Abstract
Background
Cohort studies evaluating increased serum uric acid (SUA) level as a chronic kidney disease (CKD) risk factor have yielded variable results. We aimed to assess the association between the pattern of longitudinal changes in SUA and incident CKD [estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2].
Methods
A population-based cohort study was conducted on 3,605 participants who were followed prospectively for a mean of 5.18 years. The longitudinal changes in SUA were categorized into three subgroups: persistently low, fluctuated (reduced or elevated), and persistently high. The primary outcome of interest was the development of CKD at a follow-up examination. Cox proportional hazards analysis was used to test the hypothesis.
Results
After adjustment for potential confounders, participants with fluctuated SUA with progressively elevated level and persistently high SUA level had significantly higher risk of developing CKD compared to subjects with persistently low SUA level: adjusted hazard ratio (95 % confidence interval) was 2.05 (1.24–3.38) vs. 1.90 (1.34–2.71). This longitudinal relationship was independent of sex, age, body mass index, and hypertension status.
Conclusions
Longitudinally elevated SUA independently predicts the risk of new-onset CKD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The worldwide rise in the number of patients affected by chronic kidney disease (CKD) together with the progressive nature of chronic kidney failure and ensuing end-stage renal disease (ESRD) is placing a substantial burden on global health care resources [1]. Taiwan has been recognized as an epidemic area of CKD with the highest incidence and prevalence rates of ESRD [2]. Thus, it is extremely important to identify individuals who are at risk for CKD.
Serum uric acid (SUA) level is commonly elevated in subjects with CKD, but was historically viewed as an issue of limited interest [3]. Recently, uric acid has been resurrected as a potential contributory risk factor in the development and progression of CKD [4]. However, cohort studies in humans have shown mixed results as to whether uric acid is an independent risk factor for the development of CKD [5–14]. Critically, epidemiological reports examining uric acid as a risk factor have used single time point measurements and therefore ignored physiological and pathological changes over time. In this report, we investigate the effect of longitudinal changes in SUA level on the development of incident CKD through a prospective cohort study with repeated measurements of SUA level over time.
Patients and methods
Study population
Data for this study came from the Taiwanese Survey on Prevalence of Hypertension, Hyperglycemia, and Hyperlipidemia (TwSHHH) conducted in 2002 and a subsequent follow-up program (TwSHHH-II) carried out in 2007. The study design and subject recruitment have been described elsewhere [15]. Participants in the TwSHHH were drawn from the National Health Interview Survey (NHIS). Individuals selected for the NHIS were based on a multistage stratified systematic sampling scheme with the method of probability proportional to size. In total, 10,292 individuals were randomly selected for the TwSHHH. Of these, 7,578 (73.6 %) completed a questionnaire and 6,600 (64.1 %) permitted additional blood pressure measurement and blood examination for biomarkers. A subsequent follow-up study, TwSHHH-II, was initiated in 2007 to estimate the incidence of hypertension, hyperglycemia, and hyperlipidemia for people in Taiwan. A total of 6,600 individuals who completed all examinations in the TwSHHH were eligible for recruitment in the TwSHHH-II. Among them, 242 subjects had died and 581 persons could not be contacted. The remaining 5,777 individuals were invited to participate in TwSHHH-II. A total of 1,095 subjects refused to take part in the study, resulting in 4,682 persons enrolled in the TwSHHH-II (a response rate of 70.9 %). The TwSHHH and TwSHHH-II studies were approved by the Institutional Review Board Ethics Committee from the Bureau of Health Promotion, Department of Health. Written informed consent was also obtained from all participants in TwSHHH and TwSHHH-II. Both TwSHHH and TwSHHH-II studies were in adherence with the Declaration of Helsinki.
Basic measurements
At study entry in the TwSHHH and TwSHHH-II, participants underwent questionnaire interviews and anthropometric measurements by well-trained nurses under a standardized protocol. Body mass index (BMI) was calculated as weight divided by height squared (kg/m2). Arterial blood pressure was also taken from each participant using an electric sphygmomanometer (BP3AC1-1, Microlife Corporation, Berneck, Switzerland).
Participants were instructed to fast for at least 12 h before the blood was drawn. NaF plasma was collected for glucose analysis, and serum was collected for measurement of lipids. The blood samples were centrifuged immediately and were transported at −10 °C in dry ice to the central research laboratory at the Chiu Clinic in Taipei City, stored at −20 °C and analyzed within 2 weeks. In addition, a spot urine sample was collected for evaluation of proteinuria. In this study, proteinuria was captured by urine dipstick measurement and defined as normal (urine dipstick reading negative), mild (urine dipstick reading trace or 1+), or heavy (urine dipstick reading ≥2+) [16]. Additionally, standard enzymatic methods were used to determine serum levels of total cholesterol and triglycerides. Electrophoresis was performed to measure high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C). Fasting plasma glucose (FPG) level was measured by the hexokinase glucose-6-phosphate dehydrogenase procedure. SUA level was assayed using the colorimetric uricase-peroxidase principle. Serum creatinine was measured enzymatically (VITROS® 5, 1 FS, Ortho-Clinical Diagnostics Inc., Rochester, NY, USA). The calibrator value of the enzymatic creatinine method was traceable to isotope dilution mass spectrometry by the two-level reference materials NIST (National Institute of Standards and Technology) SRM 914. Blind duplicate coefficients of variation of these measurements were approximately 5 %. All biochemical tests were performed using automatic analyzers (TBA-200FR, Toshiba Corporation, Tokyo, Japan).
Definitions of CKD and covariates
In this study, the estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) Study equation [17]. When serum creatinine (Scr) is expressed in mg/dl and age in years, the CKD-EPI equation for eGFR = 141 × min (Scr/k, 1)α × max (Scr/k, 1)−1.209 × 0.993age × 1.018 (if female), where k is 0.7 for females and 0.9 for males, α is −0.329 for females and −0.411 for males, min indicates minimum of Scr/k or 1, and max indicates maximum of Scr/k or 1. In addition, proteinuria is the cardinal sign of kidney disease [16]. Accordingly, a CKD event was defined as a reduced eGFR (<60 ml/min per 1.73 m2) [17] or proteinuria with urine dipstick reading ≥2+ [16].
In this study, hyperuricemia was defined as a SUA concentration ≥7.0 mg/dl for men and ≥6.0 mg/dl for women [18]. The SUA levels measured at study entry in 2002 and at a follow-up examination in 2007 were combined to define the pattern of longitudinal changes in SUA level. SUA concentration <7.0 mg/dl for men and <6.0 mg/dl for women both at study entry (2002) and follow-up examination (2007) was categorized as persistently low SUA. SUA concentration ≥7.0 mg/dl for men and ≥6.0 mg/dl for women both at study entry and follow-up examination was categorized as persistently high SUA. SUA measured at the aforementioned two time points was categorized as fluctuated if SUA concentrations exhibited a progression or a regression trend. The group with fluctuated SUA was further divided into two subgroups: reduced (a decrease in SUA concentration from ≥7.0 to <7.0 mg/dl for men and from ≥6.0 to <6.0 mg/dl for women) and elevated (an increase in SUA level from <7.0 to ≥7.0 mg/dl for men and from <6.0 to ≥6.0 mg/dl for women). Additionally, hypertension was defined as systolic blood pressure (SBP) of at least 140 mmHg, diastolic blood pressure (DBP) of at least 90 mmHg, or self-reported treatment for hypertension [19]. General obesity was defined as BMI ≥27.0 kg/m2, which corresponds to a similar degree of fat composition to whites with a BMI ≥30.0 kg/m2 [20].
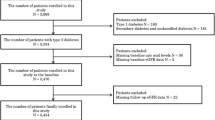
For study purposes, participants with a self-reported history of CKD (n = 133), an eGFR <60 ml/min per 1.73 m2 as measured at baseline enrollment in 2002 (n = 146), without data on blood pressure, BMI, eGFR, serum triglycerides, or FPG measurements at baseline recruitment in 2002 (n = 339), and without data on eGFR at follow-up examination in 2007 (n = 360) were excluded from data analysis. Given that uric acid-lowering medications used to treat gout such as allopurinol may affect the association observed between SUA and CKD, we excluded subjects who were taking uric acid-lowering medications (n = 99). The final analytic sample included 3,605 participants [mean ± standard deviation (SD) age, 39.52 ± 14.63 years; males = 45.6 %].
Statistical methods
In this study, the primary outcome measure was CKD incidence. The person-years of follow-up used in calculating incidence rate went from the date of enrollment in TwSHHH to the date of CKD diagnosis, death, or the end of 2007, whichever came earlier. The Cox proportional hazards model was used to analyze the association between the pattern of longitudinal changes in SUA level and incident CKD adjusted for potential confounding covariates. The potential confounding variables included in the multivariable model were sex, age, hypertension status, BMI, total cholesterol, triglycerides, FPG, and eGFR, as measured at baseline enrollment in 2002. It is known that the use of diuretics has an increasing effect on SUA levels in hypertensive patients [21]. There were 53 out of 509 hypertensive patients taking diuretic medications in our study sample. Thus, the confounding effect of diuretics would be controlled for after adjustment for hypertension status in the multivariable analysis. The association of the pattern of longitudinal changes in SUA with the risk of CKD was further evaluated using analyses stratified by several CKD risk factors, including sex, age group (divided by the median value: 39 years of age), general obesity status, and hypertension status. All statistical analyses were performed with SAS version 9.2 (SAS Institute, Cary, NC, USA), and all of the statistical tests were 2-tailed with an α level of 0.05.
Results
The distribution of the pattern of longitudinal changes in SUA among 3,605 participants was as follows: 55.8 % of subjects had persistently low SUA, 30.9 % had fluctuated SUA (27.7 % were reduced and 3.2 % were elevated), and 13.3 % had persistently high SUA. During the follow-up interval of 18,689 person-years, there were 233 incident cases of CKD. Among patients with CKD, 39.1 % (91/233) had proteinuria. Overall, the incidence rate of CKD among participants was 12.47 per 1,000 person-years.
Baseline characteristics of participants stratified by the pattern of longitudinal changes in SUA are shown in Table 1. Longitudinally elevated SUA, including fluctuated SUA with progressively elevated level and persistently high SUA, was significantly associated with increasing levels of age, BMI, SBP, DBP, serum levels of total cholesterol, LDL-C, triglycerides, and FPG. Longitudinally elevated SUA was also significantly associated with a high proportion of male gender and hypertension. However, longitudinally elevated SUA was significantly associated with reduced levels of HDL-C and eGFR.
Association of the pattern of longitudinal changes in SUA and risk of CKD incidence is presented in Table 2. After adjustment for potential confounders, participants with fluctuated SUA with progressively elevated level and persistently high SUA had a significantly higher risk of developing CKD when compared with subjects with persistently low SUA: adjusted hazard ratio (HR) [95 % confidence interval (CI)] was 2.05 (1.24–3.38) and 1.90 (1.34–2.71), respectively.
We then performed stratified analyses of the association between the pattern of longitudinal changes in SUA and the risk of incident CKD according to several risk factors of CKD (Table 3). When we calculated the HRs for incident CKD by stratifying subjects according to selected risk factors of CKD, the HRs associated with longitudinally elevated SUA were consistently significant.
Discussion
In this study, we have demonstrated that longitudinally elevated SUA was a significant risk predictor for incident CKD in a Chinese population over a lengthy follow-up period. In fact, several [6–12], but not all [5, 13, 14], epidemiological studies have suggested a significant association of hyperuricemia with the risk of CKD. In this study, longitudinally elevated SUA was revealed as an important independent risk factor for new-onset kidney disease. Of note, this association seems to be independent of a broad number of risk factors such as sex, age, hypertension, and obesity. Hyperuricemia may be associated with CKD through several mechanisms. Elevated uric acid may be directly toxic to the kidney [22], or hyperuricemia may exacerbate other risk factors for CKD, specifically hypertension [12]. Indeed, the best way to evaluate the role of uric acid in the pathogenesis of CKD is to determine whether lowering uric acid slows renal progression. At least one recent trial involving a small number of patients did just that [23]. Further clinical trials of uric acid lowering are needed to evaluate the causal influence of uric acid on the risk of CKD.
The strengths and limitations of our study deserve comment. The present study is a large representative population-based cohort study with repeated measurements of SUA. Thus, this study provides sufficient statistical power to answer our research question and could be generalized to the general population. In terms of limitations of this study, the outcome measure of CKD was based on the CKD-EPI Study equation calculated at follow-up examination. Accordingly, the current study cannot define serum creatinine elevation or eGFR decline due to acute renal failure or progression of CKD.
In conclusion, this cohort study supports longitudinally elevated SUA as a significant risk predictor for incident CKD.
References
El Nahas AM, Bello AK (2005) Chronic kidney disease: the global challenge. Lancet 365:331–340
Kuo HW, Tsai SS, Tiao MM, Yang CY (2007) Epidemiological features of CKD in Taiwan. Am J Kidney Dis 49:46–55
Kang DH, Chen W (2011) Uric acid and chronic kidney disease: new understanding of an old problem. Semin Nephrol 31:447–452
Johnson RJ, Nakaqawa T, Jalal D, Sanchez-Lozada LG, Kang DH, Ritz E (2013) Uric acid and chronic kidney disease: which is chasing which? Nephrol Dial Transplant 28:2221–2228
Hunsicker LG, Adler S, Caggiula A et al (1997) Predictors of the progression of renal disease in the Modification of Diet in Renal Disease Study. Kidney Int 51:1908–1919
Chonchol M, Shlipak MG, Katz R et al (2007) Relationship of uric acid with progression of kidney disease. Am J Kidney Dis 50:239–247
Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS (2008) Uric acid and incident kidney disease in the community. J Am Soc Nephrol 19:1204–1211
Obermayr RP, Temml C, Gutjahr G, Knechtelsdorfer M, Oberbauer R, Klauser-Braun R (2008) Elevated uric acid increases the risk for kidney disease. J Am Soc Nephrol 19:2407–2413
Yamada T, Fukatsu M, Suzuki S, Wada T, Joh T (2011) Elevated serum uric acid predicts chronic kidney disease. Am J Med Sci 342:461–466
Wang S, Shu Z, Tao Q, Yu C, Zhan S, Li L (2011) Uric acid and incident chronic kidney disease in a large health check-up population in Taiwan. Nephrology 16:767–776
Mok Y, Lee SJ, Kim MS, Cui W, Moon YM, Jee SH (2012) Serum uric acid and chronic kidney disease: the Severance cohort study. Nephrol Dial Transplant 27:1831–1835
Sedaghat S, Hoorn EJ, van Rooij FJA et al (2013) Serum uric acid and chronic kidney disease: the role of hypertension. PLoS ONE 8:1–8
Sturm G, Kollerits B, Neyer U, Ritz E, Kronenberg F (2008) MMKD Study Group. Uric acid as a risk factor for progression of non-diabetic chronic kidney disease? The Mild to Moderate Kidney Disease (MMKD) Study. Exp Gerontol 43:347–352
See LC, Kuo CF, Chuang FH et al (2008) Serum uric acid is independently associated with metabolic syndrome in subjects with and without a low estimated glomerular filtration rate. J Rheumatol 36:1691–1698
Yang T, Chu CH, Bai CH et al (2012) Uric acid level as a risk marker for metabolic syndrome: a Chinese cohort study. Atherosclerosis 220:525–531
Lamb EJ, MacKenzie F, Stevens PE (2009) How should proteinuria be detected and measured? Ann Clin Biochem 46:205–217
Levey AS, Stevens LA, Schmid CH et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612
Chuang SY, Chen JH, Yeh WT, Wu CC, Pan WH (2012) Hyperuricemia and increased risk of ischemic heart disease in a large Chinese cohort. Int J Cardiol 154:316–321
Chobanian AV, Bakris GL, Black HR et al (2003) The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 289:2560–2571
Deurenberg P, Deurenberg-Yap M, Guricci S (2002) Asians are different from Caucasians and from each other in their body mass index/body fat percent relationship. Obes Rev 3:141–146
Young JH, Alonso A, Coresh J (2012) Diuretic use, increased serum urate levels, and risk of incident gout in a population-based study of adults with hypertension: the Atherosclerosis Risk in Communities Cohort Study. Arthritis Rheum 64:121–129
Kang DH, Nakaqawa T, Feng L et al (2002) A role for uric acid in the progression of renal disease. J Am Soc Nephrol 13:2888–2897
Siu YP, Leung KT, Tong MK, Kwan TH (2006) Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis 47:51–59
Acknowledgments
This study was supported by a grant from the Bureau of Health Promotion, Department of Health (DOH95-HP-2103), Executive Yuan, Taiwan, Republic of China.
Conflict of interest
The authors stated that they have no conflicts of interest regarding the publication of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chou, YC., Kuan, JC., Yang, T. et al. Elevated uric acid level as a significant predictor of chronic kidney disease: a cohort study with repeated measurements. J Nephrol 28, 457–462 (2015). https://doi.org/10.1007/s40620-014-0158-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-014-0158-9