta-adrenergic receptor stimulation, mediated by endogenous (i.e. trauma, burns, sepsis) or exogenous (i.e. beta-adrenergic receptor agonists) catecholamines, or in presence of rapid cell proliferation (e.g., acute leukaemia, rapid proliferation of immune cells during severe infections) [3, 5, 42]. As regard to the last mechanism, phosphate depletion may occur as a consequence of increased renal and/or extra-renal body losses. In normal conditions, the kidney represents the main regulator of phosphate balance, essentially throughout the modulation of tubular reabsorption mediated by the effects of parathyroid hormone, serum phosphate levels and urinary pH variations [3]. Several drugs and/or conditions may be able to disrupt the regulation of renal tubular reabsorption leading to increased urinary losses of phosphate and hypophosphatemia (e.g., diuretics, theophylline, paracetamol overdose, glucocorticoids, acidosis, polyuria) [5]. Moreover, a negative phosphate balance may be also due to excessive intestinal (i.e. diarrhea) or skin (i.e. burn-wound patients) losses [5]. Finally, as mentioned, phosphate loss with RRTs represents the most relevant cause of hypophosphatemia in critically ill patients with severe AKI [3, 5, 28].
In summary, all the above described mechanisms may play a role at different times in the pathogenesis of hypophosphatemia in ICU patients; indeed, one or more of these mechanisms may be involved in different conditions frequently observed in this specific setting and commonly associated to phosphate depletion (e.g., sepsis, post-operative phase, trauma, refeeding, acid–base derangements, drugs, RRTs) [3, 5].
RRT-induced hypophosphatemia
Acute kidney injury is a widespread and serious complication in hospitalized patients [43] and RRTs, commonly as continuous modalities, are often required in ICU patients with severe AKI [44]. Indeed, CRRTs provide a slow and steady solute clearance, approaching the physiologic state, and an optimal fluid balance control; for this reason, CRRTs are frequently selected as the preferred option in ICU patients with hemodynamic instability [44, 45]. On the other hand, CRRTs usually provide a high daily solute clearance potentially leading to electrolyte derangements (e.g., hypokalemia, hypophosphatemia) if preventive measures are not implemented [28]. In this regard, it should be underlined that phosphorus is not a standard component of dialysis or replacement fluids; thus, hypophosphatemia usually occurs after 24–48 h from CRRT start if conventional phosphate-free dialysis solutions are used [14, 28, 37].
Phosphate is mainly an intracellular ion with a poor equilibrating ability between intra- and extra-cellular pools and with a complex and multi-compartmental kinetic during RRT [46]. The main limiting factor for phosphate clearance is the small mass transfer coefficient between the “remote” compartment (cells/interstitium) and the intravascular compartment that is known to be accessible to dialysis; on this ground, considering the slow equilibration between the different pools, phosphate clearance through RRT is time-dependent. In this regard, Ratanarat et al. conducted a study to assess, in critically ill patients, the kinetic of phosphate removal during different RRT modalities: single sessions of intermittent hemodialysis (IHD) were compared to SLED, and continuous veno-venous hemofiltration (CVVH) performing all treatments with standard phosphate-free dialysis/replacement solutions [46]. The Authors found that, although instantaneous phosphate clearance was higher in intermittent modalities (IHD 126.9 ± 18.4 ml/min, SLED 58.0 ± 15.8 ml/min, CVVH 31.5 ± 6.0 ml/min; p < 0.0001), the total phosphate removal was higher in prolonged intermittent or continuous RRT modalities (CVVH 56.7 ± 18.9 mmol, SLED 37.6 ± 9.6 mmol, IHD 29.9 ± 7.7 mmol; p = 0.001). These results are consistent with the typical behaviour of phosphate kinetics (two-pool) and confirm that the duration of RRT treatment is the main factor (r = 0.7, p < 0.0001) determining the amount of phosphate removal [46].
Moreover, phosphate clearance during RRT is highly influenced by the delivered dialysis dose, with the highest phosphate clearance observed with high intensity RRT. Indeed, in two large clinical trials patients receiving the most intensive RRT modalities experienced a higher incidence of hypophosphatemia [7, 8]. In particular, in acute renal failure trial network study, in which different RRT modalities was employed (IHD, SLED, CRRT), hypophosphatemia occurred in 17.6% of patients in the intensive-therapy group as compared to 10.9% in the less intensive-therapy group [7]. In the randomized evaluation of normal vs. augmented level (RENAL) replacement therapy trial, in which all patients were treated with CRRT, hypophosphatemia was much more frequently observed, with an incidence of 54% and 65.1% in the low- and high-intensity groups, respectively [8].
Effects and prognostic impact
Phosphorus is essential for many physiological functions. Indeed, it is a component of cellular structures and genetic material (i.e. membrane phospholipids, nucleic acids, nucleoproteins) and acts as a factor of intermediate metabolism (i.e. source of high-energy bond of the adenosine triphosphate). Furthermore, it is a component of 2,3-diphosphoglycerate (2,3-DPG), which is involved in the regulation of oxygen delivery. Lastly, phosphorus as phosphates acts as a buffer for the maintenance of plasma and urinary pH [3]. Thus, hypophosphatemia is associated with several clinical manifestations mainly due to cellular dysfunction in multiple organ systems (e.g., respiratory, cardiovascular, neuromuscular) induced by an impaired energy metabolism linked to phosphorus depletion. In the specific setting of ICU, respiratory failure, muscle weakness, lethargy, confusion, myocardial dysfunction and arrhythmias have been frequently described in patients with severe and prolonged hypophosphatemia [3, 5, 47, 48]. In particular, respiratory muscle weakness may impact on the weaning from mechanical ventilation; indeed, in 321 ICU patients, Demirjian et al. reported that CRRT-induced phosphate depletion was associated with a higher incidence of prolonged respiratory failure requiring tracheostomy [10]. Furthermore, in 34 hypophosphatemic patients with early stage of sepsis, Schwartz et al. found that routine intravenous phosphorus replacement therapy significantly reduced the incidence of new onset cardiac arrhythmias, at the same time reducing the need for antiarrhytmic drugs [47]. Moreover, it has been reported a link between hypophosphatemia and a depletion of red blood cell 2,3-DPG, leading to a leftward shift in the O2-haemoglobin dissociation curve and reduced oxygen unloading in the peripheral tissues [49,50,51].
Regarding clinical outcome, hypophosphatemia was associated with a significantly higher ICU and in-hospital mortality rates in a large and heterogeneous cohort of critically ill patients [1]. However, the Authors highlighted that hypophosphatemia was not an independent risk factor for mortality after adjusting for illness severity and suggested that low phosphate levels are likely a marker of illness severity rather than an independent determinant of outcome [1]. In critically ill patients undergoing CRRT for AKI, the ratio of CVVH days with hypophosphatemia to total days on CVVH was independently associated with 28-day mortality, so underlining the need to prevent CRRT-related phosphate depletion [13].
Management of hypophosphatemia in the critically ill
Phosphate supplementation
The treatment of hypophosphatemia still represents a matter of debate, and the threshold phosphate level for intervention remains unclear. Indeed, although phosphate supplementation is more commonly prescribed in presence of severe hypophosphatemia, the need for serum phosphate level correction may be considered in daily clinical practice also in mild to moderate hypophosphatemia, in order to prevent clinical manifestations [5, 17, 40, 47, 52]. Phosphate supplementation can be given via oral or intravenous routes; it can be administered as a part of nutritional prescription (i.e. enteral or total parenteral nutrition) or as a dedicated phosphate infusion. Especially in patients who receive feeding after a starvation period, additional phosphate should be added to nutritional preparations, while caloric intake must be gradually increased to avoid the risk of refeeding syndrome [5, 40]. As to intravenous administration of phosphate solutions, it is generally recommended in case of moderate to severe hypophosphatemia and/or in presence of symptoms associated with low serum phosphate levels. This strategy is not totally free of complications and should be cautiously applied. Indeed, phosphate may precipitate with calcium and magnesium resulting in electrolyte derangements such as hypocalcemia and hypomagnesemia [3, 18, 53]; moreover, potassium phosphate formulations should be used with a strict monitoring in patients at higher risk for hyperkalemia [53]. In reference to dose and correction rate, several replacement schedules have been reported. A phosphate dose calculation has been proposed based on serum phosphate levels and apparent phosphate distribution volume [additional phosphate dose in mmol = 0.5 × body weight x (1.25-actual serum phosphate expressed in mmol/l)] [17]. Thus, on the basis of calculated phosphate deficit, a solution containing 0.6 mmol/ml of phosphate could be safely administered at a phosphate infusion rate up to 10 mmol/h [17]. This approach appears safe and effective especially for moderate hypophosphatemia, but it results in a suboptimal correction in case of severe hypophosphatemia [54]. A lower infusion rate, up to 4 mmol/h, has been more cautiously suggested elsewhere, in order to avoid electrolyte derangements related to phosphate precipitation [18, 47].
Prevention of RRT-induced hypophosphatemia
In the specific case of critically ill patients undergoing RRT, it seems more appropriate to prevent rather than correct RRT-related hypophosphatemia. Indeed, standard dialysis/replacement solutions do not contain phosphate, and the use of these solutions is frequently associated with the onset of hypophosphatemia, especially during CRRT or PIRRT.
There are two possible approaches in the management of CRRT-induced hypophosphatemia: phosphate supplementation by intravenous route or the use of phosphate-containing CRRT solutions (home-made phosphate enriched solutions or commercially available phosphate-containing solutions) (Fig. 2). Unfortunately, due to the complexity of phosphate kinetics, the amount of phosphate removal during CRRT is not easily predictable. This very fact complicates the task of maintaining stable serum phosphate levels throughout the use of intravenous supplementation. In this regard, it has been reported that parenteral phosphate supplementation failed to prevent CVVH-related hypophosphatemia in most of the patients, suggesting that the use of CRRT phosphate-containing solutions could represent a more appropriate approach [13].
The idea of adding phosphate to conventional RRT fluids starts from the experience established in IHD for the treatment of intoxications caused by dialyzable toxins [55, 56]. Indeed, in these cases, the need for maintaining high clearances over many hours may lead to the development of severe hypophosphatemia, while phosphate addition to the acid concentrate has been proven to be a simple and safe measure to prevent phosphorus depletion. The low pH of the acid concentrate prevented phosphate precipitation with calcium, making feasible this preventive strategy [55, 56]. Unlike IHD acid concentrates, CRRT solutions are calcium-rich, lactate- and/or bicarbonate-based, so the risk of calcium precipitation with phosphate addition cannot be excluded. Despite this, the feasibility and safety of adding phosphate to conventional CRRT fluids have been successfully tested in both adult and pediatric patients [6, 9]. In particular, the addition of 2 ml of potassium phosphate (K2HPO4/KH2PO4, which contains 4.4 mmol/ml of potassium and 3.0 mmol/ml of phosphate) to the 5 l bag of Hemosol LG2® solution (Baxter; sodium 142, potassium 2, calcium 1.75, magnesium 0.75, lactate 40, bicarbonate 0, chloride 109, glucose 6.1, phosphate 0 mmol/l) and Hemosol B0® solution (Baxter; sodium 140, potassium 0, calcium 1.75, magnesium 0.50, lactate 3, bicarbonate 32, chloride 109.5, glucose 0, phosphate 0 mmol/l) was safe. Indeed, no reduction in calcium or phosphate concentrations occurred in the solutions after 5 h, and no signs of precipitation were observed in the bag after 48 h. Moreover, a physiological serum phosphate concentration of 1.2 mmol/l was maintained, and hypophosphatemia onset was effectively prevented in 20 ICU patients undergoing CRRT [6]. Similar results were obtained in a pediatric population of 38 patients undergoing CVVH/continuous veno-venous hemodiafiltration (CVVHDF) by adding monosodium phosphate to standard dialysate and/or replacement fluid [9]. The same group conducted a study to determine the in vitro stability of CRRT solutions up to 48 h after the addition of different concentrations of monosodium phosphate, demonstrating that phosphate concentration remained stable without significant changes in the pH of the solution or in the concentrations of the other components (calcium, magnesium, sodium, glucose) [57]. However, home-made phosphate-enriched solutions carry potential issues. Indeed, electrolytes addition to CRRT fluids requires controlled sterile conditions and, even with proper techniques, contamination remains a theoretical concern, as well as the risk of human error with incorrect additives or doses [58, 59].
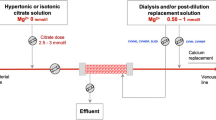
On the basis of the above reported evidences, commercially available phosphate-containing CRRT solutions have been introduced in many countries over the last few years (Table 2). The efficacy and safety of these solutions have been tested both in CVVH [19, 21, 22] and CVVHDF [11, 23,24,25] (Table 3). The use of a newly introduced commercial phosphate-containing dialysis fluid was tested for the first time in 42 ICU patients undergoing CRRT for AKI; they were divided into 3 subgroups who received, at different times, CVVHDF with standard dialysate and replacement solutions (group 1) or with phosphate-containing dialysate and standard replacement solution (group 2) or with phosphate-containing solution as both dialysate and replacement fluid (group 3) [11]. The incidence of hypophosphatemia was 79% in group 1; it was significantly lower (35%) in group 2, while no episodes of hypophosphatemia occurred in group 3. However, a mild hyperphosphatemia (> 1.9 mmol/l) occurred in 14% of patients receiving only the phosphate-containing solution (group 3) [11]. Moreover, the new phosphate-containing CRRT solution allowed an easier serum phosphate level control, also avoiding the rapid phosphate fluctuations usually observed with intravenous bolus administration [11]. It has been later reported that a commercially available phosphate-containing CRRT solution effectively prevented the decline in serum phosphate level, avoiding the need for additional intravenous phosphate supplementation [19, 20] (Fig. 3). However, the use of this solution as the sole CRRT fluid contributed to induce mild iatrogenic hyperphosphatemia, relative metabolic acidosis and hypocalcemia [19, 20]. Based on this evidence, the use of a phosphate-containing CRRT solution as roughly 50% of the whole dialysis dose was for the first time described in the specific setting of regional citrate anticoagulation (RCA) in a single case report of a patient undergoing RCA-CVVH for severe AKI after cardiac surgery [21]. This specifically designed protocol allowed to adequately control acid–base status and was effective in preventing hypophosphatemia [21]. Its efficacy has been later confirmed in larger samples both in RCA-CVVH [22] and RCA-CVVHDF [23, 25]. In particular, in 75 heart surgery patients treated for a minimum of 72 h with CVVHDF (roughly 60% for at least 7 days) using a low concentration citrate solution (18 mmol/l) combined with a commercially available phosphate-containing solution (1.2 mmol/l), serum phosphate levels were progressively corrected and maintained in a narrow normality range throughout the RCA-CRRT days of treatment, regardless of baseline phosphate levels and in absence of other electrolyte and acid–base derangements (Fig. 3). Moreover, most of the patients (> 85%) had normal serum phosphates levels within 72 h from CRRT start [25]. Considering the whole observation period (19,891 h), less than 5% of serum phosphate determinations met the criteria for hypophosphatemia avoiding the need for phosphate supplementation in most of the patients [25]. The efficacy of the same phosphate-containing dialysate solution in reducing episodes of hypophosphatemia has been confirmed, in combination with phosphate-free replacement fluid, also in standard CVVHDF [24]; in particular, during the observation period, the number of treatment days resulting in hypophosphatemia was significantly lower for patient treated with the new solution (7.4%) if compared with control group (15.6%). In addition, no impact on acid–base and calcium balance was detected [24].
Schematic representation of two CRRT protocols designed by using commercially available phosphate-containing solutions in standard CVVH setting (a) and in RCA-CVVHDF setting (b). Standard CVVH was performed by using the phosphate-containing solution as 100% of dialysis dose, while RCA-CVVHDF was performed by using the phosphate-containing solution as roughly 50% of dialysis dose. The main findings reported with the two different protocols are displayed in the table
Although specific studies are not yet available, the use of phosphate-containing solutions could be proposed and successfully implemented also in PIRRT.
Conclusion
Hypophosphatemia is a frequent, but often underrated issue in the critically ill. Multiple risk factors can be involved in the onset of hypophosphatemia in the ICU patients; among them, the negative phosphate balance which characterizes prolonged intermittent or continuous RRT modalities with standard dialysis/replacement solutions represents the main causal factor of phosphate depletion in critically ill patients with severe AKI. Persistent hypophosphatemia, especially if severe, may worsen main outcomes, and may lead to relevant clinical manifestations such as respiratory muscle weakness, delay in weaning from mechanical ventilation, and cardiac arrhythmias. Thus, any strategy aimed at reducing the incidence and the severity of hypophosphatemia should be timely implemented in ICUs. In particular, in the specific case of RRT-related hypophosphatemia, it appears reasonable and more appropriate to anticipate its occurrence through the use of phosphate-containing dialysis solutions. The efficacy and safety of this approach was successfully tested in different CRRT modalities and even in the setting of RCA-CRRT; indeed, the adoption of phosphate-containing solutions allows to effectively prevent hypophosphatemia, minimizing or avoiding the need for additional phosphate infusion by parenteral route.
On the basis of the more recent experiences, it could be suggested that the modulation of the proportion of CRRT dose given as phosphate-containing solution may allow to tailor CRRT phosphate balance according to the single patient needs. Indeed, although it should be taken into account that the fixed concentration of commercially available phosphate-containing solutions, actually ranging around 1–1.2 mmol/l, could not be able to fit all clinical situations, the variable combination of phosphate-containing and conventional CRRT solutions, according to the rapidly evolving clinical setting of AKI patients, may allow to meet clinical needs in most of the patients.
References
Suzuki S, Egi M, Schneider AG, Bellomo R, Hart GK, Hegarty C (2013) Hypophosphatemia in critically ill patients. J Crit Care 28:536
Ronco C, Ricci Z, De Backer D, Kellum JA, Taccone FS, Joannidis M, Pickkers P, Cantaluppi V, Turani F, Saudan P, Bellomo R, Joannes-Boyau O, Antonelli M, Payen D, Prowle JR, Vincent JL (2015) Renal replacement therapy in acute kidney injury: controversy and consensus. Crit Care 19:146
Bugg NC, Jones JA (1998) Hypophosphataemia. Pathophysiology, effects and management on the intensive care unit. Anaesthesia 53:895–902
Kim SY, Kim YN, Shin HS, Jung Y, Rim H (2017) The influence of hypophosphatemia on outcomes of low- and high-intensity continuous renal replacement therapy in critically ill patients with acute kidney injury. Kidney Res Clin Pract 36:240–249
Geerse DA, Bindels AJ, Kuiper MA, Roos AN, Spronk PE, Schultz MJ (2010) Treatment of hypophosphatemia in the intensive care unit: a review. Crit Care 14:R147
Troyanov S, Geadah D, Ghannoum M, Cardinal J, Leblanc M (2004) Phosphate addition to hemodiafiltration solutions during continuous renal replacement therapy. Intensive Care Med 30:1662–1665
VA/NIH Acute Renal Failure Trial Network, Palevsky PM, Zhang JH, O’Connor TZ, Chertow GM, Crowley ST, Choudhury D, Finkel K, Kellum JA, Paganini E, Schein RM, Smith MW, Swanson KM, Thompson BT, Vijayan A, Watnick S, Star RA, Peduzzi P (2008) Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med 359:7–20
RENAL Replacement Therapy Study Investigators, Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, Lo S, McArthur C, McGuinness S, Myburgh J, Norton R, Scheinkestel C, Su S (2009) Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med 361:1627–1638
Santiago MJ, López-Herce J, Urbano J, Bellón JM, del Castillo J, Carrillo A (2009) Hypophosphatemia and phosphate supplementation during continuous renal replacement therapy in children. Kidney Int 75:312–316
Demirjian S, Teo BW, Guzman JA, Heyka RJ, Paganini EP, Fissell WH, Schold JD, Schreiber MJ (2011) Hypophosphatemia during continuous hemodialysis is associated with prolonged respiratory failure in patients with acute kidney injury. Nephrol Dial Transplant 26:3508–3514
Broman M, Carlsson O, Friberg H, Wieslander A, Godaly G (2011) Phosphate-containing dialysis solution prevents hypophosphatemia during continuous renal replacement therapy. Acta Anaesthesiol Scand 55:39–45
Maynar Moliner J, Honore PM, Sánchez-Izquierdo Riera JA, Herrera Gutiérrez M, Spapen HD (2012) Handling continuous renal replacement therapy-related adverse effects in intensive care unit patients: the dialytrauma concept. Blood Purif 34:177–185
Yang Y, Zhang P, Cui Y, Lang X, Yuan J, Jiang H, Lei W, Lv R, Zhu Y, Lai E, Chen J (2013) Hypophosphatemia during continuous veno-venous hemofiltration is associated with mortality in critically ill patients with acute kidney injury. Crit Care 17:R205
Park JT, Lee H, Kee YK, Park S, Oh HJ, Han SH, Joo KW, Lim CS, Kim YS, Kang SW, Yoo TH, Kim DK, HICORES Investigators (2016) High-dose versus conventional-dose continuous venovenous hemodiafiltration and patient and kidney survival and cytokine removal in sepsis-associated acute kidney injury: a randomized controlled trial. Am J Kidney Dis 68:599–608
Lim C, Tan HK, Kaushik M (2017) Hypophosphatemia in critically ill patients with acute kidney injury treated with hemodialysis is associated with adverse events. Clin Kidney J 10:341–347
Talakoub R, Bahrami M, Honarmand A, Abbasi S, Gerami H (2017) The predicting ability of serum phosphorus to assess the duration of mechanical ventilation in critically ill patients. Adv Biomed Res 6:51
Bech A, Blans M, Raaijmakers M, Mulkens C, Telting D, de Boer H (2013) Hypophosphatemia on the intensive care unit: individualized phosphate replacement based on serum levels and distribution volume. J Crit Care 28:838–843
Agarwal B, Walecka A, Shaw S, Davenport A (2014) Is parenteral phosphate replacement in the intensive care unit safe? Ther Apher Dial 18:31–36
Chua HR, Baldwin I, Ho L, Collins A, Allsep H, Bellomo R (2012) Biochemical effects of phosphate-containing replacement fluid for continuous venovenous hemofiltration. Blood Purif 34:306–312
Chua HR, Schneider AG, Baldwin I, Collins A, Ho L, Bellomo R (2013) Phoxilium vs hemosol-B0 for continuous renal replacement therapy in acute kidney injury. J Crit Care 28:884.e7–884.e14
Morabito S, Pistolesi V, Tritapepe L, Zeppilli L, Polistena F, Fiaccadori E, Pierucci A (2013) Regional citrate anticoagulation in CVVH: a new protocol combining citrate solution with a phosphate-containing replacement fluid. Hemodial Int 17:313–320
Morabito S, Pistolesi V, Tritapepe L, Vitaliano E, Zeppilli L, Polistena F, Fiaccadori E, Pierucci A (2013) Continuous veno-venous hemofiltration using a phosphate-containing replacement fluid in the setting of regional citrate anticoagulation. Int J Artif Organs 36:845–852
Morabito S, Pistolesi V, Tritapepe L, Vitaliano E, Zeppilli L, Polistena F, Fiaccadori E, Pierucci A (2013) Continuous venovenous hemodiafiltration with a low citrate dose regional anticoagulation protocol and a phosphate-containing solution: effects on acid-base status and phosphate supplementation needs. BMC Nephrol 14:232
Godaly G, Carlsson O, Broman M (2016) Phoxilium(®) reduces hypophosphataemia and magnesium supplementation during continuous renal replacement therapy. Clin Kidney J 9:205–210
Pistolesi V, Zeppilli L, Polistena F, Sacco MI, Pierucci A, Tritapepe L, Regolisti G, Fiaccadori E, Morabito S (2017) Preventing continuous renal replacement therapy-induced hypophosphatemia: an extended clinical experience with a phosphate-containing solution in the setting of regional citrate anticoagulation. Blood Purif 44:8–15
Fiaccadori E, Coffrini E, Ronda N, Vezzani A, Cacciani G, Fracchia C, Rampulla C, Borghetti A (1990) Hypophosphatemia in course of chronic obstructive pulmonary disease. Prevalence, mechanisms, and relationships with skeletal muscle phosphorus content. Chest 97:857–868
Fiaccadori E, Coffrini E, Fracchia C, Rampulla C, Montagna T, Borghetti A (1994) Hypophosphatemia and phosphorus depletion in respiratory and peripheral muscles of patients with respiratory failure due to COPD. Chest 105:1392–1398
Jung SY, Kim H, Park S, Jhee JH, Yun HR, Kim H, Kee YK, Yoon CY, Oh HJ, Chang TI, Park JT, Yoo TH, Kang SW, Lee H, Kim DK, Han SH (2016) Electrolyte and mineral disturbances in septic acute kidney injury patients undergoing continuous renal replacement therapy. Medicine (Baltimore) 95:e4542
Thongprayoon C, Cheungpasitporn W, Mao MA, Sakhuja A, Erickson SB (2018) Admission hyperphosphatemia increases the risk of acute kidney injury in hospitalized patients. J Nephrol 31:241–247
Barak V, Schwartz A, Kalickman I, Nisman B, Gurman G, Shoenfeld Y (1998) Prevalence of hypophosphatemia in sepsis and infection: the role of cytokines. Am J Med 104:40–47
Berger MM, Rothen C, Cavadini C, Chiolero RL (1997) Exudative mineral losses after serious burns: a clue to the alterations of magnesium and phosphate metabolism. Am J Clin Nutr 65:1473–1481
Polderman KH, Bloemers FW, Peerdeman SM, Girbes AR (2000) Hypomagnesemia and hypophosphatemia at admission in patients with severe head injury. Crit Care Med 28:2022–2025
Buell JF, Berger AC, Plotkin JS, Kuo PC, Johnson LB (1998) The clinical implications of hypophosphatemia following major hepatic resection or cryosurgery. Arch Surg 133:757–761
Cohen J, Kogan A, Sahar G, Lev S, Vidne B, Singer P (2004) Hypophosphatemia following open heart surgery: incidence and consequences. Eur J Cardiothorac Surg 26:306–310
Salem RR, Tray K (2005) Hepatic resection-related hypophosphatemia is of renal origin as manifested by isolated hyperphosphaturia. Ann Surg 241:343–348
Morimatsu H, Uchino S, Bellomo R, Ronco C (2002) Continuous veno-venous hemodiafiltration or hemofiltration: impact on calcium, phosphate and magnesium concentrations. Int J Artif Organs 25:512–519
Song YH, Seo EH, Yoo YS, Jo YI (2019) Phosphate supplementation for hypophosphatemia during continuous renal replacement therapy in adults. Ren Fail 41:72–79
Sun Z, Ye H, Shen X, Chao H, Wu X, Yang J (2014) Continuous venovenous hemofiltration versus extended daily hemofiltration in patients with septic acute kidney injury: a retrospective cohort study. Crit Care 18:R70
Albino BB, Balbi AL, Abrão JM, Ponce D (2015) Dialysis complications in acute kidney injury patients treated with prolonged intermittent renal replacement therapy sessions lasting 10 versus 6 hours: results of a randomized clinical trial. Artif Organs 39:423–431
Terlevich A, Hearing SD, Woltersdorf WW, Smyth C, Reid D, McCullagh E, Day A, Probert CS (2003) Refeeding syndrome: effective and safe treatment with phosphates polyfusor. Aliment Pharmacol Ther 17:1325–1329
Chanchal R, Gupta S, Kanta C, Singh K, Koonwar S (2018) Hypophosphataemia in severe acute malnutrition: a prospective observational study. Br J Nutr 17:1–6
Bollaert PE, Levy B, Nace L, Laterre PF, Larcan A (1995) Hemodynamic and metabolic effects of rapid correction of hypophosphatemia in patients with septic shock. Chest 107:1698–1701
Sykes L, Nipah R, Kalra P, Green D (2018) A narrative review of the impact of interventions in acute kidney injury. J Nephrol 31:523–535
Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group (2012) KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl S2:1–138
Pistolesi V, Di Napoli A, Fiaccadori E, Zeppilli L, Polistena F, Sacco MI, Regolisti G, Tritapepe L, Pierucci A, Morabito S (2016) Severe acute kidney injury following cardiac surgery: short-term outcomes in patients undergoing continuous renal replacement therapy (CRRT). J Nephrol 29:229–239
Ratanarat R, Brendolan A, Volker G, Bonello M, Salvatori G, Andrikos E, Yavuz A, Crepaldi C, Ronco C (2005) Phosphate kinetics during different dialysis modalities. Blood Purif 23:83–90
Schwartz A, Brotfain E, Koyfman L, Kutz R, Gruenbaum SE, Klein M, Zlotnik A (2014) Association between hypophosphatemia and cardiac arrhythmias in the early stage of sepsis: could phosphorus replacement treatment reduce the incidence of arrhythmias? Electrolyte Blood Press 12:19–25
Ariyoshi N, Nogi M, Ando A, Watanabe H, Umekawa S (2017) Cardiovascular consequences of hypophosphatemia. Panminerva Med 59:230–240
Chanutin A, Hermann E (1969) The interaction of organic and inorganic phosphates with hemoglobin. Arch Biochem Biophys 131:180–184
Lichtman MA, Miller DR, Cohen J, Waterhouse C (1971) Reduced red cell glycolysis, 2, 3-diphosphoglycerate and adenosine triphosphate concentration, and increased hemoglobin-oxygen affinity caused by hypophosphatemia. Ann Intern Med 74:562–568
Sharma S, Brugnara C, Betensky RA, Waikar SS (2015) Reductions in red blood cell 2,3 diphosphoglycerate concentration during continuous renal replacement therapy. Clin J Am Soc Nephrol 10:74–79
Rosen GH, Boullata JI, O’Rangers EA, Enow NB, Shin B (1995) Intravenous phosphate repletion regimen for critically ill patients with moderate hypophosphatemia. Crit Care Med 23:1204–1210
Shajahan A, Ajith Kumar J, Gireesh Kumar KP, Sreekrishnan TP, Jismy K (2015) Managing hypophosphatemia in critically ill patients: a report on an under-diagnosed electrolyte anomaly. J Clin Pharm Ther 40:353–354
Engwerda E, Van den Berg M, Blans M, Bech A, De Boer H (2018) Efficacy and safety of a phosphate replacement strategy for severe hypophosphatemia in the ICU. Neth J Med 76:437–441
Chow MT, Lin HJ, Mitra EA, Singh S, Lee E, Leehey DJ, Ing TS (1998) Hemodialysis-induced hypophosphatemia in a normophosphatemic patient dialyzed for ethylene glycol poisoning: treatment with phosphorus-enriched hemodialysis. Artif Organs 22:905–907
Dorval M, Pichette V, Cardinal J, Geadah D, Ouimet D, Leblanc M (1999) The use of an ethanol- and phosphate-enriched dialysate to maintain stable serum ethanol levels during haemodialysis for methanol intoxication. Nephrol Dial Transplant 14:1774–1777
Santiago MJ, López-Herce J, Muñoz R, del Castillo J, Urbano J, Solana MJ, Botrán M (2011) Stability of continuous renal replacement therapy solutions after phosphate addition: an experimental study. Ther Apher Dial 15:75–80
Heung M, Mueller BA (2018) Prevention of hypophosphatemia during continuous renal replacement therapy—an overlooked problem. Semin Dial 31:213–218
Shaw AR, Chaijamorn W, Clark JS, Mueller BA (2018) Preparation times and costs for various solutions used for continuous renal replacement therapy. Am J Health Syst Pharm 75:808–815
Author information
Authors and Affiliations
Contributions
VP, LZ and SM had the idea for the review, performed the literature search and data analysis, and drafted the work. EF, GR and LT critically revised the work.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pistolesi, V., Zeppilli, L., Fiaccadori, E. et al. Hypophosphatemia in critically ill patients with acute kidney injury on renal replacement therapies. J Nephrol 32, 895–908 (2019). https://doi.org/10.1007/s40620-019-00648-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-019-00648-5