Abstract
Aldosterone is a mineralocorticoid hormone with a well-known effect on the renal tubule leading to water retention and potassium reabsorption. Other major effects of the hormone include the induction of proinflammatory activity that leads to progressive fibrotic damage of the target organs, heart and kidney. Blocking the aldosterone receptor therefore represents an important pharmacological strategy to avoid the clinical conditions deriving from heart failure (CHF) and chronic kidney disease (CKD). However, steroidal mineralocorticoid receptor antagonists (MRA) have a low safety profile, especially in CKD patients due to the high incidence of hyperkalemia. A new generation of nonsteroidal MRA has recently been developed to obtain a selective receptor block avoiding side-effects like hyperkalemia and thereby making the drugs suitable for administration to CKD patients. This review summarizes the results of published preclinical and clinical studies on the nonsteroidal MRA, apararenone esaxerenone and finerenone. The trials showed a better safety profile with maintained drug efficacy compared with steroidal MRA. For this reason, nonsteroidal MRA represent an interesting new therapeutic approach for the prevention of CHF and CKD progression. Some basic research findings also yielded interesting results in acute clinical settings such as myocardial infarction and acute kidney injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aldosterone is the most important mineralocorticoid hormone produced in the adrenal cortex. It is synthesized from cholesterol by the enzyme aldosterone synthase in the zona glomerulosa of the adrenal gland. Aldosterone synthesis is regulated by several factors including angiotensin II, extracellular potassium levels and corticotropin activity.
Aldosterone acts through two main pathways: one receptor-dependent, the other receptor-independent. Both are characterized by genomic and non-genomic effects. The hormone’s principal and best known effect is the regulation of fluid and electrolyte balance, which is mediated by the interaction between aldosterone and its receptor on the epithelial cells of the distal tubule and collecting duct. However, aldosterone overexpression exerts many detrimental effects in other tissues and organs like the heart, vessels, central nervous system, and adipocytes [1,2,3]. Indeed, aldosterone mineralocorticoid receptor (MR) binding promotes cardiac and renal remodeling by inducing myocardial fibrosis and glomerular and tubular sclerosis. Myocardial fibrosis is involved in uremic cardiomyopathy, the morphological substrate of the main cardiological complications of patients with chronic kidney disease (CKD) (myocardial infarction, atrial fibrillation, valvulopathy etc.) whatever the status of the illness. Glomerular and tubular sclerosis represent the mainstays of chronic disease progression [4,5,6,7]. Moreover, aldosterone causes endothelial dysfunction and vasoconstriction, sympathetic activation and oxidative stress [8, 9].
Although MR blockade has been demonstrated to be beneficial in patients with heart failure (CHF) and CKD (REF), traditional mineralocorticoid receptor antagonists (MRA) present important side-effects like hyperkalemia that limit their use in clinical practice.
To overcome this obstacle a new generation of MRA has recently been developed with better efficacy and fewer side-effects. The aim of this review is to summarize the pharmacological characteristics and preclinical and clinical evidence of these new drugs.
The mineralocorticoid receptor
The aldosterone MR is a member of the nuclear receptor family and has two main ligands: aldosterone and progesterone. MR-mediated effects can be classified into genomic and non-genomic.
While the genomic effects are better known, the mechanisms underlying non-genomic action are not clearly understood, but appear to be related to reactive oxygen species (ROS) inhibition in target tissues. There is evidence that aldosterone-induced ROS generation is associated with NADPH oxidase activation and that elevated ROS levels transactivate the epidermal growth factor receptor (EGFR) leading to activation of the Na+/H+ exchanger (NHE) [10, 11]. A common but not necessary property of these effects is their rapid time scale: indeed, the response is often seen after minutes [12].
The genomic effects stem from the interaction between aldosterone and MR. MR is a steroidal intracellular receptor located in the cytoplasm. The ligand-receptor complex migrates into the nucleus, recognizes specific DNA regions containing mineralocorticoid responsive elements and activates target gene transcription [13]. Although the genomic effects were observed within 30 min after aldosterone administration in some studies, they usually take several hours because of the need for the MR to translocate intracellularly, recruit transcription factors, activate gene expression and subsequently accumulate a sufficient amount of protein [12].
MR is known to be expressed in the kidney, not only on tubular epithelial cells but also on podocytes and in mesangial cells. Once MR binding occurs in tubular epithelial cells, genes such as Sgk1, Chif, and Ki-RasA are transcribed. These genes are then translated into proteins, which play an important role in water reabsorption in the kidneys, mainly by increasing ENaC, Na+/K+-ATPase transport activity and Na+/K+-ATPase synthesis in the principal cells of renal collecting duct. Other proteins influenced by aldosterone include the sodium-hydrogen antiporter (NHE) and sodium-chloride symporter (Na+/Cl−) [14].
Furthermore, aldosterone in the principal cells of the collecting duct activates the nuclear factor kB (NFkB), which regulates inflammation and the transepithelial transport of sodium, suggesting a correlation between sodium reabsorption in the collecting duct and inflammatory stimulus [15].
The effects of MR activation on the kidney are complex and extend beyond the tubular epithelial cells. Although the precise mechanisms remain undefined, aldosterone shows deleterious effects on podocytes, inducing apoptosis or altering their adhesive properties. Since these cells are highly differentiated, they are not considered to have proliferative capacity, so their reduction causes glomerular basement membrane denudation and adhesion to the Bowman capsule, a common pathway leading to glomerulosclerosis [16]. The MR is also found in other kidney cells, such as mesangial and fibroblast cells, modulating their activity towards a profibrotic phenotype or alterations of cell cycle regulators.
MR distribution is even wider as the receptor is expressed in endothelial and smooth muscle cells (SMC), cardiomyocytes, adipocytes, fibroblasts, macrophages and central nervous system cells [13, 16].
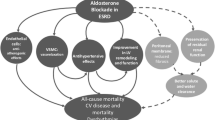
MR activation has been widely evaluated at cardiac level and is known to induce myocardial fibrosis by promoting inflammation. MR stimulation increases oxidative stress in the vessel wall and the expression of chemoattractant proteins like chemokine ligand 2, chemokine ligand 1, chemokine ligand 5 and adhesion molecules like intercellular adhesion molecule 1 and vascular cell adhesion molecule-1 determining the recruitment of inflammatory cells in heart tissue. This process is enhanced by pro-inflammatory cytokine secretion by macrophages and T-cells (TNF-alpha, inducible nitric oxide synthase, osteopontin). Resolution of the inflammatory process is crucial to preserve heart structure. It involves the expression of pro-fibrotic factors like TGF-beta, platelet-derived growth factor and connective tissue growth factor [17, 18] but when over-activated, as in CHF, it leads to myocardial fibrosis and dysfunction [19, 20]. In addition, oxidative stress has been demonstrated to trigger MR activation in a ligand-independent manner by Ras-related C3 botulinum toxin substrate 1, promoting cardiac fibrosis [21, 22]. Detrimental effects of MR activation on target organs are summarized in Fig. 1.
Mineralcorticoid receptor antagonists in CKD and CHF: indications and limits
MRA exert an anti-fibrotic and anti-inflammatory effect on the kidneys and other target organs like the heart (reduced cardiac fibrosis and left ventricular remodeling) and vessels (reduced collagen deposition) [13].
Spironolactone was the first MRA to be produced, followed some time later by eplerenone. Spironolactone was synthetized in 1957 and approved as a diuretic drug to treat edematous states, hypertension and primary hyperaldosteronism. Its therapeutic indications were later expanded in the wake of growing evidence of the systemic pro-fibrotic and pro-inflammatory effects of aldosterone especially in the kidneys, heart and vessels.
The RALES trial [23] was a landmark study highlighting an effect of MR antagonists to well beyond its modest diuretic activity. The trial demonstrated an impressive mortality rate reduction in patients with CHF with reduced left ventricular ejection fraction (LVEF) (< 35%) and NYHA class III–IV treated with low dose spironolactone (up to 50 mg/day) compared to placebo, administered on top of standard of care (ACE-I or ARB plus a loop diuretic). These data formed the basis of international guidelines for the treatment of CHF that recommend MRA in patients with CHF with reduced LVEF (< 35%) and NYHA class II–IV on top of an ACE I or ARB and a beta blocker [24].
Aldosterone levels usually increase as the glomerular filtration rate (GFR) falls in CKD, so that CKD and end-stage renal disease (ESRD) are considered states of relative hyperaldosteronism [25,26,27].
Spironolactone showed a good nephroprotective effect in several clinical trials, as summarized in the review by Bomback et al. [28]. This meta-analysis investigated 15 studies, evaluating the effects of MRA (spironolactone and eplerenone) in addition to ACE-I/ARB therapy on albuminuria, kalemia and arterial pressure in patients with diabetic and non-diabetic protenuric nephropathy. The studies lasted from 8 weeks to a year and showed a 15–54% reduction of proteinuria in the treatment groups compared to baseline, with hyperkalemia (K + > 5.5 mmoL/L) occurring in 5.5% of cases. Only six of the studies reported a significant reduction in blood pressure during treatment, without a clear correlation between proteinuria and blood pressure fall, demonstrating an independent action of the molecule from the hypotensive effect. The authors concluded that the data analyzed provided sufficient evidence to formulate the hypothesis that aldosterone blockade represents a safe and effective strategy for the treatment of proteinuric CKD patients who do not fully respond to ACE-I/ARB therapy.
Mineralocorticoid receptor antagonists (MRAs) hypotensive effects with no diuretic mechanism have also been reported [29, 30]. However, targeting aldosterone in the CKD population should yield benefits beyond proteinuria and blood pressure reduction, given the numerous harmful effects of aldosterone on the cardiovascular system, making ESRD patients a potentially prime target population for MRA therapy [31].
Although MRA have been advocated as beneficial in patients with diabetic kidney disease, this therapeutic approach may be considered equally beneficial in non-diabetic proteinuric kidney disease along with other emerging treatment for CKD progression [32,33,34,35]. MRA may also benefit patients on treatment with renin–angiotensin-aldosterone system (RAAS) blockade: an initial decline in aldosterone levels may be followed by the aldosterone breakthrough phenomenon represented by persistently elevated aldosterone levels that may be particularly noxious, with an increased risk of proteinuria and worsening left ventricular hypertrophy [27, 32, 33].
Spironolactone has two main side-effects which are substantially related to its low specificity for MR. The first comprise gynecomastia, erectile dysfunction and dysmenorrhea which are related to MRA binding with androgen or estrogen receptors; the second, particularly in subjects with kidney failure, is hyperkalemia probably related to the greater concentration of potassium in kidney tissue (sixfold higher than in the heart) [36].
Eplerenone, an MRA synthesized in the 1980s, is much more selective for MR than spironolactone and its affinity for MR is 40 times lower. These features reduce the burden of side-effects but limit the drug’s clinical efficacy. Weinberg et al. [37] found 100 mg/day the maximum effective dose of eplerenone for the treatment of hypertension. They also demonstrated a 25–50% lower efficacy compared to the same spironolactone dose. This disadvantage is counteracted in vivo by a greater bioavailability conferred by a lower plasma protein binding (50% vs 94% of spironolactone). Like spironolactone, eplerenone tends to be more concentrated in the kidneys than in other tissues but to a lesser extent (concentration in the kidneys threefold higher than in heart tissue) [36].
The number of patients meeting the indication for MRA in daily clinical practice is lower than expected because these drugs are frequently not prescribed. Albert et al. [38] showed that only a third of a sample of 12,565 patients hospitalized for CHF had received an MRA at 1 year after discharge. In addition, almost 70% of the cohort had been treated with a suboptimal dose of MRA. The main reason for not prescribing MRA is hyperkalemia [39]. Epstein et al. [40] recently demonstrated that the incidence of hyperkalemia is higher in “real world” studies compared to randomized controlled trials (RCT) (RALES for spironolactone and EMPHASIS-HF for eplerenone). In addition, hyperkalemia is the reason for the non-prescription of MRA in more than 35% of CHF patients. Among the CKD population, Shirazian et al. showed that despite being indicated in 40% of cases, RAAS blockade is not prescribed in 23% of patients with CKD stages 3–5 due to the risk of hyperkalemia [41].
Nonsteroidal MRA (new MRA)
The high side-effect burden of first (spironolactone) and second (eplerenone) generation MRA has led to the development of new molecules whose features seem to reduce the risk of hyperkalemia. The new drugs tested in the most important clinical trials are apararenone, esaxerenone and finerenone. No published preclinical or clinical data are currently available for the nonsteroidal MRA apararenone (Table 1).
Esaxerenone is an 18- and 260-fold more potent human MRA than spironolactone and eplerenone, respectively. It did not exert any agonistic effect on human and rat MR, whereas spironolactone and eplerenone exhibited a weak but significant agonist activity for these two receptors. Moreover, esaxerenone is at least 1400-fold more selective for human MR than other human steroid hormone receptors [42]. Pharmacokinetic studies in rats and monkeys showed that esaxerenone is well absorbed and widely distributed after oral administration, and mainly excreted in feces. The major elimination pathway is considered to be oxidation in rats, and oxidation combined with glucuronidation in monkeys [43]. The preventive effect of the molecule on hypertension and cardiorenal injury was tested in Dahl salt-sensitive hypertensive rats where the administration of 0.5 mg/kg and higher doses suppressed the elevation of systolic blood pressure (SBP). Moreover, the same dose was able to inhibit the increase in urinary protein excretion which appeared after 7 weeks of salt loading [44]. The same authors showed an improvement in existing renal injury with no fall of SBP in deoxycorticosterone acetate (DOCA)/salt-induced hypertensive rats (DOCA rats) [45]. Esaxerenone is also being tested in humans in phase II and III RCT but no results have been published to date [46] (Table 2).
More preclinical and clinical data are available on finerenone, a non-steroidal MRA belonging to the dihydropyridine family (Table 3). This molecule is a potent (IC50 17.8 nM) MR antagonist with greater selectivity for MR than spironolactone or eplerenone (over 500-fold more selective for MR than for any other steroid receptor). Finerenone is a full antagonist in different cell types including the gain-of function S810L mineralocorticoid receptor mutant, [47] responsible for early onset hypertension in men and gestational hypertension in women [36].
Finerenone acts as a “bulky-passive antagonist” that binds to MR in a different way from steroidal MRA. This interaction leads to a protrusion of helix 12 in the c-terminal activating the function domain on MR. This MR structural modification destabilizes the ligand-receptor complex preventing co-regulator recruitment and translocation into the nucleus, and thereby accelerating the MR degradation process. Structurally different MRA present different pharmacological characteristics.
Another important characteristic of finerenone is its distribution: target organ distribution of the drug is more balanced and uniform making the concentration of the molecule almost the same in the kidney and heart. The different ratio of distribution between tissues, associated with a greater selectivity of the molecule, could underlie the drug’s better safety profile and therefore its more extensive use in daily clinical practice.
A preclinical study in DOCA rats showed that the use of finerenone at a dose of 1 mg/kg prevented cardiac and renal functional and structural impairment regardless of the reduction in SBP. Finerenone also reduced cardiac hypertrophy, pro-BNP and proteinuria levels more efficiently than eplerenone when comparing doses with equivalent natriuretic effects [48]. Grune et al. [49] compared the effects of finerenone and eplerenone on cardiac hypertrophy in a mouse model, showing a different gene expression of troponin 2 and pro-BNP and a significant prevention of the increase in left ventricle mass in the group receiving finerenone.
Finerenone has been tested in many phase II RCTs. The ARTS study, focused on safety, enrolled patients with CHF, reduced LVEF (EF < 40%) and CKD with eGFR 30–90 ml/min and serum potassium ≤ 4.8 mmoL/l. The aim of the study was to compare proteinuria, eGFR and kalemia between finerenone and placebo in the first part (65 patients) and between finerenone, placebo and spironolactone in the second (393 patients). The study showed that serum potassium levels were higher in patients randomized to finerenone 10 mg/day or 5 mg/day compared to placebo but this was not true for lower finerenone doses (5 and 2.5 mg/day). The comparison between finerenone and spironolactone showed that the incidence of hyperkalemia was higher in the spironolactone group and that the eGFR decrease over time was lower in the finerenone group. Mean potassium values, however, remained within normal range and hyperkalemia events were lower than those in the spironolactone group (1.5–7.8% vs. 11.1%). Moreover, finerenone determined the same degree of proteinuria reduction as spironolactone except for low doses (5 mg/day).
ARTS-HF was a phase IIb RCT with a safety and dose-ranging purpose in a population of 1058 subjects with CHF. Inclusion criteria were ejection fraction < 40%, type 2 diabetes mellitus and/or eGFR 30–60 ml/min. Patients were randomized to finerenone or eplerenone at growing doses on the basis of serum potassium and eGFR. The primary end-point of the study was the reduction of NT-proBNP, for which finerenone showed the same level of efficacy as eplerenone. A surrogate end-point was a composite of all-cause death, cardiovascular hospitalization or CHF worsening. Almost all finerenone dosages (apart from the lowest dose of 5 mg/day) were superior to eplerenone, especially the highest dosages (10 mg/day, uptitrated to 20 mg/day). Hyperkalemia (defined as serum potassium ≥ 5.6 mmol/l) was a rare event in all study groups although there was a mild increase in serum potassium [50].
ARTS-DN was a phase IIb RCT focused on 823 patients with type 2 diabetes mellitus and CKD. Inclusion criteria were persistent proteinuria (UACR ≥ 30 mg/g) and serum potassium ≤ 4.8 mmol/l. Finerenone was administered on top of a RAAS blocker and compared to a placebo group. After 90 days ARTS-DN reached its primary end-point of UACR reduction. The results showed that finerenone reduced UACR in a dose-dependent manner (21% in the 7.5 mg/day group and 38% in the 20 mg/day group compared to placebo). Hyperkalemia (serum potassium ≤ 5.6 mmol/l) was present in 1.8% of patients and mean eGFR decline was 1.8, 2.6, 2.2 and 2.4 ml/min respectively in patients treated with 7.5, 10, 15 and 20 mg/die of finerenone with a reversible effect 30 days after drug withdrawal. The effects of finerenone on blood pressure were minimal. The difference between this drug and steroidal MRA was attributed to the inability of non-steroidal MRA to pass the blood–brain barrier and consequently lower blood pressure at central nervous system level [51].
This aspect was analyzed further in the ABPN study, an ARTS-DN substudy in which a group of 120 patients underwent stricter blood pressure monitoring by Holter. Masked uncontrolled hypertension (MUCH) was defined as normal office blood pressure but BP ≥ 135/85 mmHg upon awakening and nocturnal hypertension as a blood pressure ≥ 120/70 mmHg during the night-time. MUCH was diagnosed in 52.2% of patients and nocturnal hypertension in 76.5%. After 90 days patients treated with finerenone showed a significant reduction in MUCH and nocturnal hypertension compared to the placebo group.
Two double-blind placebo-controlled phase III RCTs, FIDELIO (NCT02540993) and FIGARO (NCT 02545049), are currently ongoing. The FIDELIO study is focused on evaluating the efficacy of finerenone in slowing CKD progression in 4800 patients with type 2 diabetes mellitus, very high albuminuria (UACR > 300 mg/g) and eGFR 25–60 ml/min. The aim of the trial is to test finerenone’s effects on cardiovascular morbidity and mortality in 6400 subjects with type 2 diabetes mellitus and high albuminuria (UACR 30–300 mg/g). The FIDELIO study recruitment phase ended in June 2018 while for FIGARO study recruitment is still ongoing. Interactions between Old and New MRAs and their receptors on target organs and main side effects mechanisms are summarized in Fig. 2.
Interactions between old and new MRAs and their receptors on target organs. a, c New and old MRAs act on renal tubular cells and myocardiocytes determining main therapeutic and side effects. Finerenone is equally concentrated as in heart (less hyperkalemia risk and has got high selectivity and high affinity for MR. Spironolactone and eplerenone are more concentrated in kidney than in heart (higher hyperkalemia risk). b Mechanism of MRAs induced hyperkalemia. d Mammary gland: new MRAs has lower selectivity for estrogens receptors compared to old MRAs (less gynecomastia risk)
Future perspectives on nonsteroidal MRA
Interesting data have emerged on the possible protective effect of finerenone against acute kidney injury (AKI). In a murine model, Lattenist et al. induced ischemia–reperfusion AKI after pre-treatment with finerenone for 3 days. They observed reduced AKI severity, lower levels of AKI biomarkers (NGAL and KIM 1) and less severe tubular damage on histology [52]. Authors from the same group identified vessel SMC as the possible main targets of finerenone in ischemia–reperfusion AKI prevention by a mechanism mediated by reduced ROS production and increased nitric oxide synthesis by endothelial cells [53].
Barrera-Chimal et al. also demonstrated in vivo that finerenone has a protective effect against the development of chronic kidney injury after AKI mediated by ischemia–reperfusion. This protection was associated with increased expression of M2-antinflammatory markers in macrophages from finerenone-treated mice. Moreover, finerenone increased IL-4 receptor expression and activation in the whole kidney and in isolated macrophages [54].
Recently, Martinez et al. studied neutrophil gelatinase-associated lipocalin (NGAL) as a downstream MR activation target in 119 post-myocardial infarction patients. They found that both higher baseline NGAL and a greater increase in NGAL levels during follow-up were associated with lower 6-month LVEF recovery due to increased myocardial fibrosis and that this phenomenon was prevented by finerenone [55].
A recent in vitro study incubated human coronary artery SMC and human umbilical vein endothelial cells (EC) with aldosterone and with or without finerenone. The authors showed that finerenone prevented aldosterone-induced EC apoptosis and SMC proliferation. Moreover, they used an in vivo model to demonstrate that finerenone accelerates the re-endothelialization process following vascular injury, reducing leukocyte recruitment and the inflammatory response and exerting a positive effect in preventing vascular remodeling [56].
In conclusion, MRA inhibit aldosterone by a receptor-dependent mechanism and reduce fibrosis in many target organs including heart, kidney and vessels. Their use, however, is limited because of a high side-effect burden (overall hyperkalemia). Phase II RCTs have demonstrated that the latest generation of non-steroidal MRA have a superior safety profile to spironolactone and eplerenone in patients with heart and kidney failure. The aim of the ongoing phase III RCTs is to evaluate the efficacy of these drugs on hard outcomes like mortality, cardiovascular events, proteinuria and renal death reduction.
References
Funder JW (2005) Mineralocorticoid receptors: distribution and activation. Heart Fail Rev 10(1):15–22. https://doi.org/10.1007/s10741-005-2344-2
Funder JW (2005) Mineralocorticoid-receptor blockade, hypertension and heart failure. Nat Clin Pract Endocrinol Metab 1(1):4–5. https://doi.org/10.1038/ncpendmet0016
Funder JW (2010) Minireview: aldosterone and mineralocorticoid receptors: past, present, and future. Endocrinology 151(11):5098–5102. https://doi.org/10.1210/en.2010-0465
Vashistha V, Lee M, Wu YL, Kaur S, Ovbiagele B (2016) Low glomerular filtration rate and risk of myocardial infarction: a systematic review and meta-analysis. Int J Cardiol 223:401–409 https://doi.org/10.1016/j.ijcard.2016.07.175
Bansal N, Hsu CY, Go AS (2014) Intersection of cardiovascular disease and kidney disease: atrial fibrillation. Curr Opin Nephrol Hypertens 23(3):275–282. https://doi.org/10.1097/01.mnh.0000444820.80249.56
La Manna G, Boriani G, Capelli I, Marchetti A, Grandinetti V, Spazzoli A, Dalmastri V, Todeschini P, Rucci P, Stefoni S (2013) Incidence and predictors of postoperative atrial fibrillation in kidney transplant recipients. Transplantation 96(11):981–986. https://doi.org/10.1097/TP.0b013e3182a2b492
Guerraty MA, Chai B, Hsu JY, Ojo AO, Gao Y, Yang W, Keane MG, Budoff MJ, Mohler ER 3rd, CRIC Study Investigators (2015) Relation of aortic valve calcium to chronic kidney disease (from the Chronic Renal Insufficiency Cohort Study). Am J Cardiol 115(9):1281–1286. https://doi.org/10.1016/j.amjcard.2015.02.011
Funder JW (2010) Aldosterone and mineralocorticoid receptors in the cardiovascular system. Prog Cardiovasc Dis 52(5):393–400. https://doi.org/10.1016/j.pcad.2009.12.003
Jaisser F, Farman N (2016) Emerging roles of the mineralocorticoid receptor in pathology: toward new paradigms in clinical pharmacology. Pharmacol Rev 68(1):49–75. https://doi.org/10.1124/pr.115.011106
He BJ, Anderson ME (2013) Aldosterone and cardiovascular disease: the heart of the matter. Trends Endocrinol Metab 24(1):21–30. https://doi.org/10.1016/j.tem.2012.09.004
Namsolleck P, Unger T (2014) Aldosterone synthase inhibitors in cardiovascular and renal diseases. Nephrol Dial Transplant 29(1):i62–i68. https://doi.org/10.1093/ndt/gft402
Lösel R, Schultz A, Boldyreff B, Wehling M (2004) Rapid effects of aldosterone on vascular cells: clinical implications. Steroids 69(8–9):575–578
Hermidorff MM, Assis LDe, Isoldi LVM MC (2017) Genomic and rapid effects of aldosterone: what we know and do not know thus far. Heart Fail Rev 22(1):65–89. https://doi.org/10.2174/1389450119666180326125926
Chen R, Sun W, Gu H, Cheng Y (2015) Aldosterone-induced expression of ENaC-a is associatedwith activity of p65/p50 in renal epithelial cells. 30(1):73–79. https://doi.org/10.1007/s40620-015-0231-z
Sato A. Saruta T (2004) Aldosterone-induced organ damage: plasma aldosterone level and inappropriate salt status. Hypertens Res 27(5):303–310
Shavit L, Lifschitz MD, Epstein M (2012) Aldosterone blockade and the mineralocorticoid receptor in the management of chronic kidney disease: current concepts and emerging treatment paradigms. Kidney Int 81(10): 955–968. https://doi.org/10.1038/ki.2011.505
Rickard AJ, Young MJ (2009) Corticosteroid receptors, macrophages and cardiovascular disease. J Mol Endocrinol 42(6):449–459
Shen JZ, Morgan J, Tesch GH, Rickard AJ, Chrissobolis S, Drummond GR, Fuller PJ, Young MJ (2016) Cardiac tissue injury and remodeling is dependent upon MR regulation of activation pathways in cardiac tissue macrophages. Endocrinology 157(8):3213–3223. https://doi.org/10.1210/en.2016-1040
Fraccarollo D, Berger S, Galuppo P, Kneitz S, Hein L, Schütz G, Frantz S, Ertl G, Bauersachs J (2011) Deletion of cardiomyocyte mineralocorticoid receptor ameliorates adverse remodeling after myocardial infarction. Circulation 123(4):400–408. https://doi.org/10.1161/CIRCULATIONAHA.110.983023
Tesch GH, Young MJ (2017) Mineralocorticoid receptor signaling as a therapeutic target for renal and cardiac fibrosis. Front Pharmacol 8:313. https://doi.org/10.3389/fphar.2017.00313
Nagase M, Ayuzawa N, Kawarazaki W, Ishizawa K, Ueda K, Yoshida S, Fujita T (2012) Oxidative stress causes mineralocorticoid receptor activation in rat cardiomyocytes: role of small GTPase Rac1. Hypertension 59(2):500–506. https://doi.org/10.1161/HYPERTENSIONAHA.111.185520
Ayuzawa N, Nagase M, Ueda K, Nishimoto M, Kawarazaki W, Marumo T, Aiba A, Sakurai T, Shindo T, Fujita T (2016) Rac1-mediated activation of mineralocorticoid receptor in pressure overload-induced cardiac injury. Hypertension 67(1):99–106. https://doi.org/10.1161/HYPERTENSIONAHA.115.06054
Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J (1999) The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 341(10):709–717
McMurray JJ et al (2012) ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 14(8):803–869. https://doi.org/10.1093/eurjhf/hfs105
Berl T, Katz FH, Henrich WL, de Torrente A, Schrier RW (1978) Role of aldosterone in the control of sodium excretion in patients with advanced chronic renal failure. Kidney Int 14(3):228–235
Hené RJ, Boer P, Koomans HA, Mees EJ (1982) Plasma aldosterone concentrations in chronic renal disease. Kidney Int 21(1):98–101
Reams GP, Bauer JH (1986) Effect of enalapril in subjects with hypertension associated with moderate to severe renal dysfunction. Arch Intern Med 146(11):2145–2148
Bomback AS, Kshirsagar AV, Amamoo MA, Klemmer PJ (2008) Change in proteinuria after adding aldosterone blockers to ACE inhibitors or angiotensin receptor blockers in CKD: a systematic review. Am J Kidney Dis 51(2):199–211. https://doi.org/10.1053/j.ajkd.2007.10.040
Gross E, Rothstein M, Dombek S, Juknis HI (2005) Effect of spironolactone on blood pressure and the renin–angiotensin–aldosterone system in oligo-anuric hemodialysis patients. Am J Kidney Dis 46(1):94–101
Shavit L, Neykin D, Lifschitz M, Slotki I (2011) Effect of eplerenone on blood pressure and the renin–angiotensin–aldosterone system in oligo-anuric chronic hemodialysis patients—a pilot study. Clin Nephrol 76(5):388–395
Bomback AS (2016) Mineralocorticoid receptor antagonists in end-stage renal disease: efficacy and safety. Blood Purif 41(1–3):166–170. https://doi.org/10.1159/000441262
Mehdi UF, Adams-Huet B, Raskin P, Vega GL, Toto RD (2009) Addition of angiotensin receptor blockade or mineralocorticoid antagonism to maximal angiotensin-converting enzyme inhibition in diabetic nephropathy. J Am Soc Nephrol 20(12):2641–2650. https://doi.org/10.1681/ASN.2009070737
Mavrakanas TA, Gariani K, Martin PY (2014) Mineralocorticoid receptor blockade in addition to angiotensin converting enzyme inhibitor or angiotensin II receptor blocker treatment: an emerging paradigm in diabetic nephropathy: a systematic review. Eur J Intern Med 25(2):173–176. https://doi.org/10.1016/j.ejim.2013.11.007
Bellizzi V, Conte G, Borrelli S, Cupisti A, De Nicola L, Di Iorio RB, Cabiddu G. Mandreoli M, Paoletti E, Piccoli GB, Quintaliani G, Ravera M, Santoro D, Torraca S, Minutolo R (2016) On behalf of the “conservative treatment of CKD” study group of the Italian society of nephrology. 30(2):159–170. https://doi.org/10.1007/s40620-016-0338-x
Stefoni S, Cianciolo G, Baraldi O, Iorio M, Angelini ML (2014) Emerging drugs for chronic kidney disease. Expert Opin Emerg Drugs 19(2):183–199. https://doi.org/10.1517/14728214.2014.900044
Ruilope LM, Tamargo J (2017) Renin-angiotensin system blockade: finerenone. Nephrol Ther 13(Suppl 1):S47–S53. https://doi.org/10.1016/j.nephro.2017.02.003
Weinberger MH, Roniker B, Krause SL, Weiss RJ (2002) Eplerenone, a selective aldosterone blocker, in mild-to-moderate hypertension. Am J Hypertens 15(8):709–716
Albert NM, Yancy CW, Liang L, Zhao X, Hernandez AF, Peterson ED, Cannon CP, Fonarow GC (2009) Use of aldosterone antagonists in heart failure. JAMA 302(15):1658–1665. https://doi.org/10.1001/jama.2009.1493
De Nicola L, Di Lullo L, Paoletti E, Cupisti A, Bianchi S (2018) Chronic hyperkalemia in non-dialysis CKD: controversial issues in nephrology practice. https://doi.org/10.1007/s40620-018-0502-6(Epub ahead of print)
Epstein M (2016) Hyperkalemia constitutes a constraint for implementing renin–angiotensin–aldosterone inhibition: the widening gap between mandated treatment guidelines and the real-world clinical arena. Kidney Int Suppl 6(1):20–28
Shirazian S, Grant CD, Mujeeb S, Sharif S, Kumari P, Bhagat M, Mattana J (2015) Underprescription of renin–angiotensin system blockers in moderate to severe chronic kidney disease. Am J Med Sci 349(6):510–515. https://doi.org/10.1097/MAJ.0000000000000475
Arai K, Homma T, Morikawa Y, Ubukata N, Tsuruoka H, Aoki K, Ishikawa H, Mizuno M, Sada T (2015) Pharmacological profile of CS-3150, a novel, highly potent and selective non-steroidal mineralocorticoid receptor antagonist. Eur J Pharmacol, 761:226–234. https://doi.org/10.1016/j.ejphar.2015.06.015
Yamada M, Takei M, Suzuki E, Takakusa H, Kotsuma M, Washio T, Murayama N, Inoue SI, Izumi T (2017) Pharmacokinetics, distribution, and disposition of esaxerenone, a novel, highly potent and selective non-steroidal mineralocorticoid receptor antagonist, in rats and monkeys. Xenobiotica 47(12):1090–1103. https://doi.org/10.1080/00498254.2016.1263766
Arai K, Tsuruoka H, Homma T (2015) CS-3150, a novel non-steroidal mineralocorticoid receptor antagonist, prevents hypertension and cardiorenal injury in Dahl salt-sensitive hypertensive rats. Eur J Pharmacol 769:266–273. https://doi.org/10.1016/j.ejphar.2015.11.028
Arai K, Morikawa Y, Ubukata N, Tsuruoka H, Homma T (2016) CS-3150, a novel nonsteroidal mineralocorticoid receptor antagonist, shows preventive and therapeutic effects on renal injury in deoxycorticosterone acetate/salt-induced hypertensive rats. J Pharmacol Exp Ther 358(3):548–557. https://doi.org/10.1124/jpet.116.234765
Kolkhof P, Bärfacker L (2017) 30 Years of the mineralocorticoid receptor: Mineralocorticoid receptor antagonists: 60 years of research and development. J Endocrinol 234(1):T125–T140. https://doi.org/10.1530/JOE-16-0600
Amazit L, Le Billan F, Kolkhof P, Lamribet K, Viengchareun S, Fay MR, Khan JA, Hillisch A, Lombès M, Rafestin-Oblin ME, Fagart J (2015) Finerenone impedes aldosterone-dependent nuclear import of the mineralocorticoid receptor and prevents genomic recruitment of steroid receptor coactivator-1. J Biol Chem 290(36):21876–21889. https://doi.org/10.1074/jbc.M115.657957
Kolkhof P, Delbeck M, Kretschmer A, Steinke W, Hartmann E, Bärfacker L, Eitner F, Albrecht-Küpper B, Schäfer S (2014) Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist protects from rat cardiorenal injury. J Cardiovasc Pharmacol 64(1):69–78. https://doi.org/10.1097/FJC.0000000000000091
Grune J, Benz V, Brix S, Salatzki J, Blumrich A, Höft B, Klopfleisch R, Foryst-Ludwig A, Kolkhof P, Kintscher U (2016) Steroidal and nonsteroidal mineralocorticoid receptor antagonists cause differential cardiac gene expression in pressure overload-induced cardiac hypertrophy. J Cardiovasc Pharmacol 67(5):402–411. https://doi.org/10.1097/FJC.0000000000000366
Filippatos G. Anker SD, Böhm M, Gheorghiade M, Køber L, Krum H, Maggioni AP, Ponikowski P, Voors AA, Zannad F, Kim SY, Nowack C, Palombo G, Kolkhof P, Kimmeskamp-Kirschbaum N, Pieper A, Pitt B (2016) A randomized controlled study of finerenone vs. eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease. Eur Heart J 37(27): 2105–2114. https://doi.org/10.1093/eurheartj/ehw132
Bakris GL, Agarwal R, Chan JC, Cooper ME, Gansevoort RT, Haller H, Remuzzi G, Rossing P, Schmieder RE, Nowack C, Kolkhof P, Joseph A, Pieper A, Kimmeskamp-Kirschbaum N, Ruilope LM, Mineralocorticoid Receptor Antagonist Tolerability Study-Diabetic Nephropathy (ARTS-DN) Study Group (2015) Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA 314(9):884–894. https://doi.org/10.1001/jama.2015.10081
Lattenist L, Lechner SM, Messaoudi S, Le Mercier A, El Moghrabi S, Prince S, Bobadilla NA, Kolkhof P, Jaisser F, Barrera-Chimal J (2017) Nonsteroidal mineralocorticoid receptor antagonist finerenone protects against acute kidney injury-mediated chronic kidney disease: role of oxidative stress. Hypertension 69(5):870–878. https://doi.org/10.1161/HYPERTENSIONAHA.116.08526
Barrera-Chimal J, André-Grégoire G, Nguyen Dinh Cat A, Lechner SM, Cau J, Prince S, Kolkhof P, Loirand G, Sauzeau V, Hauet T, Jaisser F (2017) Benefit of mineralocorticoid receptor antagonism in AKI: role of vascular smooth muscle Rac1. J Am Soc Nephrol 28(4):1216–1226. https://doi.org/10.1681/ASN.2016040477
Barrera-Chimal J, Estrela GR, Lechner SM, Giraud S, El Moghrabi S, Kaaki S, Kolkhof P, Hauet T, Jaisser F (2018) The myeloid mineralocorticoid receptor controls inflammatory and fibrotic responses after renal injury via macrophage interleukin-4 receptor signaling. Kidney Int 93(6):1344–1355. https://doi.org/10.1016/j.kint.2017.12.016
Martínez-Martínez E, Buonafine M, Boukhalfa I, Ibarrola J, Fernández-Celis A, Kolkhof P, Rossignol P, Girerd N, Mulder P, López-Andrés N, Ouvrard-Pascaud A, Jaisser F (2017) Aldosterone target NGAL (neutrophil gelatinase-associated lipocalin) is involved in cardiac remodeling after myocardial infarction through NFκB pathway. Hypertension 70(6):1148–1156. https://doi.org/10.1161/HYPERTENSIONAHA.117.09791
Dutzmann J, Musmann RJ, Haertlé M, Daniel JM, Sonnenschein K, Schäfer A, Kolkhof P, Bauersachs J, Sedding DG (2017) The novel mineralocorticoid receptor antagonist finerenone attenuates neointima formation after vascular injury. PLoS One 12(9):0184888. https://doi.org/10.1371/journal.pone.0184888
Funding
The authors declare no funding. Authors declare that this work has not been published before, that it is not under consideration for publication anywhere else and that that its publication has been approved by all co-authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical commitment
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Capelli, I., Gasperoni, L., Ruggeri, M. et al. New mineralocorticoid receptor antagonists: update on their use in chronic kidney disease and heart failure. J Nephrol 33, 37–48 (2020). https://doi.org/10.1007/s40620-019-00600-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-019-00600-7