Abstract
Background
Primary hyperoxaluria (PH) is a rare autosomal recessive disease commonly arising in childhood and presenting with nephrolithiasis, nephrocalcinosis and/or chronic renal failure. Three genes are currently known as responsible: alanine-glyoxylate aminotransferase (AGXT, PH type 1), glyoxylate reductase/hydroxypyruvate reductase (GRHPR, PH type 2), and 4-hydroxy-2-oxoglutarate aldolase (HOGA1, PH type 3). In our Centre, at the end of 2014 molecular diagnosis of PH1 had been performed in 80 patients, while one patient received a PH2 diagnosis.
Materials and methods
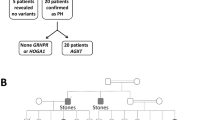
Fifteen patients referred to our Centre and suspected to have PH on clinical grounds were negative for pathogenic variants in the entire coding sequence and exon–intron boundaries of the AGXT gene. Therefore, we extended the analysis to the AGXT promoter region and the GRHPR and HOGA1 genes.
Results
Two patients were heterozygous for two novel AGXT-promoter variants (c.-647C > T, c.-424C > T) that were probably non pathogenic. One patient was homozygous for a novel HOGA1 variant of intron 2 (c.341-81delT), whose pathogenicity predicted by in silico splicing tools was not confirmed by a minigene splicing assay in COS-7 and HEK293T cells.
Conclusion
New genetic subtypes of PH can be hypothesized in our patients, that may be caused by mutations in other gene encoding proteins of glyoxylate metabolism. Alternatively, some kind of mutations (e.g., deletions/duplications, deep intronic splicing regulatory variants) could be missed in a few cases, similarly to other genetic diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary hyperoxaluria (PH) is a rare autosomal recessive disease (prevalence 1–3 × 10−6) with onset mainly in childhood [1]. It is characterized by increased levels of urinary and plasma oxalate, nephrolithiasis and/or nephrocalcinosis leading to end-stage renal disease (ESRD) and systemic oxalosis [2]. Three types of PH are currently known, but further genetic heterogeneity has been hypothesized, since in some patients the clinical suspicion of PH is not confirmed by molecular analysis [1].
PH1 (MIM #259900), the most frequent and severe form, is caused by an impaired function of the alanine-glyoxylate aminotransferase enzyme (AGT), encoded by the AGXT gene (MIM *604285) [3, 4]. Deficiency of the glyoxylate reductase/hydroxypyruvate reductase enzyme, encoded by the GRHPR gene (MIM *604296), is responsible for PH2 (MIM #260000) [5]. PH2 patients rarely develop systemic oxalosis and only 20 % of them progress to ESRD [5–7]. A peculiar biochemical PH2 characteristic is the presence of increased urinary l-glyceric acid [8]. PH3 (MIM #613616) is caused by mutations in the HOGA1 gene (MIM *613597), that encodes the mitochondrial enzyme 4-hydroxy-2-oxoglutarate aldolase (HOGA), catalysing the final step of 4-hydroxy-2-oxoglutarate catabolism to glyoxylate and pyruvate [9]. The age at onset of signs and symptoms is similar to those of PH1 and PH2, but in most PH3 patients a clinical remission is observed in the adult, despite the persistence of high oxalemia and oxaluria levels [10, 11].
High 24-h urinary oxalate (Uox) levels are suggestive of PH in the absence of secondary causes of hyperoxaluria, such as inflammatory bowel diseases or intestinal malabsorption [1, 4]. Another important parameter is the molar urinary oxalate/creatinine ratio, that should always be related to the age of the subject, since higher ratios are observed in children due to lower urinary creatinine concentrations [12]. In patients with ESRD, Uox can be within the normal range or even below because of the decreased glomerular filtration; in such cases, plasma oxalate (Pox) measurement it is preferable [1].
The assay of AGXT activity on hepatic tissue, once considered as the gold standard in PH1 diagnosis, is at present rarely performed, since the less invasive genetic testing has become available. In addition, the clinical presentation of the three PH types is largely overlapping; thus genetic analysis is an important tool to differentiate the three subtypes. According to the current recommendations, a different treatment is indicated for each PH type: combined kidney–liver transplantation is recommended only for PH1 [3], while isolated kidney transplantation is mostly performed in PH2, and no specific need of transplantation has been so far reported for PH3. Moreover, an AGXT genotype can help in predicting the effectiveness of vitamin B6 supplementation [1].
Here we report data on the 80 Italian PH1 patients and only one PH2 patients currently known in our country, collected up to the end of 2014, and we describe a group of 15 patients with a clinical suspicion of PH but without a mutation in the three known genes. In addition, we characterize the functional assay of a HOGA1 variant of unknown pathogenicity.
Materials and methods
Major clinical criteria for suspecting PH included nephrolithiasis, nephrocalcinosis and precocious age at onset of symptoms, while macro/micro haematuria and recurrent urinary infections were considered as indirect signs of renal stone disease. Glomerular filtration rate (GFR) in children was evaluated using the appropriate Schwartz’s formula according to creatinine dosage assay, while for adults the Modification of Diet in Renal Disease (MDRD) or Chronic Kidney Failure—Epidemiology Collaboration (CKD-EPI) formulae were employed. Urinary tract anomalies and secondary causes of hyperoxaluria, such as intestinal malabsorption and inflammatory bowel disease, were excluded. Biochemical parameters included oxalemia, oxaluria, urinary glycolate (see Ref. [12] for normal values) and renal function (plasma creatinine and GFR). Data about parents’ consanguinity and the familiar history were collected.
Sanger sequencing of AGXT, GRHPR and HOGA1 genes
Genomic DNA was extracted from peripheral blood using Maxwell System (Promega, Madison, WI, USA). Amplification and sequencing primers and protocols are available on request. Analysis of AGXT was performed at the first step; in the case of negative results, GRPHR, HOGA1 and the AGXT promoter-region were analyzed.
Minigene assay
In vitro analysis of the potential splice-site HOGA1 c.341-81delT variant was performed using the pSPL3 splicing assay previously described [10, 13, 14].
A 449 bp genomic fragment of DNA centred on the change c.341-81delT from the patient and one from a wild type (WT) control sample were amplified and the polymerase chain reaction (PCR) products were cloned into a TA-vector (pGEM-T Easy Vector—Promega) and transformed into DH5a bacterial cells (RBC Bioscience, Chung Ho City, Taiwan). Plasmids containing the WT or the variant sequence were extracted and digested with EcoRI and NotI restriction endonuclease. Inserts were sub-cloned into a pSPL3 exon-trapping vector (Life Technologies, Paisley, UK). PSPL3 minigene constructs containing the genomic change c.341-81delT and the WT sequence were extracted from DH5a bacterial cells and transfected into both COS-7 and HEK293T cells. After 24 h, total RNA was extracted and retro-transcribed with the Cells-To-Ct kit (Life Technologies). The cDNA was amplified by standard and nested PCR and the different amplicons were separated on a TBE-agarose 1.8 % gel (Online Resource, protocols available upon request).
Results
Clinical and biochemical data of the 105 patients referred to our Centre were evaluated according to recent guidelines [3] and the clinical suspicion of PH was supported by clinical findings in 96 of them. A different diagnosis was established in 9 patients: 4 were affected by secondary hyperoxaluria, 3 by kidney malformations, 1 was diagnosed with Dent and 1 with pseudo-Bartter syndrome.
Clinical, biochemical and genetic characteristics of PH1 and PH2 patients
At the end of 2014, the Italian database included 80 PH1 patients (28 females and 52 males) and 1 PH2 patient. Thirteen patients (16 %) had consanguineous parents (information not available for 16 patients), a familiar history was reported in 30 cases (24.6 %).
Symptoms at onset were mainly nephrolithiasis (52.5 %) and nephrocalcinosis (35.2 %). Three patients were diagnosed at family screening before symptoms developed. Median age at onset was 4 years (range 0.5–11.5), median age at diagnosis was 11 years (range 4–30).
Biochemical measurements at diagnosis were not available for 11 patients. Median Pox at diagnosis was 130 umol/L (24–187; normal values (nv): <2.5 umol/L; data not available in 23 patients) and median Uox 230 uM/mM creatine/day (151–435.5; data not available in 41 patients).
Liver biopsy for AGT activity measurement was available for 35 patients: mean residual AGT activity was negligible in 27 only in 8/35 (22.9 %) samples was a partial AGT activity detected (mean 39 ± 13 % of the normal).
At the end of 2014, 55 patients had ESRD and the median age was 14 years (range 4–31).
Ten patients had a kidney-only transplant and two of them had a second kidney-only transplant. In another 6 cases a second kidney transplant was combined with liver transplant. Median age at first kidney transplant was 18 years (range 10–35). In 17 patients a combined kidney–liver transplant was performed and in one a sequential kidney–liver transplant. Median age at kidney–liver transplant was 13 years (range 4–27). In two cases, a second kidney transplant was performed after the combined kidney–liver. One patient underwent two combined kidney–liver transplants. In one case, a preemptive liver transplant was performed.
As reported in the literature, also in the Italian cohort the most common AGXT mutation wais p.Gly170Arg (28 % of the alleles) detected in 14 patients in homozygosis, in 11 in heterozygosis with a missense mutation and in four with a null mutation.
The patient affected by PH2 had suffered from recurrent nephrolithiasis since 9 years of age. Neither familiar history of nephrolithiasis nor parentals consanguinity was referred. Uox was 77 uM/mM creatinine per day at 31 years of age. Urinary l-glycerate was 4600 uM/24 h (nv <30). His renal function was still normal at the end of 2014. Molecular analysis revealed the c.295C>T, p.Arg99* mutation in the GRHPR gene in homozygosis.
Clinical, biochemical and genetic characteristics of non-PH1 non-PH2 patients
Among the 15 mutation-negative patients (6 males), 11 (73 %) had nephrolithiasis (2 bilateral), 5 (33 %) had nephrocalcinosis and one 1 was affected with both. The median age at onset of symptoms was 5.3 years (range: 2 months–32 years). Renal function was normal in 9; 3 had already progressed to ESRD; data were not available for 3 patients. Three patients had recurrent urinary infections, 4 had haematuria (3 of them microhaematuria).
Uox was available for 8 patients; in children <12 years, mean oxaluria was 190.3 mmol/mmol creatinine (range 106–366) while in adults it was 78 mmol/mmol creatinine (nv <40 mmol/mmol creatinine [12]).
Mean Pox was available in 4 children (7.3 µmol/L) and 3 adults (33.9 µmol/L) in ESRD state.
Urinary calcium in patients with preserved renal function was in the normal range.
Four patients had family history of nephrolithiasis (Online Resource Table 1).
Among these patients no pathogenic mutations in the three PH genes were found.
Sequencing of the AGXT promoter revealed two novel variants: one patient was heterozygous for the c.-647C>T variant and another patient resulted heterozygous for the c.-424C>T variant. Neither variants was reported in dbSNP141, 1000 Genomes (http://www.1000genomes.org, 29/07/2015), Exome variant server (EVS, 29/07/2015), or the Exome Aggregation Consortium Browser (ExAC, 29/07/2015) and, because they do not appear in any known regulatory site, they were classified as probably non pathogenic.
A third patient carried a homozygous variant in the intron 2 of HOGA1 (c.341-81delT). This variant is not reported in the ExAC Browser or EVS, but it is reported in dbSNP and 1000 Genomes (http://www.1000genomes.org 29/07/2015) as a variant of unknown pathogenicity (rs570638580, MAF/minor allele: 0.0030/15).
In silico tools for splicing prediction [15–19] reported c.341-81delT as pathogenic, because it generates a novel acceptor splicing site (AG in c.341-79 position). Four out of five tools (SSF, MaxEntScan, Gene Splicer, HSF) predicted a possible damaging role of the variant, with an alternative transcript 79 bp longer r.(340_341ins341-79_341-1). This transcript would cause a reading frameshift and a truncated protein product [premature stop codon in exon 3: p.(Thr115Glufs*43)].
Clinical characteristics of the patient homozygous for the c.341-81delT HOGA1 variant
The patient homozygous for the HOGA1 variant had suffered from nephrocalcinosis since the age of 2 months and died at 4 months of respiratory failure. Pox levels were normal (<1 mmol/L), while values of oxaluria/urinary creatinine were at the upper limit of the normal range (366 µmol/mmol, nv for 0–6 months 325–360 µmol/mmol creatinine). Urinary calcium level was at the upper limit (uCa/uCr 0.56 mmol/mmol, nv for children <1 years old:<0.4). The renal function at age 4 months was decreased (GFR 53 mL/min/1.73 m2). His parents were not consanguineous.
HOGA1 minigene assay
In order to substantiate the predicted effect of the HOGA1 c.341-81delT variant on splicing we set up a minigene assay by cloning the intron 2 and exon 3 WT and variant sequences into a pSPL3 vector (Fig. 1). We expected a 391 bp product resulting from the canonical splicing of HOGA1 exon 3, a 471 bp product from the alternative splicing generated by the deletion of T in c.341-81, and a 263 bp product resulting from the complete skipping of the inserted exon. Analysis of the cDNA showed only the presence of the two 263 and 391 bp bands, without any difference from WT (Fig. 2).
Minigene assay: the WT or mutant fragments of the human HOGA1 gene containing exon 3 were cloned into the pSPL3 splicing vector to construct plasmids pSPL3-HOGA1-Exon3-WT and pSPL3-HOGA1-delT-Exon3. White boxes mark the pSPL3 exons A and B (derived from the B-globin gene) and HOGA1 exon 3, and the adjacent black lines depict the flanking intronic sequence of pSPL3. The grey box represents the 79 bp insertion expected from the generation of a novel splice-site by the c.341-81delT variant. The interrupted black lines mark the intronic sequences expected to be spliced out
Discussion
The genetic basis of PH has been widened in recent years thanks to second line studies of AGXT negative patients, leading to identify PH2 and PH3 [1, 9].
Nevertheless, a grey zone of cases in which suspicion based on clinical and biochemical findings is not confirmed by genetic testing still exists. The exact prevalence of this subset is currently unknown: several authors have reported the existence of undefined PH, but did not specify its prevalence or its clinical and genetic characteristics [1, 3, 20].
In our cohort of patients, 80 patients resulted homozygous or compound heterozygous for AGXT mutations (83.3 %) and one homozygous for a recurrent GRHPR mutation (1.1 %). Of subjects clinically suspect for PH, 15.6 % resulted negative for any PH mutation. A recent UK study performed in 200 patients detected mutations in AGXT, GRHPR and HOGA1 in 50, 8 and 8 % of the patients, respectively, while in 25 % of the subjects no mutations were found [21]. In the USA, an analysis of 301 PH families revealed PH1 in 68.4 % of them, PH2 in 9.3 % and PH3 in 11.0 % [20]. Compared to these results, PH1 in Italy seems to be far more frequent than the other PH types and, surprisingly, no Italian PH3 cases were identified up to the end of 2014.
In two patients two novel heterozygous AGXT-promoter variants, c.-647C>T and c.-424C>T, were identified. We considered these variants not to be pathogenic since they do not appear in any known regulatory site. The validity of this conclusion is limited by the scanty literature on the AGXT promoter [22], that precludes comparison with previous studies.
In one patient we detected a homozygous HOGA1 variant in intron 2 (c.341-81delT); we could not perform genetic analysis of the HOGA1 variant in the patient’s parents, since their DNA samples were not accessible.
The c.341-81delT variant was predicted to affect splicing by in silico analysis and to introduce a frameshift change (p.Thr115Glufs*43) in isoform 1, disruptive of HOGA enzymatic activity. Indeed, the two minor HOGA isoforms described [23] include exons 1, 5, 6, 7 (isoform 2), and exons 1, 6,7 (isoform 3) but not exons 2 and 3, and are probably not functional.
Even if the clinical characteristics of the c.341-81delT homozygous child were suggestive of PH3, a definite diagnosis could not be established on a clinical basis alone. High urinary or plasma levels of dihydroxyglutarate and hydroxyoxoglutarate are indicative of PH3 [24]; however the measurement of these metabolites is not yet performed in Italy.
Neither RNA nor HOGA enzymological studies were possible because further biological samples of the patient were not available. To evaluate the effect of the variant, we set up a minigene assay that, in contrast to in silico predictions, did not reveal any difference between the product of WT and c.341-81delT variant constructs. A possible explanation is that the power of the splicing site may be weaker than the canonical splicing site, so it could not be revealed by the simple minigene protocol in COS-7 and HEK293T cells.
In the Primary Hyperoxaluria Database [25], a HOGA1 c.341-82delA variant of undetermined significance has been reported: this description and our finding of the delT at c.341-81 highlight the potential pathogenic effect of variations in this region, as pointed out also by in silico predictions. No functional studies are available for the c.341-82delA and we believe that this report could be a useful base for further functional studies, despite the limitations of the minigene approach.
Negative findings in our cohort of patients with a clinical diagnosis of hyperoxaluria may be ascribed to the presence of gross rearrangements (e.g. deletions/duplications), not identifiable by Sanger sequencing. However, such mutations implicate homozygosis of the single-nucleotide polymorphism (SNP) haplotype and this is in contrast with our finding of heterozygous patients for several known AGXT, GRHPR and HOGA1 SNPs (Online Resource Table 2).
In our patients the presence of rare deep intronic splicing mutations in the three PH genes could not be excluded, as we did not sequence the whole intronic regions. However, such mutations are rarely searched for in genetic testing and their prevalence is unknown; in the literature, there are few data reported regarding deep intronic mutations: AGXT and GRHPR promoter analysis did not identify any causative mutations, while no study has analyzed the whole intronic regions of the three PH genes [22, 26].
In 4 out of 15 cases we carried out genetic analysis on the basis of a suggestive clinical history alone, although neither plasma nor urinary oxalate determinations were available since the collection was difficult; in these patients, secondary causes were excluded and a genetic etiology could be hypothesized because of their early onset of nephrolithiasis/nephrocalcinosis.
The large number of undefined patients without a definitive diagnosis (11 patients) could be explained by other nephropathies or by other hypothetical PH type(s) for which the gene/s responsible are still unknown, as postulated by several authors [1, 10, 20].
Conversely, a putatively causative homozygous or compound heterozygous genotype may not always lead to phenotypic expression, and a recent extensive population genetic screen suggested an unexpectedly high frequency of such asymptomatic/underdiagnosed cases [20].
Oxalate metabolism is complex and not fully described, and it is possible that more causative or modifier genes exist [1]. As candidates, the three human lactate dehydrogenases (LDHA, LDHB, LDHC) have been proposed, because of their important role in excessive oxalate production: LDH is able to convert glyoxylate to oxalate, glyoxylate to glycolate and hydroxypyruvate to L-glycerate, using NAD(H) as a cofactor [27]. Genetic variants in these three genes could influence the total LDH activity and the oxalate accumulation in PH patients.
Another candidate involved in the pathogenesis of PH is the hepatocytes peroxisomal enzyme glycolate oxidase, (GO, encoded by HAO1 gene, OMIM *605023), also known as “hydroxyacid oxidase 1”. This enzyme catalyses the oxidation of glycolate to glyoxylate, the AGT substrate. The absence of GO activity causes isolated glycolic aciduria [28], but it could be hypothesized that hypermorphic genetic HAO1 variants could modulate the enzyme activity leading to a different production of glyoxylate, responsible for the variable phenotype in PH patients.
Finally, even a multigenic PH inheritance has been suggested [10, 29], on the basis of the wide clinical variability among patients with the same AGXT genotype [30]. Therefore, further studies are needed to evaluate the relative contribution of genotype and environment in the development of the clinical phenotype.
To explore this hypothesis and discover novel genes involved in oxalate metabolism, whole exome sequencing and SNPs array techniques and also further phenotype–genotype studies are needed.
In conclusion, this study is the first report of HOGA1 genetic analysis in the Italian population. Despite PH3 being frequently reported both in Europe and in USA [10, 20, 21], no PH3 Italian cases have so far been identified. It could be possible that some PH3 cases have been missed because of the clinical remission of PH3 in adult age.
In this study the analysis of GRHPR and HOGA1 genes after AGXT analysis did not increase the sensitivity of PH genetic testing. This negative report should prompt a closer interaction between clinicians and geneticists in evaluating the clinical and biochemical data and we propose a multiple step diagnostic algorithm (Fig. 3) based on clinical and biochemical data to achieve this goal.
Abbreviations
- AGT:
-
Alanine-glyoxylate aminotransferase
- ESRD:
-
End stage renal disease
- GFR:
-
Glomerular filtration rate
- GRHPR:
-
Glyoxylate reductase/hydroxypyruvate reductase
- GO:
-
Glycolate oxidase
- HOGA:
-
4-Hydroxy-2-oxoglutarate aldolase
- LDH:
-
Lactate dehydrogenase
- Nv:
-
Normal value
- PH:
-
Primary hyperoxaluria
- PH1:
-
Primary hyperoxaluria type 1
- PH2:
-
Primary hyperoxaluria type 2
- PH3:
-
Primary hyperoxaluria type 3
- Pox:
-
Plasma oxalate
- Uox:
-
Urinary oxalate
- WT:
-
Wild type
References
Beck BB, Hoyer-Kuhn H, Goebel H et al (2013) Hyperoxaluria and systemic oxalosis: an update on current therapy and future directions. Expert Opin Investig Drugs 22(1):117–129
Leumann E, Hoppe B (2001) The primary hyperoxalurias. J Am Soc Nephrol 12(9):1986–1993
Cochat P, Hulton SA, Acquaviva C et al (2012) Primary hyperoxaluria Type 1: indications for screening and guidance for diagnosis and treatment. Nephrol Dial Transplant 27(5):1729–1736
Hoppe B, Beck BB, Milliner DS (2009) The primary hyperoxalurias. Kidney Int 75(12):1264–1271
Cregeen DP, Williams EL, Hulton S, Rumsby G (2003) Molecular analysis of the glyoxylate reductase (GRHPR) gene and description of mutations underlying primary hyperoxaluria type 2. Hum Mutat 22(6):497
Milliner DS, Wilson DM, Smith LH (2001) Phenotypic expression of primary hyperoxaluria: comparative features of types I and II. Kidney Int 59(1):31–36
Marangella M, Petrarulo M, Cosseddu D (1994) End-stage renal failure in primary hyperoxaluria type 2. N Engl J Med 330(23):1690
Williams H, Smith L (1968) l-glyceric aciduria: a new genetic variant of primary hyperoxaluria. N Enl J Med 278:233–239
Belostotsky R, Seboun E, Idelson GH et al (2010) Mutations in DHDPSL are responsible for primary hyperoxaluria type III. Am J Hum Gen 87(3):392–399
Beck B, Baasner A, Buerscher A et al (2012) Novel findings in patients with primary hyperoxaluria type III and implications for advanced molecular testing strategies. Eur J Hum Genet 21(2):162–172
Allard L, Cochat P, Leclere AL et al (2015) Renal function can be impared in children with primary hyperoxaluria type 3. Pediatr Nephrol 30(10):1807–1813
Milliner DS (2005) The primary hyperoxalurias: an algorithm for diagnosis. Am J Nephrol 25(2):154–160
Cavalieri S, Pozzi E, Gatti AR, Brusco A (2012) Deep intronic ATM mutation detected by genomic resequencing and corrected in vitro by antisense morpholino oligonucleotide (AMO). Eur J Hum Genet 21(7):774–778
Mancini C, Vaula G, Scalzitti L et al (2012) Megalencephalic leukoencephalopathy with subcortical cysts type 1 (MLC1) due to a homozygous deep intronic splicing mutation (c.895-226T > G) abrogated in vitro using an antisense morpholino oligonucleotide. Neurogenetics 13(3):205–214
SpliceSiteFinder (SSF) http://www.genet.sickkids.on.ca/~ali/splicesitefinder.html. Accessed 29 July 2015
GeneSplicer http://www.cbcb.umd.edu/software/GeneSplicer/gene_spl.shtml. Accessed 29 July 2015
NNSplice http://www.fruitfly.org/seq_tools/splice.html. Accessed 29 July 2015
HumanSplicingFinder (HSF) http://www.umd.be/HSF/. Accessed 25 July 2015
MaxEntScan http://genes.mit.edu/burgelab/maxent/Xmaxentscan_scoreseq.html. Accessed 29 July 2015
Hopp K, Cogal AG, Bergstrahl EJ et al (2015) Phenotype–genotype correlations and estimated carrier frequencies of primary hyperoxaluria. J Am Soc Nephrol 26(10):2559–2570
Williams EL, Bagg EA, Mueller M et al (2015) Performance evaluation of Sanger sequencing for the diagnosis of primary hyperoxaluria and comparison with targeted next generation sequencing. Mol Genet Genomic Med 3(1):69–78
Sato M, Toné S, Ishikawa T et al (2002) Functional analysis of the 5′-Flanking region of the human alanine:glyoxylate aminotransferase gene AGXT. Biochim Biophys Acta 1574(2):205–209
Bunker RD, Loomes KM, Baker EN (2012) Purification, crystallization and preliminary crystallographic analysis of human dihydrodipicolinate synthase-like protein (DHDPSL). Acta Cryst 68(Pt 1):59–62
Clifford-Mobley O, Hewitt L, Rumsby G (2015) Simultaneous analysis of urinary metabolites for preliminary identification of primary hyperoxaluria. Ann Clin Biochem. doi:10.1177/0004563215606158
Primary hyperoxaluria mutation database. www.uclh.nhs.uk/OURSERVICES/SERVICEA-Z/PATHBIOMED/CBIO/Pages/Phmdatabase.aspx. Accessed 10 Dec 2015
Fu Y, Rope R, Fargue S et al (2014) A mutation creating an out-of-frame alternative translation initiation site in the GRHPR 5′UTR causing primary hyperoxaluria type II. Clin Genet 88(5):494–498
Mdluli K, Booth MP, Brady RL, Rumsby G (2005) A preliminary account of the properties of recombinant human glyoxylate reductase (GRHPR), LDHA and LDHB with glyoxylate, and their potential roles in its metabolism. Biochim Biophys Acta 1753(2):209–216
Frishberg Y, Zeharia A, Lyakhovetsky R et al (2014) Mutations in HAO1 encoding glycolate oxidase cause isolated glycolic aciduria. J Med Genet 51(8):526–529
Monico CG, Rossetti S, Belostotsky R et al (2011) Primary hyperoxaluria type III gene HOGA1 (formerly DHDPSL) as a possible risk factor for idiopathic calcium oxalate urolithiasis. Clin J Am Soc Nephrol 6(9):2289–2295
Mandrile G, van Woerden CS, Berchialla P et al (2014) Data from a large European study indicate that the outcome of primary hyperoxaluria type 1 correlates with the AGXT mutation type. Kidney Int 86(6):1197–1204
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pelle, A., Cuccurullo, A., Mancini, C. et al. Updated genetic testing of Italian patients referred with a clinical diagnosis of primary hyperoxaluria. J Nephrol 30, 219–225 (2017). https://doi.org/10.1007/s40620-016-0287-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-016-0287-4