Abstract
Background
Hyperhomocysteinaemia, an independent risk factor for cardiovascular diseases, is common in hemodialysis patients (HD) and particularly in those homozygous for polymorphism of the 5,10‐methylenetetrahydrofolate reductase (MTHFR) gene. B vitamins supplementation has been shown to lower plasma total homocysteine (tHcy), but this has been contreversed in several groups. The aim of our study was to explore the response of tHcy in hemodialysis (HD) patients to individual supplementation with folic acid (B9) and/or vitamin B12, based on carrier status for the (MTHFR) polymorphism.
Methods
132HD were randomized according to C677TMTHFR genotypes into 2 groups (AandB). The group (A) was treated initially with B9 (10mg/day orally) for 2 months (t1) and then with B12 vitamin (cyanocobalamin ampoule of 1000 μg) for the following 2 months (t2), then association of B9 and B12 for 2 months (t3). The group (B) was supplemented initially with vitamin B12 (t1), then with folic acid (t2) and then B9 + B12 for 2 months (t3). A wash-out period of 2 months followed the treatment in both groups (t4). We determined tHcy, B9 and B12 concentrations at each time.
Results
In group A, we noted that the decrease in tHcy becomes significant for CC when patients were supplemented with vit B12 only (p = 0.009). While, B9 + vit B12 supplementation did not seem to improve a significant effect compared with B12 alone. For genotypes (CT) and (TT) we noticed a significant decrease in tHcy at t1 (p = 0.038; 0.005 respectively) and at (t3; CT p = 0.024; TT p = 0.017). In group B, for genotypes CC, the decrease in tHcy became significant at t3 (vit B12 + B9; p = 0.031). For genotypes (CT) and (TT), at the replacement of vit B12 by B9, tHcy was significantly decreased (p = 0.036; 0.012, respectively). The combination of the 2 vitamins (t3) showed no difference compared to folate alone. In the 2 groups (t4), there was an significant increase of tHcy again for 3 genotypes.
Conclusion
Supplementation with B vitamins correlated to the MTHFR genotypes has been shown to lower significantly tHcy in HD patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hyperhomocysteinemia (HHcy), a common characteristic in chronic kidney disease patients [1] and in hemodialysis (HD) [2], is an independent risk factor for the development of several diseases such as premature arterial fibrosis, cerebrovascular and heart disease, coronary occlusion, myocardial infarction and venous thromboembolism [3].
The cardiovascular disease mortality in HD is 10 to 20-fold greater than that in the general population, even after adjustment for factors such as age and sex.
In 1995, a common genetic variant of the gene encoding for methylenetetrahydrofolate-reductase (MTHFR), consisting of a cystosine (C) to thymidine (T) transition at nucleotide position 677 leading to exchange of a highly conserved alanine (A) to valine (V), was described. This polymorphism is correlated to HHcy in both normal [4] and impaired [5] kidney function.
Several attempts have been made to reduce total homocysteine (tHcy) levels in end-stage renal disease (ESRD) patients. Folate, which is vital to humans for its involvement in several metabolic reactions including the remethylation pathway, is considered by far the most important vitamin to reduce tHcy levels. In contrast to the lowering effect of routine folate supplementation in the general population, the moderate HHcy characteristic of dialysis patients has been shown to be resistant to larger doses of folate [6].
The protocols of folate and vitamin B12 supplementation in HD are controversial and appear to be dependent on C677T MTHFR genotypes.
The aim of our work was to study in HD the response of tHcy concentrations to supplementation with folate and/or vitamin B12 according to C677T MTHFR genotypes.
Subjects and methods
Patients
We recruited 132 patients (aged > 18 years, under hemodialysis for more than 3 months, without any severe pathology at 1 month before the study) from the Dialysis Unit of the University Hospital Sahloul in Sousse, Tunisia. All clinical data were collected on a spreadsheet. None of the patients had received B vitamins before the study. In this study, our patients received 2 drugs: cyanocobalamin (ampoule 1 ml/intramuscular injection, siphat) and folicum (folic acid, 5 mg/tablet; Julphar, Tunisia).
The study was approved by the Hospital Medical Ethics Committee and all patients involved in the study provided their consent.
Genotyping
DNA was extracted from blood samples by the salting out method. The C677T polymorphism was determined by polymerase chain reaction (PCR) followed by HinfI digestion.
Protocol
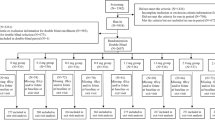
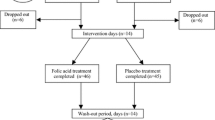
Patients were randomized according to C677T MTHFR genotypes into 2 groups of 66 individuals each (in each: 34 CC, 22 CT and 10 TT). The first group (protocol A) was treated initially with folic acid (B9) (10 mg/day orally) for 2 months (t1). Secondly, the patients received 2 B12 vitamin ampoules per week (cyanocobalamin ampoule of 1 ml = 1000 μg, intramuscular injection during the next 2 months (t2) at the end of dialysis. Thirdly, for the following 2 months (t3), they received B9 and B12 vitamins together (10 mg of B9/day orally and 2000 μg of B12 vitamin/week via intramuscular injection).
The second group (protocol B) was supplemented initially with vitamin B12 (t1), secondly with folic acid during the next 2 months (t2) and thirdly with B9 and B12 vitamins together for the following 2 months (t3) with the same doses as in protocol A.
A 2-month wash-out period followed the treatment in both groups (t4) (last 2 months of the study). We determined tHcy concentrations at each time-point (Fig. 1).
Samples
The samples were collected in EDTA tubes. Sampling from fasting patients was carried out throughout the study at different times. Tubes were quickly transported in ice, centrifuged at 4 °C at 3000 G for 10 min. The obtained plasma was stored at − 80 °C.
Assays
tHcy, vitamin B12 and B9 levels were measured in plasma by an AxSYM® autoanalyzer (Abbott Laboratories, Chicago, IL, USA) with the respective reagent references: 69-5372/R2, B8K202 and B3C812. tHcy was assayed by the fluorescence polarization immunoassay (FPIA) technique. Folate and vitamin B12 levels were determined by microparticle enzyme immunoassay (MEIA).
Normative references were 5–15 µmol/l for tHcy, > 187 pg/ml for vitamin B12 and 3.5–16.1 ng/ml for folate. At all 5 time-points, patient samples were assayed in the same series in order to reduce the analytical variations.
Statistical methods
Statistical analyses were performed on SPSS (Vers.17.0.0). Quantitative variables are reported as means ± standard deviation (SD) and compared by the Student’s t test if the distribution was Gaussian. If not, they are reported as median (min–max) and compared by the Mann–Whitney U test. A paired Student’s test was used for the comparison between tHcy at different times. The categorical variables were expressed numerically (n) and as a percentage (%), and compared by the Chi square test. For all tests, a p value <0.05 was considered statistically significant.
Results
The two groups were similar in terms of the main patient characteristics: age, gender, medical history, duration of chronic kidney disease, and number of hemodialysis sessions/week (Table 1).
Concerning patients’ characteristics by genotype, none of the patients had received folate and/or vitamin B12 before the study. The tHcy level at baseline differed significantly between the genotype groups and was ≥ 20 µmol/l (Table 2). At t0, no significant difference in tHcy, vitamin B12 and folate concentrations was observed between the two protocols (groups A and B) whatever the genotype (Table 3). This shows the similarity between the two groups at the study baseline.
tHcy values at the different time-points of the 2 protocols and according to genotype
Variation of tHcy concentrations according to the three genotypes in HD patients during the vitamin treatments in protocols A and B are shown in Table 4.
Student’s paired test p values between tHcy at the different time-points for protocols A and B are reported in Tables V and VI. The tHcy evolution is reported in Figs. 2 and 3 as the percentage relative to the baseline value for protocol A and protocol B.
Protocol A (Table 5; Fig. 2)
For CC carriers, no significant change was observed in tHcy when patients were supplemented with folate only (at t1, p = 0.155). The decrease became significant in CC carriers when supplemented with vitamin B12 only (t2, p = 0.009). However, the association of folate and vitamin B12 (t3) did not seem to significantly improve the effect of the supplementation compared to B12 alone (p = 0.638) in CC. Among CT and TT carriers, at t1 (folate only) a significant decrease in tHcy was observed (p = 0.038 and 0.005, respectively). Replacing folate by vitamin B12 (t2) did not seem to be beneficial for CT and TT (p = 0.169 and p = 0.224, respectively). The association of folate with B12 vitamin (t3) significantly decreased tHcy (p = 0.024 for CT and p = 0.017 for TT). At the wash out period (t4), there was a genotype-independent increase of tHcy (p < 0.05).
Protocol B (Table 6; Fig. 3)
For CC carriers, the decrease in tHcy became significant with the two vitamins (t3, p = 0.031). At t1, vitamin B12 alone had no significant effect for CT and TT genotypes (p = 0.189 and p = 0.247, respectively). When vitamin B12 was replaced by folate, tHcy significantly decreased (p = 0.036 and p = 0.012, respectively). The combination of the two vitamins (t3) showed no difference compared to folate alone (p = 0.197 for CT and p = 0.181 for TT). At the end of the study (t4), tHcy increased for all genotypes (t4, p < 0.05).
Discussion
Patient characteristics were similar in the two groups. None of the patients had received folic acid and/or vitamin B12 before the study and all had baseline tHcy concentration >20 µmol/l.
HHcy is a common hallmark of ESRD, reported in 80–100 % of HD patients [7, 8]. It is mainly due to impairment in methylation of homocysteine caused by uremia, as well as to a deficiency of the co-factors such as folate [9], and dialysis does not usually normalize its levels [10].
However, lowering tHcy concentrations in patients with HD is desirable. In fact, HHcy may partially account for thrombotic disease in dialysis patients. This correlation is confounded by malnutrition, inflammation and atherosclerosis syndrome [8], and cardiovascular events [11].
Homocysteine induces oxidative stress, inhibits antioxidant enzymes, accelerates endothelial dysfunction and increases thrombogenicity [12]. The MTHFR C677T polymorphism can also affect homocysteine metabolism [13]. In fact, it leads to a thermolabile variant of MTHFR with 30 % less enzyme activity [14].
In our study, genotype frequencies were 51.52 % CC, 33.33 % CT and 15.15 % TT. This genotypic distribution is quite similar to that reported in other Tunisian studies [15, 16]. The baseline vitamin B12 and folate levels did not differ statistically between the 3 genotypes. But tHcy concentrations were 26.07 ± 8.63, 38.86 ± 13.62 and 63.74 ± 44.15 µmol/l (p < 0.001), respectively. The increase in the initial concentrations of tHcy whatever the MTHFR genotype might be clinically relevant to HD [7].
In patients on HD, the Hcy conversion to methionine is substantially decreased and a state of folate resistance exists in uremic patients and was explained by downregulation of the folate receptor [17]. An altered tHcy pathway is amplified in subgroups of patients with MTHFR mutation [5]. Födinger et al. [5] have reported a direct correlation between MTHFR genotype and tHcy concentrations with higher values for the TT genotype. The reduction of tHcy levels could thus be critical in the TT and CT subgroups of uremic patients whose baseline tHcy is higher than normal.
Also, the effect of diet on lowering tHcy in nephropathic patients seems to be influenced by C677T MTHFR genotypes [18].
In our study, HD TT carriers displayed higher basal tHcy than did CC and CT carriers, but among each genotype tHcy at baseline did not differ statistically between the two protocols (Table 3).
For folate supplementation, we observed a significant decrease among CT and TT carriers in both groups when the treatment was started with folate (t1: protocol A; CT: p = 0.038; TT: p = 0.05) and at t2 after the vitamin B12 treatment (protocol B. CT: p = 0.036; TT: p = 0.012). The decrease was greater with TT genotypes in both groups. For CC carriers, the folate supplementation did not have a significant effect in either group.
Vitamin B12 seemed to be beneficial to the CC carriers only after folate supplementation (t2: p = 0.009; protocol B). In CT and TT genotypes, vitamin B12 did not appear to have a significant effect in reducing tHcy concentration, alone at t1 (protocol B) or after folate supplementation (t2, protocol A). The two vitamins supplementation at t3 significantly decreased tHcy in CT and TT carriers (CT: p = 0.024; TT: p = 0.017) in protocol A when the treatment started with folate, and in CC carriers in protocol B when the treatment started with vitamin B12 (t3. CC: p = 0.031).
We found a C677T MTHFR genotype-dependent influence of vitamin B supplementation as previously reported [19–25].
The present study suggests that supplementation of vitamin B12 and folate together seems to be useful only for CC carriers. In fact vitamin B12 supplementation seems to be beneficial for CC carriers after a first folate supplementation and to have a side-effect on CT and TT carriers. FA decreases tHcy in HD patients whatever the MTHFR 677 genotype.
The tHcy reduction was significantly higher in TT carriers than in other groups. The alternating vitamin treatment demonstrated the importance of folate therapy and the secondary contribution of vitamin B12 in lowering tHcy in HD patients.
Accordingly, Bostom et al. [20] reported the important effect of folate in reducing HHcy in HD patients.
Dierkers et al. indicated that, after B12 supplementation, tHcy reduction in CC carriers is higher than in CT or TT carriers. They presumed that the essential role of cobalamin in remethylation of tHcy was the reason behind the simultaneous reduction of homocysteine and folate levels. They concluded that folate is used as a substrate and this cycle is influenced by genotype [21].
Pastore et al. [22] demonstrated for the first time the importance of folate therapy and the secondary contribution of vitamin B12 in lowering tHcy in HD patients, and the supplementation with both vitamin B12 and folate was useful in patients with the wild-type genotype. A Canadian study [23] found that the greatest tHcy reduction after folate therapy was in the TT carriers. Obeid et al. [24] demonstrated the same results. They assumed that folate may diffuse and be lost due to its reduced size. In addition, an accelerated depletion of folate is expected because of its lower storage compared to vitamin B12.
Therefore, in the presence of adequate vitamin B12 status at the end of the wash-out period, folate could become a rate-limiting factor, which may partly explain the increase in tHcy concentrations, after stopping vitamin therapy, particularly, in patients whose MTHFR enzymatic activity was lower (CT and TT genotypes).
On the other hand, we noted that with folate supplementation TT carriers were more likely than CC or CT carriers to normalize their tHcy. Accordingly, Malinow et al. [25] reported that the MTHFR 677 → T transition has some influence on the response to folic acid therapy in subjects without renal failure. In contrast, Gere-Sunder-Plassmann et al. [26] did not discern any independent influence of these MTHFR polymorphisms on the response to folate treatment. Gere-Sunder-Plassmann et al. [26] also demonstrated that doses of 30–60 mg of folic acid per day are not more effective than 15 mg/day in reducing HHcy in regular HD patients. They demonstrated that HHcy in ESRD patients cannot be treated only by folate therapy regardless of the dosage. Azadibakhsh et al. [27] also demonstrated that oral supplementation with 15 mg/day folic acid along with 1 mg/day of vitamin B12 is effective in reducing tHcy levels in HD patients.
Some studies [28, 29] have demonstrated that 5-methyltetrahydofolate is essential for Hcy methylation using vitamin B12-dependent methionine synthase. Supplementation with vitamin B12 increases the intracellular demand for 5-methyltetrahydrofolate which results in a decrease in folate and an increase in 5-methyl tetrahydrofolate in the serum. The distribution of folate between the intra and extracellular compartments severely influences the serum folate levels, because serum folate accounts for only 1–2 % of extracellular folate.
Dierkes et al. [21] found that the increase of folate consumption results in higher serum vitamin B12 levels. Low-dose folate supplementation results in substitution of vitamin B12 in some metabolic pathways. However, the increase of dosage decreases the utility of vitamin B12 and thus inhibits the decrease of levels of this vitamin in serum.
Different publications [17, 30, 31] have described metabolisms of folate. In fact, FA is a synthetic form of the vitamin which is only found in fortified foods, supplements and pharmaceuticals. It locks coenzyme activity and reduces the metabolically active tetahydrofolate (l-5-methyl-THF). It is the predominant form of dietary folate and the form normally found in the circulation, and hence it is normally transported to peripheral tissues to be used for cellular metabolism. Studies comparing l-5-methyl-THF and folic acid have found that the two compounds have comparable physiological activity, bioavailability and absorption at equimolar dose [32] but some studies report a difference between the two forms according to genotypes [33, 34].
Verhaar et al. [30] indicated that 5-MTHFR can restore endothelial function in hypercholesterolemic patients probably by affecting cellular oxidative metabolism. This mode of action suggests that 5-MTHF effect on nitric oxide (NO) activity could be deduced from other clinical conditions that have been associated with impaired NO activity. So, folate exerts a direct antioxidant effect during endothelial function restoration, probably by affecting cellular oxidative metabolism.
A potential beneficial effect of folic acid supplementation in renal-failure patients is the normalization of the [adenosylmethionine]/[adenosylhomocysteine] ratio. Penna et al. [31] demonstrated convincingly that treatment with 5-methyltetrahydofolate (the active form of folate) resulted in a more pronounced increase of S-adenosylmethionine than S-adenosylhomocysteine leading to a higher [S-adenosylmethionine]/[S-adenosylhomocysteine] ratio. This would indicate some improvement of methylation reactions in uremic subjects. The reduction of this ratio is in good agreement with the degree of impairment of membrane protein repair [31].
In fact, the MTHF induces an increase in cells’ methionine intake through an ATP-dependent reaction elevating adenosylmethionine levels. Treatment with MTHF in HD patients increases the [adenosylmethionine]/[adenosylhomocysteine] ratio indicating that the blockage in the normal flow of transmethylation is eased if not dispelled by treatment with MTHF. An increase in transmethylation should therefore induce a diminished accumulation of altered proteins, with the relevant consequences in terms of protein function.
Concerning vitamin B12, we found that it seems to be beneficial to CC carriers only after a first folate supplementation and to have a secondary effect on the CT and TT carriers.
Billion et al. [7] found that oral vitamin B12 in itself does not appear to have a significant tHcy lowering effect. However, parenteral vitamin B12 seems to lower the tHcy plasma level in dialysis patients. Vitamin B12 might reduce tHcy concentration through a pharmacological mechanism presumably analogous to that of the ‘megadose’. FA is used to lower tHcy concentrations in non folate deficient ESRD patients with oral vitamin B12 (1 mg/day). This pharmacological action does not appear to be obvious in hemodialysis patients. Billion et al. [7] believe that oral vitamin B12 should be given to ESRD patients despite its lack of tHcy lowering effect for three reasons: (a) cobalamin deficiency assessed by high methylmalonic acid concentrations is observed in spite of normal vitamin B12 serum concentrations; (b) the high flux hemodialysis treatment and the use of erythropoeitin could induce subnormal serum vitamin B12 levels; (c) folate supplementation in vitamin B12 deficient patients masks hematological features of cobalamin deficiency (megaloblastosis) while its neurological consequences are not curbed, and therefore its diagnosis can be grossly delayed.
In conclusion, we have demonstrated the importance of folate therapy and the secondary contribution of vitamin B12 in lowering tHcy in HD patients. The decrease of tHcy concentration as a result of these therapies is correlated to the MTHFR genotype. We also demonstrated that supplementation with both vitamin B12 and folate is useful only for CC carriers. Having a secondary role in reducing tHcy levels does not exclude the potentially beneficial action of vitamin B12 on many neuropathies in chronic hemodialysis.
References
Chao MC, Hu SL, Hsu HS, Davidson LE, Lin CH et al (2014) Serum homocysteine level is positively associated with chronic kidney disease in a Taiwan Chinese population. J Nephrol 27(3):299–305
Sen U1, Mishra PK, Tyagi N, Tyagi SC (2010) Homocysteine to hydrogen sulfide or hypertension. Cell Biochem Biophys 57(2–3):49–58
Foley RN, Parfrey PS, Samak MJ (1998) Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 32(5 Suppl 3):S112
Brattstrom L, Wilcken DEL (1998) O¨ hrvik J and Brudin L. Common methylentetrahydrofolate reductase gene mutation leads to Hyperhomocysteinemia but not to vascular disease: the result of a meta-analysis. Circulation 98:2520–2526
Födinger M, Mannhalter C, Wölfl G, Pabinger I, Muller E, Schmid R et al (1997) Mutation (677 C to T) in the methylentetrahydrofolate reductase gene aggravates hyperhomocysteinemia in hemodialysis patients. Kidney Int 52:517–523
Bostom AG, Shemin D, Lapane KL et al (1996) High-dose-B-vitamin treatment of hyperhomocysteinemia in dialysis patients. Kidney Int 49:147–152
Billion S, Tribout B, Cadet E, Queinnec C, Rochette J, Wheatley P et al (2002) Hyperhomocysteinaemia, folate, and vitamin B12 in unsupplemented haemodialysis patients: effect of oral therapy with folic acid and vitamin B12. Nephrol Dial Transplant 17:455–461
Nair AP, Nemirovsky D, Kim M et al (2005) Elevated homocysteine levels in patients with end-stage renal disease. Mt Sinai J Med 72(6):365–373
Righetti M (2007) Homocysteine-lowering vitamin B treatment decreases cardiovascular events in hemodialysis patients. Clin Chem Lab Med 45:1586–1589
Righetti M, Ferrario GM, Milani S et al (2003) effects of folic acid treatment on homocysteine levels and vascular disease in hemodialysis patients. Med Sci Monit 9:137–142
Suliman ME, Stenvinkel P, Heimburger O et al (2002) Plasma sulfur amino acids in relation to cardiovascular disease, nutritional status, and diabetes mellitus in patients with chronic renal failure at start of dialysis therapy. Am J Kidney Dis 40:480–488
Arnadottir M, Gudnason V, Hultberg B (2000) Treatment with different doses of folic acid in haemodialysis patients: effects on folate distribution and aminothiol concentrations. Nephrol Dial Transplant 15:524–528
Weisberg I, Tran P, Christensen B, Sibani S, Rozen R (1998) A second genetic polymorphism in methylenetetrahydrofolate reductase (MTHFR) associated with decreased enzyme activity. Mol Genet Metab 64:169–172
Sharp L, Little J (2004) Polymorphisms in genes involved in folate metabolism and colorectal neoplasia : a huge review. Am J Epidemiol 159:423–443
Belkahla R, Omezzine A, Kchok K, Rebhi L, Ben Hadj Mbarek I, Rejeb J et al (2008) Effect of polymorphisms on key enzymes in homocysteine metabolism, on plasma homocysteine level and on coronary artery-disease risk in a Tunisian population. Annales de Cardiologie et d’Angéiologie 5(4):219–224
BelhadjMbarek I, Omezzine A, Abroug S, Belkahla R, Rebhi L, Benrejeb N and al. Supplementation in vitamin B response correlated to C 677T MTHFR genotype in hemodialised children. Immuno-analyse et biologie spécialisée (2008)23:368-374
Perna AF, Lanza D, Sepe I, Conzo G, Altucci L, Ingrosso D (2013) Altered folate receptor 2 expression in uraemic patients on haemodialysis: implications for folate resistance. Nephrol Dial Transplant 28(5):1214–1224
Di Daniele N, Di Renzo L, Noce A, Lacopino L, Ferraro FM, Rizzo M et al (2014) Effects of Italian Mediterranean organic diet vs. low-protein diet in nephropathic patients according to MTHFR genotypes. J Nephrol 27(5):529–536
Yong H, Jianping L, Xianhui Q, Yining H, Xiaobin W, Rebecca F et al (2015) Efficacy of Folic Acid Therapy in Primary Prevention of Stroke Among Adults With Hypertension in China. Am Med Assoc 313:1325–1335
Bostom AG, Shemin D, Bagley P, Massy ZA, Zanabli A, Christopher K et al (2000) Controlled comparison of L-5-methyltetrahydrofolate versus folic acid for the treatment of hyperhomocysteinemia in hemodialysis patients. Circulation 101:2829–2832
Dierkes J, Domrose U, Ambrosch A, Schneede J, Guttormsen AB, Neumann KH et al (1999) Supplementation with vitamin B12 decreases homocystein and methylmalonic acid but also serum folate in patients with end stage renal disease. Metabolism 48:631–635
Pastore A, De Angelis S, Casciani S et al (2006) Effects of folic acid before and after vitamin B12 on plasma homocysteine concentrations in hemodialysis patients with known MTHFR genotypes. Clin Chem 52(1):145–148
Tremblay R, Bonnardeaux A, Geadah D et al (2000) Hyperhomocysteinemia in hemodialysis patients: effects of 12-month supplementation with hydrosoluble vitamins. Kidney Int 58:851–858
Obeid R, Kuhlmann MK (2005) Ko¨hler H, Herrmann W. Response of homocysteine, cystathionine, and methylmalonic acid to vitamin treatment in dialysis patients. Clin Chem 51:196–201
Malinow MR, Nieto FJ, Kruger WD, Duell PB, Hess DL, Gluckman RA, Block PC, Holzgang CR, Anderson PH, Seltzer D, Upson B, Lin QR (1997) The effects of folic acid supplementation on plasma total homocysteine are modulated by multivitamin use and methylenetetrahydrofolate reductase genotypes. Arterioscler Thromb Vasc Biol 17:1157–1162
Sunder-Plassmann G, Födinger M, Buchmayer H et al (2000) Effect of high dose folic acid therapy on hyperhomocysteinemia in hemodialysis patients: results of the Vienna multicenter study. J Am Soc Nephrol 11:1106–1116
Azadibakhsh N, Hosseini RS, Atabak S, Nateghiyan N, Golestan B, Rad AH (2009) Efficacy of folate and vitamin B12 in lowering homocysteine concentrations in hemodialysis patients. Saudi J Kidney Dis Trans 20(5):779–788
Loehrer FM, Haefeli WE, Angst CP et al (1996) Effect of methionine loading on 5 methyltetrahydrofolate, S-adenosylmethionine and Sadenosyl homocysteine in plasma of healthy humans. Clin Sci (Colch) 91:79–86
Mansoor MA, Kristensen O, Hervig T et al (1997) Low concentrations of folate in serum and erythrocytes of smokers. Clin Chem 43:2193–2194
Verhaar MC, Wever RM, Kastelein JJ, van Dam T, Koomans HA, Rabelink TJ (1998) 5-methyltetrahydrofolate, the active form of folic acid, restores endothelial function in familial hypercholesterolemia. Circulation 97(3):237–241
Perna AF, D’Aniello A, Lowenson JD, Clarke S, De Santo NG, Ingrosso D (1997) D-aspartate content of erythrocyte membrane proteins is decreased in uremia: implications for the repair of damaged proteins. J Am Soc Nephrol 8:95–104
Pietrzik K, Bailey L, Shane B (2010) Folic acid and l-5-Methyltetrahydrofolate. Clin Pharmacokinet 49(8):535–548
Litynski P, Loehrer F, Linder L et al (2002) Effect of low dose of 5-methyltetrahydrofolate and folic acid on plasma homocysteine in healthy subjects with or without the 677C-T polymorphism of methylenetetrahydrofolate reductase. Eur J Clin Invest 32:662–668
Fohr IP, Prinz-Langenohl R, Bronstrup A et al (2002) 5,10-Methylenetetrahydrofolate reductase genotype determines the plasma homocysteine-lowering effect of supplementation with 5-methyltetrahydrofolate or folic acid in healthy young women. Am J Clin Nutr 75(2):275–282
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the Hospital Medical Ethics Committee.
Informed consent
All patients involved in the study provided their consent.
Rights and permissions
About this article
Cite this article
Achour, O., Elmtaoua, S., Zellama, D. et al. The C677T MTHFR genotypes influence the efficacy of B9 and B12 vitamins supplementation to lowering plasma total homocysteine in hemodialysis. J Nephrol 29, 691–698 (2016). https://doi.org/10.1007/s40620-015-0235-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-015-0235-8