Abstract
Background
Recent advances in neuroscience tools for single-cell molecular profiling of brain neurons have revealed an enormous spectrum of neuronal subpopulations within the neuroendocrine hypothalamus, highlighting the remarkable molecular and cellular heterogeneity of this brain area.
Rationale
Neuronal diversity in the hypothalamus reflects the high functional plasticity of this brain area, where multiple neuronal populations flexibly integrate a variety of physiological outputs, including energy balance, stress and fertility, through crosstalk mechanisms with peripheral hormones. Intrinsic functional heterogeneity is also observed within classically ‘defined’ subpopulations of neuroendocrine neurons, including subtypes with distinct neurochemical signatures, spatial organisation and responsiveness to hormonal cues.
Aim
The aim of this review is to critically evaluate past and current research on the functional diversity of hypothalamic neuroendocrine neurons and their plasticity. It focuses on how this neuronal plasticity in this brain area relates to metabolic control, feeding regulation and interactions with stress and fertility-related neural circuits.
Conclusion
Our analysis provides an original framework for improving our understanding of the hypothalamic regulation of hormone function and the development of neuroendocrine diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The hypothalamus, with its intricate bidirectional communication between neurons and hormones, holds a central position in regulating a multitude of physiological outputs and behaviours essential for survival. These encompass vital functions such as food and water consumption [1, 2], energy balance and thermal regulation [3, 4], osmotic balance [5], circadian rhythm [6], reproduction [7, 8], stress response [9, 10], and goal-oriented behaviours [11, 12]. The orchestration of multiple outputs requires considerable functional adaptability, which is reflected in the organization of endocrine neurons into multiple subpopulations. Traditionally, subpopulations of hypothalamic neuroendocrine neurons have been classified into functionally homogeneous clusters based on their molecular, topological and electrophysiological properties. However, this conventional view is now being challenged. The advancement of the single-cell molecular profiling technique has revealed the presence of sub-clusters in each neuronal subpopulation that may be involved in distinct physiological roles and modes of action, unravelling a more intricate and subtle working model [13,14,15,16,17,18]. The identification of new molecular markers within ‘defined’ neuroendocrine subpopulation and the development of genetically engineered mouse models that allow precise manipulation of these subpopulations using chemogenetic and optogenetic techniques have led to the emergence of new perspectives on the functional heterogeneity of the hypothalamus.
Amidst this progress, several critical questions emerge: Does heterogeneity among endocrine neuronal populations remain static throughout an organism's lifespan? Can physiological and environmental stimuli dynamically influence hormonal responses by modifying the heterogeneity of endocrine neurons? How does neuronal heterogeneity influence the onset and progression of neuroendocrine diseases? In this review, we address these central questions by discussing emerging models of hypothalamic neuroendocrine neuron heterogeneity. We will review potential molecular and cellular mechanisms underlying the functional heterogeneity and adaptability of hypothalamic neurons involved in hormonal regulation. We will also investigate the impact of environmental and internal cues on shaping neuronal diversity and adaptability in the hypothalamus across the lifespan, and explore the implications for understanding the aetiology of neuroendocrine diseases such as obesity and infertility disorders.
Current and evolving models of neuronal heterogeneity in the neuroendocrine hypothalamus
Neurochemical heterogeneity
Current knowledge suggests that endocrine neurons in the hypothalamus can be divided into functionally distinct populations based on the expression of specific neuropeptidergic markers and peptide hormones that regulate specific endocrine functions. For example, in the arcuate nucleus (ARC) of the hypothalamus, proopiomelanocortin (POMC)-expressing neurons are characterised by the expression of the neuropeptidergic precursor POMC, which undergoes post-translational cleavage to produce various biologically active neuropeptides, such as α-melanocyte stimulating hormone (α-MSH), which suppress appetite in response to peripheral hormonal actions [19]. When POMC neurons are activated by an interoceptive stimulus, such as in response to changes in peripheral hormones, the resulting release of α-MSH from the axon terminals activates melanocortin-4 receptors (MC4R) on target secondary order neurons to suppress food intake and increase energy expenditure [20]. This central regulatory mechanism involves MC4Rs expressed in the paraventricular nucleus (PVN) or the dorsomedial hypothalamus (DMH) of the hypothalamus, thereby forming a crucial circuit that dynamically regulates feeding behaviour and metabolism in response to changes in the body energy status, commonly known as the central melanocortin circuit [19, 21].
The melanocortin circuit is co-regulated by a second key neuronal population defined by the co-expression of the neuropeptide agouti-related protein (AgRP) and neuropeptide Y (NPY). Unlike POMC neurons, which are typically considered excitatory, AgRP neurons are inhibitory in nature. They release AgRP, which is an endogenous antagonist of MC4R [22]. AgRP counteracts the effects of α-MSH by competing for MC4R binding; the inhibition of MC4R subsequently increases appetite and reduces energy expenditure. AgRP/NPY neurons exert their influence not only by antagonising POMC neurons at their target sites, where MC4Rs are located, but also by directly inhibiting POMC perikaryal [23]. This direct inhibition involves a 36 amino-acid neuropeptide, NPY [23,24,25], which suppresses POMC neuronal activity whenever AgRP/NPY neurons are active [23].
POMC neurons are typically inhibited under conditions of low energy availability, whereas they are activated by hormonal and nutrient-related signals indicating a surplus of body energy reserve. Activation of POMC neurons in response to energy supply, such as after a meal, is necessary to maintain a stable body weight by reducing food intake and increasing energy expenditure [26, 27]. Conversely, when energy reserves in the body are low during fasting, AgRP/NPY neurons are activated to stimulate hunger and reduce energy expenditure, thereby promoting energy repletion via both POMC neuron-dependent and independent mechanisms.
Thus, the melanocortin circuit is adept at responding to continuous changes in peripheral endocrine signals that inform the brain of changes in the whole-body energy status. Among these peripheral signals, the orexigenic hormone ghrelin is known to induce hunger by activating AgRP/NPY neurons, whereas anorexigenic endocrine signals such as insulin and leptin act in an opposite manner by inhibiting AgRP/NPY neurons while activating POMC neurons [26,27,28] respectively. The neuroendocrine significance of this feedback loop is evidenced when it fails to respond properly to anorexigenic metabolic hormones, leading to detrimental metabolic consequences. Insulin and leptin receptors are expressed in POMC and AgRP/NPY neurons [28], and disruptions in these hormonal signals pave the way to metabolic disorders. Nevertheless, despite acting on the same receptor, the mechanisms by which the same hormone, such as leptin, can exert contrasting neurobiological effects on POMC and AgRP neuronal populations remain a subject of intense research and never-ending debate, as extensively discussed in previous work [29,30,31,32]. Regardless, the heterogeneous response of different neurochemically defined neuronal populations to a specific hormonal signal is likely to be a general critical feature for normal hypothalamic function, allowing rapid and accurate monitoring of metabolic status and subsequent energetic adjustments.
A similar neuropeptidergic model can be used to explain how the hypothalamus orchestrates other neuroendocrine axes, such as stress and fertility, by ‘defined’ populations of neurons that act via the release of ‘defined’ sets of neuropeptide hormones. To regulate reproductive cycles, kisspeptin (Kiss1)-expressing neurons in the anteroventral periventricular nucleus (AVPV) influence the release of pituitary and gonadal hormones to modulate the hypothalamic-pituitary–gonadal (HPG) axis [33, 34]. During pubertal development in female mice, kisspeptin-immunoreactive fibres from the AVPV innervate gonadotropin-releasing hormone (GnRH) neurons in the organum vasculosum laminae terminalis (OV) and the median preoptic nucleus (MePO) around postnatal day 25 (P25) [35]. These AVPV fibres project directly to GnRH neurons in the OV and MePO [36], which express the kisspeptin receptor [37]. The preovulatory surge of oestradiol in the ovary triggers AVPV Kiss1 neurons to stimulate GnRH release [38], subsequently leading to a rise in luteinising hormone (LH) and follicle-stimulating hormone (FSH). This hormonal cascade plays a crucial role in guiding the onset of puberty during sexual maturation [39].
Following the same model, when eliciting fight-or-flight behaviour in response to stressors, a population of neurons in the PVN plays a central role in modulating behavioural and metabolic adaptation to cope with the cue through the release of corticotropin-releasing hormone (CRH) [40]. In response to environmental stressors. PVN CRH neurons are activated and rapidly release CRH into the pituitary portal circulation, followed by activation of the hypothalamic–pituitary–adrenal (HPA) axis. At the same time, stress-induced glucocorticoid secretion from the adrenal glands provides negative feedback to override the HPA axis and prevent system overactivation. The regulation of CRH release from PVN neurons is complex and involves multiple neurotransmitters, hormones and feedback mechanisms. Interoceptive signals such as glucocorticoids (including cortisol), vasopressin and neurotransmitters, such as serotonin and norepinephrine can modulate CRH release. In addition, feedback loops involving cortisol and other components of the HPA axis help to regulate CRH secretion to maintain neuroendocrine homeostasis [41].
These examples provide just a glimpse of the intricate mechanisms by which defined populations control specific neuroendocrine outputs. However, the complexity within neuronal populations extends far beyond neuropeptidergic control. In addition to neuropeptides, different neuronal populations exert their influence through a variety of downstream secreted factors, including fast acting neurotransmitters. These multiple interactions play a crucial role in shaping the plasticity during neurotransmission, highlighting the rich complexity of neural activity regulation within the neuroendocrine hypothalamus. As recently demonstrated by single-cell RNA sequencing data, neurochemically defined populations in the hypothalamus can express a repertoire of different neurotransmitters, including glutamate, gamma-aminobutyric acid (GABA) or both [42]. Glutamatergic signalling in the brain is recognised for its capacity to elicit excitatory responses, whereas GABAergic neurons exert inhibitory effects. Thus, despite expressing the same neuropeptidergic markers, different neurons in each ‘defined’ population may act dichotomously to modulate the excitatory-inhibitory balance of postsynaptic neurons, potentially displaying intrinsic heterogeneity. Accordingly, neuropeptides and neurotransmitters do not operate in isolation, as neuropeptides are involved in fine-tuning neurochemical transmission to elicit rapid responses while adjusting signal strength. POMC neurons, for example, are classically thought to induce satiety once activated by promoting fast-acting glutamatergic transmission to satiety-supportive neurons in the PVN [43]. However, accumulating evidence suggests that POMC neurons can be divided into GABA and glutamatergic subtypes [44,45,46,47,48,49], and that the two distinct subpopulations may play different roles in food intake regulation. Mice with a Pomc gene loss-of-function mutation typically develop obesity. When the Pomc gene is selectively re-expressed during postnatal life specifically in GABAergic POMC neurons, food intake is reduced, effectively reversing the obese phenotype [50]. This suggests that GABAergic POMC neurons may act through yet-to-be-defined mechanisms to promote food intake and weight gain, thus challenging the classical paradigm in which POMC neurons consistently act as satiety signals. Recent findings align with this notion, demonstrating that pharmacological inhibition of the mechanistic target of the rapamycin (mTOR) pathway in mice activates GABAergic POMC neurons and induces hyperphagia by mimicking low cellular energy levels of POMC neurons [44].
The presence of highly plastic neurobiological pathways connecting neuropeptides and fast-acting neurotransmitters extends beyond ARC neuronal populations. Orexin neurons (hypocretin neurons) are located primarily in the lateral hypothalamus and adjacent areas [51]. They are named after the neuropeptides they produce, orexin-A (hypocretin-1) and orexin-B (hypocretin-2), which play crucial roles in regulating various physiological processes, including sleep–wake cycles, energy homeostasis, reward processing, and arousal [52]. Synaptic transmission in orexin neurons involves a complex interplay of neurotransmitters, receptors and neural circuits. Glutamate is the primary excitatory neurotransmitter in synaptic transmission in orexin neurons [53, 54]. Glutamatergic inputs from other brain regions, such as the locus coeruleus and the basal forebrain, can excite orexin neurons and promote their firing activity [55]. This excitatory input is crucial for regulating arousal and wakefulness, as orexin neurons are most active during wakefulness and play a key role in promoting arousal and maintaining wakefulness. Notably, the activity of orexin neurons is also modulated by several neuromodulators, including dopamine [56], serotonin [57], and acetylcholine [58], which affect the excitability and synaptic transmission of orexin neurons, further regulating their activity and function. Thus, overall synaptic transmission in orexin neurons is finely tuned by a balance of excitatory and inhibitory inputs from different brain regions and neurotransmitter systems, which ultimately influence neuropeptide release and downstream actions.
In summary, the coexistence of glutamatergic and GABAergic subtypes within populations traditionally considered homogeneous functional units in the neuroendocrine hypothalamus, alongside various extracellular signals modulating neurotransmission, underscores the necessity for developing more comprehensive models of functional heterogeneity of the neuroendocrine hypothalamus. Novel conceptual frameworks are essential to elucidate how the neurochemical diversity of hypothalamic neuronal populations translates into specific functional properties, going beyond the conventional 'one population, one neuropeptide, one physiological function' paradigm.
Spatial heterogeneity of the neuroendocrine circuits in the hypothalamus
Early electrophysiological and histological studies have provided preliminary evidence that the anatomical position of a hypothalamic neuron population expressing the same peptidergic marker may determine their responses to circulating hormones [59, 60]. For example, leptin and glucagon-like peptide-1 (GLP1), which are involved in regulating glucose metabolism and stimulating satiety via POMC neurons expressing the leptin receptor (Lepr) and glucagon-like peptide-1 receptor (Glp1r), may regulate different subpopulations of POMC neurons localised in specific spatial locations Most Lepr-expressing POMC neurons are located in the anterior division of ARC, while Glp1r-expressing neurons are, to a larger extent, located in the posterior ARC [61]. Leptin is regarded as a regulator that coordinates long-term energy balance [62], whereas GLP1 exerts an acute effect on promoting satiety and suppressing food intake [63, 64]. During the progression of obesity, obese patients develop resistance towards these hormones, which exert opposing effects on POMC neurons. Leptin activates POMC neurons to promote glucose metabolism [65], while insulin can suppress hepatic gluconeogenesis by inhibiting POMC neuron activity [66]. In response to acute insulin or leptin application, leptin-excited POMC neurons are mainly located in the lateral division of the retrochiasmatic area (RCA) and medial division of the ARC. In contrast, insulin-inhibited POMC neurons are primarily localised to the medial RCA and anterior ARC, which agrees with the observed insulin receptor distribution [59]. POMC neurons in the anterior ARC project primarily to autonomic areas [67, 68], and those in the medial posterior ARC project mainly to hypothalamic nuclei [69, 70]. Thus, certain subpopulations of POMC neurons may be more or less sensitive to certain hormones, depending on their spatial organisation, thereby fine-tuning different types of downstream brain circuits.
The physiological purpose underlying the spatial arrangement of various neuroendocrine subpopulations in the hypothalamus remains enigmatic. Specific neuronal subtypes may need strategic positioning to receive and transmit specific signals from the peripheral bloodstream. Neurons in the ARC are located in close proximity to the third ventricle (3V) and the median eminence (ME), which is a region more exposed to nutrients and signalling molecules release due to the presence of fenestrated blood vessels [71] and the tanycytic barrier [72, 73]. In addition, the organisation of the extracellular matrix (ECM) in the ARC-ME region may affect the diffusion of hormones and nutrients [74, 75]. The molecular composition, pore size, and electrical charge properties of ECM influence the molecular diffusivity [76, 77]. ARC GABAergic neurons in the vicinity of ME are enveloped by a condensed form of ECM composed mainly of hyaluronan and chondroitin sulphate proteoglycans, which are known as the perineuronal net (PNN) [78]. The PNN wraps the somata of ARC GABAergic neurons but leaves space for the access of specific afferent synaptic boutons. While the functions of PNNs are still being debated in the scientific community, they are thought to play a crucial role in regulating synaptic transmission and plasticity in the brain and the proper development of neural circuits [79,80,81,82]. This includes the maturation of ARC neurons, possibly via mechanisms controlled by the metabolic hormone leptin [78]. Of note, PNNs may also serve as physical tunnels or scaffolds that facilitate the transport of hormones and nutrients.
PNNs show a heterogeneous organisation within the ARC. Approximately 80% of NPY/AGRP neurons are surrounded by PNNs, whereas only about 50% of POMC neurons are surrounded by this specific extracellular matrix [78]. This suggests that functional differences between and within populations may be influenced by the properties of the extracellular matrix, thereby affecting sensitivity to extracellular homeostatic signals. The heterogeneous distribution of PNNs around ARC neurons, located in a brain region with a permeable blood–brain barrier, supports the hypothesis that specialised mechanisms that regulate the flow of signalling molecules from the bloodstream to specific spatial locations within the brain are crucial for synaptic plasticity and neuroendocrine responses.
The spatial distribution of endocrine neurons may not only be related to the purpose of communicating with blood-borne hormonal signals. Oxytocin (OXT) neurons in PVN are located where there is a lack of contact with fenestrated blood vessels. These neurons are classified into two distinct subpopulations, including magnocellular neurons, characterised by larger somata, and parvocellular neurons, with smaller somata, each displaying unique dendritic structures and electrophysiological properties [83]. While magnocellular and parvocellular OXT neurons regulate social and emotional behaviours and physiological responses such as uterine contractions and milk ejection [84], the two populations are localised in different spatial positions in PVN [83]. Magnocellular neurons primarily reside in the anterior PVN, facilitating an efficient release of signals into the bloodstream to regulate peripheral physiological functions. Conversely, parvocellular neurons in the posterior PVN are strategically positioned to modulate central neural circuits involved in social and emotional behaviours [85]. A subset of approximately 30 parvocellular OXT neurons has been identified that have collateral projections to magnocellular OXT neurons and deep spinal cord neurons [86]. This subset has been implicated in regulating nociception and analgesia in an animal model of inflammatory pain, further demonstrating how intrinsic differences in the spatial organisation of molecularly ‘defined’ populations can lead to different physiological outcomes [86]. Thus, the inherent spatial diversity within neuropeptidergic populations is indispensable for orchestrating changes in peripheral hormonal responses alongside the modulation of secondary-order brain neurons responsible for processing behavioural and metabolic responses, among other functions.
Sex dimorphism of the neuroendocrine circuits in the hypothalamus
Sex hormones, such as testosterone and oestrogen, play a pivotal role in shaping sex-specific neural circuits in the hypothalamus during development [87], influencing sexual differentiation [88].
In adult rats, Kiss1 neurons in the AVPV are sexually dimorphic, with females having more Kiss1-expressing cells than males [89]. This sexual dimorphism of the AVPV Kiss1 circuit is influenced by the hormonal environment, independent of neurogenesis [90], during the postnatal period. The AVPV neuronal population is susceptible to testosterone or its aromatised metabolite oestradiol during perinatal development [89, 91, 92]. During P7-10 days, male neonates produce testosterone, while females produce negligible levels of sex steroids [93]. The testosterone surge during this critical period potentially triggers cell death of AVPV Kiss1 neurons in male neonates [94], and the apoptotic event can be prevented by gonadectomy [92]. A similar phenomenon can be recapitulated in female neonates upon exogenous testosterone administration [89, 92, 95]. Without perinatal testosterone surge in female mice and rats, Kiss1 mRNA transcript and protein expression become detectable between P10 and P15 [96, 97]. From P15 to adulthood, AVPV Kiss1 expression increases steadily [96], while AVPV Kiss1 fibres innervate GnRH neurons in female mice at P25 [35]. Ovariectomy at P15 significantly reduces AVPV Kiss1 levels in adult females [96], indicating the essential role of ovarian sex hormone secretion in the maturation of the AVPV Kiss1 circuit, which can be restored by oestrogen replacement [96]. Other studies demonstrate the involvement of oestrogen receptor alpha (ERα). Furthermore, blunting oestrogen signalling by ERα deletion in Kiss1 neurons diminishes AVPV Kiss1 expression in adult females [98], confirming the involvement of oestrogen signalling in the postnatal development of the AVPV Kiss1 circuit. It is currently uncertain why males require fewer AVPV Kiss1 neurons than females, probably due to the absence of ovulation in males [8].
Other than the AVPV kisspeptin circuits, gonadal hormones regulate the sexual dimorphism of reprimo (Rprm), tachykinin 1 (Tac1), and prodynorphin (Pdyn) neuronal subpopulations in the ventral medial hypothalamus (VMH) via oestrogen signalling [99]. These differences contribute to the modulation of thermogenesis [99], physical activity [100], and potentially reproductive behaviours in a sex-specific manner. Female VMH is enriched by Rprm and Tac1-positive neurons, whereas male VMH is enriched by Pdyn-positive neurons. Rprm and Tac1 clusters are eliminated in males, and the Pdyn cluster is eliminated in females and is regulated by gonadal hormones but not sex-linked chromosomes [99]. In females, Rprm gene silencing in VMH during adulthood increases core temperature [99], and genetic deletion of Tac1 in VMH mildly reduces locomotor activities [101]. In males, testosterone can be aromatised to oestradiol to defeminise the VMH by suppressing Tac1 and Rprm expressions through ERα signalling and to reinforce the male-specific identity of VMH by maintaining Pdyn expression via testosterone in adulthood [99]. Pdyn-expressing cells in the VMH are the cold-sensitive cells in males, which are activated by cold shock and suppressed by heat, as shown by in vivo calcium recording [102]. Thus, sexually dimorphic circuits and nuclei shape different metabolic outcomes and reproductive behaviours in response to sex hormones, which are vital in directing the functional heterogeneity of the neuroendocrine circuits that control fertility and reproductive behaviours.
Sex dimorphism is also observed in the neural circuits that affect body energy balance. Females are more protected by hypercaloric diet-induced metabolic disorder [103, 104], but the protection is abolished when female sex hormones are depleted in menopause [105, 106] or prematurely by ovariectomy in rodent models [107, 108]. From a neuroanatomical standpoint, female mice have more ARC POMC neurons, which display higher firing rates than males [109]. At the molecular level, male and female POMC neurons may recruit different molecular machinery to regulate energy balance and diet-induced obesity susceptibility [110, 111]. Obesity or diet-induced obesity is exclusively observed in a sex specific manner when specific genes, for example, 5-hydroxytryptamine 2c receptor (5-HT2CR), in males or oestrogen receptors in females are deleted in POMC neurons [109, 112,113,114,115]. These collective findings demonstrate that sex hormone regulation can shape sexual dimorphism in molecularly defined neuronal populations in the hypothalamus to orchestrate physiological outcomes associated with both reproductive outcomes and energy balance regulation (Table 1).
One population, multiple functions: integrative models of the neuroendocrine circuits in the hypothalamus
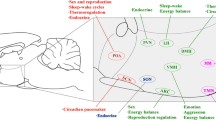
According to the central dogma of neuroscience, neurons in the mammalian brain establish their specific function, including their neurochemical identity, before birth through the action of regulatory proteins known as 'terminal selectors'. Once this developmental process is complete, each neuron is thought to maintain its function within its designated group throughout its lifetime. However, this view does not take into account that hypothalamic neurons often monitor and flexibly adapt their neuronal and hormonal regulation, as well as their neuropeptidergic machinery, to both internal physiological needs and environmental cues. Thus, while the notion of 'one population, one physiological function' still prevails in the field, there is increasing evidence that the functional roles of specific neuronal populations in the neuroendocrine hypothalamus are much more complex (Fig. 1), as discussed below.
Challenging the 'one population, one physiological function' paradigm, rodent studies have provided new insights into how populations of neuroendocrine neurons in different hypothalamic nuclei can regulate multiple physiological functions. For example, the AgRP neuronal population and many others are involved in the regulation of reproductive functions and the stress response, in addition to the previously known function of regulating food intake and energy balance. These neuronal populations integrate various external stimuli, including energy needs, reproductive needs and environmental stress, to ensure real-time coordination of multiple physiological needs
Energy balance vs reproduction
In the ARC, POMC and AgRP neurons are widely recognised as important regulators of energy balance, whereas Kiss1 neurons have a more specialised role in fertility control. However, a closer examination reveals that these different neuronal populations can often control the same physiological outcome. Sexual maturation and fertility are critical for mammalian survival, and these neuroendocrine outputs are integrated with the regulation of whole-body energy homeostasis. Nutritional status, on the other hand, plays a critical role in reproductive health and outcomes, possibly through intimate cross-talk mechanisms between melanocortin neurons, Kiss1 neurons and the HPG axis.
After a period of extended fasting, leading to diminished energy reserves, suppression of the HPG axis is observed, particularly in females, which is orchestrated by heightened activity in AgRP neurons [116, 117]. Conversely, following a meal, the surge in postprandial leptin levels suppresses the activity of AgRP/NPY neurons in the ARC, indicating an increase in energy availability and prompting meal cessation. The effect of leptin on feeding behaviour may involve a specific subset of AgRP neurons expressing Lepr [117]. In a transgenic mouse model, the absence of Lepr in AgRP neurons leads to increased adiposity. Female mutants display a discernible delay in the onset of puberty compared to controls, highlighting how distinct molecularly defined clusters of 'hunger neurons' can synergistically regulate reproductive parameters. Supporting this view, the genetic rescue of Lepr expression solely in AgRP neurons restores puberty onset, oestrous cyclicity, and fecundity in female Lepr-null mice, denoting the alternative role of AgRP neurons in regulating female fertility [118].
Following continuous chemogenetic activation of AgRP neurons for 14 days, which simulates chronic food deprivation, disrupted ovarian cycles and fertility are observed in adult female rodents [119], indicating that changes in the AgRP neuronal activity in response to long-lasting alterations in whole-body energy demands can override reproductive cycles. Similar observations have been made in a model of undernourished neonatal mice raised in large litters. Neonatal malnutrition in this model causes delayed onset of puberty, and chemogenetic inhibition of AgRP neuronal activity from postnatal day 26–30 reverses the delay in preputial separation and vaginal opening in male and female pups, respectively [120]. However, chemogenetic activation or inhibition during prepubertal development does not affect puberty onset when juveniles are nutritionally nourished during postnatal development [120], indicating how nutritional deprivation during early development can upset the energy balance, thereby dampening sexual maturation through the modulation of AgRP neuron. This multifaceted function may be controlled by AgRP neurons projecting to kisspeptin neurons in both the ARC (Kiss1ARC) and the anteroventral periventricular nucleus (Kiss1AVPV) [119], which are implicated in regulating the female ovarian cycle and initiating puberty onset, respectively [121]. Of note, kisspeptin can directly act on the melanocortin circuit by activating POMC neurons and inhibiting NPY neurons [122], indicating that AgRP neurons are part of a neuronal circuit co-regulated by Kiss1 neurons, which is sensitive to changes in central oestrogen signalling [123]. Therefore, changes in overall energy availability during early development or adulthood could disrupt fertility by affecting neurons traditionally linked to food intake or energy balance regulation. This challenges the conventional model of ‘One Population, One Physiological Function’ that has been previously established.
While energy balance impacts reproductive needs and feeding regulation, the opposite relationship has also been demonstrated. The female ovarian cycle substantially influences appetite [124, 125], mainly when food is limited [126]. Cyclic fluctuations in female sex hormones affect food intake and body weight through changes in AgRP neuron activity [127], which are likely ERα dependent [128]. Xu et al. have established a transgenic mouse line in which AgRP neurons are ablated due to AgRP neuron-specific deletion of the mitochondrial transcription factor A gene [129]. In this model, the disrupted AgRP neural activity does not affect oestrous cyclicity but abolishes the cycle-dependent, oestrogen-suppressed food intake [127]. Also, female mutants disply blunted responses to E2-inhibited food intake [127]. Peripheral oestradiol fluctuation can also influence female receptivity to reinforce reproductive success. AgRP/NPY neurons achieve this output through a multi-order circuit targeting the ventromedial hypothalamus (VMH) to facilitate lordosis behaviour and thus reproductive outcomes in female mice in response to E2 [130,131,132,133,134,135,136]. Hence, hunger neurons may have diverse roles in synchronising fluctuations in overall energy levels with hormonal and behavioural responses that regulate reproductive function via central E2 signalling.
Energy balance is defined as the state achieved when energy intake equals energy expenditure, and the second part of the equation can also be integrated with reproductive needs. A molecularly defined hypothalamic population in the ventrolateral division of VMH and expressing ERα (VMHERα) seems vital in this context. E2 acts on the VMH to regulate energy expenditure by increasing physical activity and adaptive thermogenesis [99, 100, 137]. Food deprivation inhibits the neuronal firing of VMHvlERα neurons [138], while reduced VMHvlERα neuronal firing is linked to reduced female receptive behaviours during mating [136]. Blunting oestrogen signalling by genetic deletion of ERα in VMHSF1 neurons results in weight gain, reduced metabolic rate during the inactive phase, reduced thermogenesis in brown adipose tissue, and increased susceptibility to HFD-induced obesity in a female-specific manner [113]. Oestrogen deficiency in ovariectomised females blunts the responses to cold exposure or food deprivation, indicating a need for endogenous ovarian hormones to coordinate thermoregulatory responses and nutritional states. Conversely, chemogenetic activation of VMHERα neurons during the inactive phase significantly increases spontaneous activity and promotes BAT thermogenesis in females [99]. It is noted that VMHvlERα neuronal firing is increased during the receptive period [80], indicating that VMHERα neurons promote energy expenditure during the reproductive receptive phase, while impaired oestrogen signalling in these neurons can blunt energy dissipation and abolish receptive behaviours. Collectively, the evidence emphasises the bidirectional communication of oestrogen signalling and energy balance through VMHERα neurons and suggests that activating these neurons might potentially restore metabolic homeostasis, even when oestrogen signalling is impaired.
Energy balance vs stress
Stress disrupts the delicate equilibrium between energy homeostasis and reproductive demands, a balance overseen by a central population of CRH-expressing neurons in the PVN, which are among the postsynaptic targets of AgRP neurons [139]. Food deprivation can disinhibit PVNCRH neurons by triggering AgRP neuron firing [139], suggesting that negative energy balance may activate the HPA axis with elevated circulating glucocorticoid levels. Consequently, the glucocorticoid surge allows the body to mobilise glucose to fuel energy demands [125], like food-seeking activity, and promote energy storage [140]. Notably, liver-expressed antimicrobial peptide 2 is secreted from the gut during food deprivation, whereas ghrelin is secreted after a meal. These gastrointestinal hormones can suppress and activate AgRP neurons via the growth hormone secretagogue receptor [141], suggesting how feeding status may counter-modulate the stress response via the periphery-to-brain axis.
Similar to food deprivation, when exposed to environmental or physical stressors such as foot shock, air puff, tail suspension, immobilisation or cooling, PVNCRH neurons become activated. However, neuronal activity can be rapidly and significantly reduced when exposed to appetitive or palatable stimuli [142, 143]. Thus, the reward system may prevent excessive HPA axis activation by modulating a common circuit that controls energy homeostasis. Accordingly, glucocorticoid receptors present in PVNCRH neurons facilitate the negative feedback of glucocorticoids [144] by inhibiting presynaptic norepinephrine [145] or glutamatergic [146] activation, thereby reducing CRH secretion and suppressing the HPA axis. By activating the reward system, the act of eating may help alleviate the effects of physical and environmental stressors [147]. This concept is particularly relevant to our understanding of how stress influences the consumption of 'comfort food' in eating disorders such as bulimia nervosa [148, 149]. Therefore, these studies suggest that the classically known 'stress neurons' can detect and adapt to both energy and environmental demands, whereas satiety and reward-associated feeding behaviours can reduce PVNCRH neuronal overactivation, highlighting that specific neuronal populations typically regulate more than one bodily function in an integrated manner over time.
Stress vs energy balance vs reproduction
Encountering adverse early-life experiences, such as being raised in an impoverished environment with unpredictable maternal care during a critical developmental period, is associated with altered brain maturation [150] and emotional vulnerabilities [151]. Rodent studies have shown that unpredictable maternal care during neonatal development can lead to emotional deficits, including anhedonia [152,153,154], meaning that offspring have diminished pleasure towards previously perceived rewarding stimuli [155]. In the animal kingdom, disruptions in maternal care, such as restricted access to nesting and bedding materials after birth, can result in lasting traumatic experiences for offspring, possibly via increased glutamatergic transmission to the PVNCRH circuit during neonatal development [156, 157]. Such functional identity reprogramming may be due to defective microglial action in shaping the circuit [157] and changes in the intrinsic gene expression profiles of PVNCRH neurons [158]. Conversely, optimal rearing conditions with maternal care have been shown to reduce excitatory synapses to PVNCRH neurons [159, 160] and the expression of the stress-related hormone CRH [159, 161, 162]. Neonates exposed to maternal separation exhibit changes in serum corticosterone and leptin levels. By postnatal day 4 (P4), traumatised neonates tend to have lower leptin and higher corticosterone levels compared to those receiving proper maternal care [163]. Although traumatised juveniles may initially exhibit reduced body weight before puberty [163], these changes in hormone levels during neonatal development may predispose individuals to obesity [164] and hyperactivity of the HPA axis [165] later in life.
PVNCRH neuron intrinsic excitability is regulated across the oestrous cycle with a peak in the preovulatory phase and a nadir in the oestrus phase [166], implicating the potential involvement of ovarian hormones in regulating stress response through manipulating the CRH neuron firing. Conversely, CRH neurons may also play a role in restraining reproductive receptivity during periods of stress. Pharmacological antagonism of CRH receptors before stress exposure can prevent stress-induced changes in LH pulses [167, 168]. Specifically, circulating oestradiol can potentiate the effects of stress induced by insulin-induced hypoglycaemia [169,170,171]. Exposure to acute psychosocial stress before the preovulatory phase disrupts the LH surge [172]. This finding is artificially recapitulated by exposure of female mice to acute stress upon E2 administration. CRH elicits dose-dependent effects on GnRH neuron firing activity in stress-free conditions, stimulating at lower concentrations and inhibiting at higher concentrations, depending on the CRH receptor subtypes involved [173]. Thus, CRH neurons can influence GnRH neuron activity, with oestrogen signalling playing a crucial role in determining whether CRH has stimulatory or inhibitory effects on these neurons.
The intensity and duration of stress experiences can affect food intake and reproductive cycles by involving a specific population of neuroendocrine neurons defined by CRH production. Thus, the evidence presented here supports the emerging model that the same population of neuroendocrine neurons can play a central role in orchestrating and integrating multiple physiological demands, responding adeptly to the ever-changing environmental cues encountered during both early postnatal development and adult life.
Intrinsic heterogeneity of neuroendocrine neurons: multiple subsets and multiple purposes?
Over a decade ago, pioneering ex vivo studies investigating the electrochemical properties of hypothalamic pro-opiomelanocortin (POMC) neurons revealed the remarkable intrinsic diversity of this population. This work has identified a wide range of electrophysiological properties and demonstrated a distinct responsiveness to different neurotransmitters and hormones [59, 174]. Recent advances in single-cell transcriptomic approaches have further elucidated the complexity among POMC neurons, unravelling several molecularly distinct clusters [13, 175, 176]. Thanks to these efforts, we are now uncovering that hypothalamic POMC neurons, previously thought to be a homogeneous functional pool, comprise distinct subpopulations organised based on spatial, molecular and electrophysiological features, which may play different roles in physiological conditions or the pathogenesis of hypothalamic diseases [49, 175,176,177]. This emerging framework, which has been extensively reviewed [177] and is beyond the scope of this paper, holds great promise for elucidating how different subsets of neurons in well-defined populations can affect food intake and body weight regulation. However, this inherent diversity raises new fundamental questions: Is heterogeneity an exclusive property of POMC neurons? Or can it also be found in other important hypothalamic neuroendocrine populations?
Intrinsic heterogeneity of melanocortin neurons
AgRP/NPY neurons have classically been regarded as a molecularly homogeneous cluster of neurons in the ARC, predominantly propagating GABAergic transmission [178]. However, recent studies have shown that refeeding after a period of fasting can lead to a significant decrease in spiking activity in approximately two-thirds of AgRP neurons while causing an increase in firing rates in less than one-third of AgRP neurons [179], suggesting intrinsic functional heterogeneity.
Single-cell transcriptomic analyses have identified two distinct subtypes of AgRP neurons: somatostatin-expressing (AgRPSST) and Gm8773-expressing (AgRPGm8773) neurons [176, 180] (Fig. 3a). However, the specific functional roles of these neuronal subpopulations remain elusive. Interestingly, Drop-seq analysis, which reads the gene expression profile of thousands of individual neurons and determines their molecular identity, has revealed that Gap43, a gene encoding a protein involved in axonal regeneration [181], is significantly upregulated in the AgRPGm8773 subpopulation but downregulated in the AgRPSST population following fasting [176], suggesting differential structural plasticity to food deprivation in these subpopulations.
Corticotropin-releasing hormone receptor type 1 (Crhr1) is predominantly expressed by the AgRPSST subpopulation [176]. These neurons receive innervation from PVNCRH neurons and project back to the PVN [182], potentially relaying feedback signals to the PVN [183]. Corticotropin-releasing hormone has been shown to suppress the neuronal firing of AgRPCrhr1 [182]. Additionally, genetic deletion of Crhr1 from AgRP neurons has been found to affect hepatic gluconeogenesis and thermogenesis during the active dark phase following overnight food deprivation without significant alterations in feeding behaviour or body weight [182]. Hence, the AgRPCrhr1 subpopulation may regulate glucose levels and body temperature in response to stress compared to other classical AgRP hunger neurons responding to the change in body energy status.
Dopamine signalling plays a crucial role in regulating feeding-associated behaviours, particularly in favour of palatable food consumption [184]. Dopamine deficiency may lead to failure to ingest food and subsequent death from starvation [185], while the effect of hyperactivating dopamine signalling on overconsumption remains uncertain. Among the dopamine receptor D1 (Drd1)-expressing subpopulations in the ARC, the AgRPGm8773 subpopulation constitutes the primary neuronal cluster expressing Drd1. To explore the role of Drd1 in AgRP/NPY neurons in food intake, an adenovirus expressing chemogenetic activation receptor has been injected into the ARC of Drd1::Cre; Npy::Flp mice, thereby allowing to precisely modulate by chemogenetic activation the subpopulation of AgRP/NPYDrd1 neurons [186]. Chemogenetic activation of this specific population induces acute voracious feeding behaviour. In contrast, genetic deletion of Drd1 in AgRP/NPY neurons reduces daily consumption of a high-fat diet, diminishes high-fat diet consumption following acute fasting, and attenuates foraging behaviour to access chow pellets [186], suggesting that the AgRPGm8773 populations may drive feeding behaviours through an increased dopamine level in response to energy-rich food stimulus [187,188,189].
In summary, these findings raise the possibility that different molecularly defined clusters of AgRP neurons may control divergent physiological outcomes, with some more specialised in homeostatic energy balance and others more likely to control hedonic feeding behaviour (Fig. 2a).
Possible models for intrinsic heterogeneity within different neurondocrine populations in the hypothalamus. (a) AgRP neurons may be functionally subdivided into somatostatin (SST) and newly identified Gm8773 subtypes. (b) Kiss1 neurons may be subclassified on the basis of their spatial distribution in the AVPV and ARC. Their identities are further marked by differential expression of peptidergic markers. (c) GnRH neurons may exert diverse functions by projecting to different hypothalamic subnuclei. (d) CRH neurons have recently been subdivided into the Nr3c1 subtype, which expresses the glucocorticoid receptor, and the Adarb2 subtype. The identification of neuronal subpopulations demonstrates their possible involvement in specific endocrine responses
Kisspeptin neurons
In the mouse hypothalamus, Kiss1 expression is restricted to the ARC and the AVPV regions, where each harbour functionally distinct Kiss1 subpopulations [190]. AVPV kisspeptin neurons are considered to be the impulse generators [191] responsible for the onset of puberty. In contrast, ARC Kiss1 neurons are regarded as the pulse generator [192], responsible for the cyclicity of the female ovarian cycle. These two topographically distant populations express different peptidergic markers. Indeed, tyrosine hydroxylase (TH) and metenkephalin (mENK) are found colocalised with AVPV Kiss1 neurons [193, 194], whereas ARC Kiss1 neurons are frequently co-localised with neurokinin B (NKB) and dynorphin (DYN) [195]. Recent studies on single-cell transcriptomic profiling suggest that (pro)dynorphin may not necessarily be a specific marker for Kiss1ARC neurons [176, 196].
Whether or not ARC or AVPV kisspeptin neurons display intrinsic heterogeneity is poorly understood. Approximately half of the AVPV Kiss1 neurones co-express TH with Kiss1TH innervating GnRH neurons [197]. The functional role of this kisspeptin subpopulation has yet to be discovered. However, since the specific deletion of TH in kisspeptin neurons has no effect on the onset of puberty and fertility in both males and females [198], it could be speculated that this subtype does not follow the classical functional behaviour of Kiss1 neurons.
NKB can promote kisspeptin release through NKB receptors on Kiss1 neurons via autocrine control. NKB signalling stimulates GnRH secretion [199], which is essential for puberty onset in both sexes. Impaired NKB signalling results in hypogonadotropic pubertal delay in humans [200]. Both mENK and DYN modulate opioidergic signalling of kisspeptin neurons [201]; in particular, they inhibit the release of kisspeptin through κ-opioid receptors and δ-opioid receptors, respectively, via autocrine fashion. While the role of mENK in AVPV Kiss1 neurons is uncertain, ablation of Kiss1 in ARC Pdyn neurons does not affect the onset of puberty. Still, it leads to a disrupted ovarian cycle, hypogonadism and female infertility [202], further suggesting the existence of multiple sub-populations of Kiss1 neurons that operate via divergent modes of action.
In adult mice, the AVPV and the ARC Kiss1 populations have different sensitivity to oestrogen, possibly because ERα binds to a different regulatory site on the Kiss1 locus in AVPV compared to ARC Kiss1 neurons [203]. The differential transcriptional control of kisspeptin through oestrogen signalling could thereby lead to ARC Kiss1 neurons control GnRH/LH pulses via mediating the oestrogen-negative feedback action and that the AVPV Kiss1 neurons control GnRH/LH surge via mediating the oestrogen-positive feedback action [204]. In female rodents, the ARC expresses a high level of Kiss1 when the circulating oestradiol level is low at diestrus, which can be suppressed by oestrogen treatment [205]. Conversely, the AVPV Kiss1 expression level is high during the preovulatory period and can be further augmented by oestrogen treatment in rodents [205]. Indeed, analyses from bulk RNA sequencing from laser-capture microdissection fluorescently-labelled Kiss1 neurons show that ARC Kiss1 neurons display an entirely different transcription profile than AVPV Kiss1 neurons in response to E2 treatment [206].
Interestingly, the expression of the transcription factors Nhlh2, Tbx3, and Sox14 is specific to Kiss1ARC neurons [194], suggesting that different developmental origins may account for the regulation of different downstream circuits and possibly the functional dichotomy of different subpopulations of Kiss neurons in response to oestrogen action [194] (Fig. 2b).
GnRH neurons in the hypothalamus
The presence of diverse sub-populations of gonadotropin-releasing hormone (GnRH) neurons may be central to orchestrating the patterns of gonadotropin secretion essential for puberty onset and fertility. GnRH neurons are dispersed across the anteroposterior axis of the brain, including the medial septum, rostral preoptic area (POA), anterior hypothalamus, and medial basal hypothalamus [207]. Yet, they originate from distinct pools of progenitor cells derived from Islet-1/2 or Wnt1 origins [208], located respectively in the placodal ectoderm and neural crest rather than the hypothalamus. During development, GnRH neurons embark on a long-distant migration from the nasal septum, facilitated by olfactory ensheathing cells [209], astrocytes [210], and hormonal cues [211, 212], to the forebrain, which ultimately projects dendrites towards the preoptic area, hypothalamus, and median eminence [213]. While the molecular profiles of GnRH neurons in different brain regions require further investigation at the single-cell level, it is plausible that GnRH neurons located in different anteroposterior positions express different subsets of chemotactic and hormone-sensing machinery, facilitating their migration and localisation within specific hypothalamic nuclei during development.
The majority of GnRH neurons project to the median eminence, where they release GnRH peptide into the portal blood in a pulsatile manner to control gonadotropin secretion from the pituitary gland, a mechanism crucial for the initiation of puberty and the preservation of fertility [214]. Despite challenges in the detection of GnRH neurons due to their limited cell numbers by single-cell transcriptomic analysis, the use of the more sensitive multiplexed error-robust fluorescence in situ hybridisation (MERFISH) technique has identified that GnRH-expressing clusters in the POA that highly express kisspeptin receptors [215]. Sexual dimorphism emerges in the kisspeptin fibre innervation patterns onto the dendrites of GnRH neurons. Female mice receive denser kisspeptin fibre innervation in rostral preoptic GnRH neurons than males [35], which probably originates from the AVPV Kiss1 neurons.
In addition to ME, GnRH neurons project onto kisspeptin and tyrosine hydroxylase-expressing neurons in the POA and ARC [216], denoting their structural heterogeneity (Fig. 2c). The GnRH neurons projecting to POA may have implications on regulating lactation [216]. Moreover, a subset of GnRH neurons targets tuberoinfundibular dopaminergic neurons in the ARC, showing hormonal regulation of their innervation patterns across the oestrous cycle [217]. Under physiological conditions, TH-expressing neurons in ARC received GnRH fibre innervation at proestrus and estrus but no fibre innervation at diestrus, suggesting an ovarian hormone-dependent regulation of GnRH fibre innervation.
Corticotrophin-releasing factor neurons in PVN
While the role of CRH neurons in regulating stress response is well established, surprisingly, the heterogeneity of this neuronal population has been barely investigated. In a single-cell transcriptomic study, however, Romanov et al. have revealed that CRH neurons residing in the PVN are glutamatergic, contrary to those GABAergic subtypes residing in DMH and the preoptic area [218]. By single-cell RNA-sequencing, Berkhout et al. have identified two main populations of CRH neurons, including the pituitary-projecting population expressing Nr3c1 glucocorticoid receptor and the spinal cord-projecting Adarb2 population [219] (Fig. 3d). The latter has yet to be studied, but the CRHNr3c1 population expresses glucocorticoid receptors to facilitate feedback mechanisms to suppress HPA axis overactivation [220]. This population also expresses Avp, which strongly potentiates the release of CRH-driven adrenocorticotropic hormone (ACTH) from the anterior pituitary. Arginine vasopressin (AVP) is present in both magnocellular and parvocellular divisions of the PVN [221], and the latter population of AVP is colocalised with CRH [222]. A pioneering study has demonstrated the co-release of CRH and AVP upon stressful stimuli, whereas their syntheses can be suppressed by glucocorticoid [223]. By exposing neonates to maternal separation from postnatal day 2 to 9, Short and Tai et al. showed that Avp is strongly expressed among the glutamatergic CRH neurons [158]. At P10, mice expressing tdTomato under the CRFAvp resided primarily in the dorsal region of PVN [158]. CRHNr3c1 also expresses Scgn encoding secretagogin. Secretagogin appears to be a calcium ion sensor that regulates intracellular vesicle transport of CRH and facilitates its extracellular release [224]. Gene silencing of secretagogin induces CRH accumulation in the PVN, suggesting an impairment in the intracellular translocation of CRH. Besides, ACTH and corticosterone production are abolished after secretagogin knockdown in animals exposed to acute formalin stress. Lastly, Agtr1a, encoding angiotensin type-1a receptor, also expresses the CRHNr3c1 population. Photo-stimulation of CRHAgtr1a neurons activates the HPA axis and increases systolic blood pressure, whereas inhibiting CRHAgtr1a neurons suppresses the activity of the HPA axis and reduces anxiety-like behaviour [225]. Notably, secretagogin [224] and angiotensin type-1a receptor [225] co-express with CRH axons in the ME.
The classical neurobiological view (left panel) depicts hypothalamic endocrine neurons as discrete populations, each characterised by a specific neuropeptidergic profile, programmed by development to elicit singular physiological functions throughout life. However, emerging evidence challenges this ‘One Population, One Physiological Function’ paradigm. The integrated view (right panel) presents a more in-depth understanding, illustrating that hypothalamic endocrine neurons exhibit additional layers of plasticity and functional heterogeneity. Contrary to the classical notion, these neural circuits are highly heterogeneous and interconnected, participating in a network where functions related to reproduction, stress, and hunger are intricately intertwined. This model highlights the dynamic nature of hypothalamic endocrine neurons, emphasising the need for a holistic experimental approach to dissect the neuroendocrine system and the underlying neural correlates
In conclusion, although the PVN CRH neurons share a common peptidergic marker in the same hypothalamic nucleus, differential expression of additional markers, Nr3c1 and Adarb2, may define subpopulations that project to and control different circuits, thereby conferring intrinsic functional heterogeneity (Fig. 2d).
Conclusion and open questions
The current neurobiological model used to explain how hypothalamic neuroendocrine neurons operate implies that a specific neuronal population, defined by a set of neuropeptides and other secreted molecules, is programmed by development always to elicit one physiological function throughout life. However, this traditional ‘One-Population-One-Function’ model does not take into account that additional layers of plasticity and heterogeneity are observed with each molecularly defined cluster of neuroendocrine neurons (Fig. 3). For example, ‘hunger neurons’ promote food intake when activated by integrating this behavioural output with reproductive needs and environmental cues that affect stress-related circuits. No single circuit in the brain operates as an isolated functional entity, and each population modulates different downstream circuits in response to presynaptic and postsynaptic cross-talk between neuropeptides, neurotransmitters, and other released factors such as cytokines that fine-tune neurotransmission. Since neuroendocrine neurons are in constant dialogue with hypothalamic non-neuronal cells, microglia, oligodendrocytes, astrocytes, tanycytes, to maintain body homeostasis [226,227,228], non-neuronal cells may play a key contributing role in this context, although this remains a key question to be answered.
Advancements in single-cell analysis reveal that even neuronal populations identified by their shared neurochemical or neuropeptidergic identities harbour distinct subpopulations, likely participating in distinct neurobiological mechanisms. Hence, it is plausible that neuronal populations classically known to control one unique output, such as hunger, stress, or fertility, may contain special subtypes that are capable of integrating all these different outputs.
In this evolving framework, additional important questions arise. Is functional heterogeneity a static property throughout mammalian life, or can this property dynamically change in response to environmental or internal cues? Exposure to an unbalanced diet during embryonic development induces persistent gene-specific DNA methylation changes [173,174,175,176], leading to permanent metabolic reprogramming and the development of neuroendocrine diseases such as obesity in adulthood. Following exposure to a maternal hypercaloric diet, offspring mice show increased circulating leptin levels at weaning [177] and hypermethylation of the Pomc gene, highlighting the long-term imprinting effect of maternal diet on offspring gene expression and whole-body energy homeostasis. Maternal overnutrition during lactation in mice reduces embryonic (E12.5) neurogenesis and impairs postnatal neurotrophic development in the hypothalamus. When dams consume a high-fat diet during pregnancy and early lactation, their male neonates develop more ‘hunger neurons’ and fewer ‘satiety neurons’ in the hypothalamus. This alteration in NPY-to-POMC neuron ratio sets the stage for them to become obese later in life [229], suggesting that metabolic and dietary challenges may alter the functional heterogeneity of hypothalamic circuits and influence how these circuits respond to changes in hormonal signalling across the lifespan.
The prevailing lifestyle in modern societies, characterised by a lack of physical activity, excessive calorie intake, and repetitive behaviours, is widely recognised as one of the main causes of the global burden of obesity, leading to maladaptive regulation of energy balance, eating behaviour and body weight control. When laboratory mice are subjected to environmental enrichment, which involves augmenting their rearing environment with increased physical activity and exposure to sensory, cognitive, and social stimulations, significant changes in synaptic plasticity are observed in the neuroendocrine hypothalamus. Specifically, an increase in the excitation-inhibition ratio in α-MSH neurons and a decrease in the excitation-inhibition ratio in AgRP neurons are observed, which is accompanied by an improved hypothalamic leptin sensitivity, highlighting how environmental cues can reprogram hypothalamic neuroplasticity [230].
Interestingly, hypothalamic neuronal functions can be maximally enhanced by environmental stimuli that are applied during developmental stages [230]. During post-weaning, when hypothalamic neuronal projections in animals undergo plastic changes, voluntary wheel running selectively reduces obesity in rats bred to develop diet-induced obesity compared to those bred to be diet-resistant. Remarkably, three weeks of exercise in juvenile rats (4 week old) has a sustained effect in preventing obesity development later in adult life, even when the animals stop exercising and continue to consume a diet relatively high in fat and calories [231]. The protective effect of exercise during juvenile life is associated with substantial differences in the hypothalamic expression of various neuropeptides, such as NPY, AgRP, or POMC [231] and increased central leptin signalling, suggesting functional reprogramming. Consistent with this, it has been recently shown using whole-cell patch-clamp electrophysiology that exercise training promotes a rapid reorganisation of synaptic inputs and biophysical properties in POMC and NPY/AgRP neurons [232].
These data collectively emphasise the intriguing possibility that environmental stimuli can reprogram the functional heterogeneity of hypothalamic neural circuits not only during juvenile development but also, albeit to a lesser extent, in adulthood. Such findings hold substantial promise for therapeutic applications, particularly in light of the growing interest in developing pharmacological anti-obesity treatments to emulate the beneficial effects of environmental stimuli on hypothalamic neuronal plasticity. Some of the effects of environmental enrichment or exercise on hypothalamic neurobiology and central leptin sensitivity may be mediated by the increased expression of brain-derived neurotrophic factor (BDNF) and its influence on neuronal plasticity [233]. The selective serotonin reuptake inhibitor fluoxetine is an antidepressant drug with similar effects to environmental stimulation [233]. Fluoxetine-treated obese mice experience reduced weight gain, increased energy expenditure and improved central leptin sensitivity [234]. Patch-clamp recordings from POMC neurons further indicate that fluoxetine increases the firing rate of this neuronal population and excitatory AMPA-mediated transmission while concurrently reducing presynaptic inhibitory GABAergic currents, potentially through heightened activity of the mTOR pathway [235]. The case of BDNF and fluoxetine therefore illustrates how further research into the mechanisms of hypothalamic functional plasticity may pave the way for innovative therapeutic solutions.
Elucidating the neurobiological mechanisms underlying the functional heterogeneity of neuroendocrine neurons in the hypothalamus is a complex and dynamic area of research with several important outstanding questions (see Box 1). Addressing these questions will significantly improve our understanding of the hypothalamus-mediated control of hormonal action under physiological conditions and during neuroendocrine disease.
Data availability
All data cited in this article are publicly available online.
Abbreviations
- 3V:
-
Third ventricle
- ACTH:
-
Adrenocorticotropic hormone
- AgRP:
-
Agouti-related protein
- ARC:
-
Arcuate nucleus
- AVP:
-
Arginine vasopressin
- AVPV:
-
Anteroventral periventricular nucleus
- CRH:
-
Corticotropin-releasing hormone
- Crhr :
-
Corticotropin-releasing hormone receptor
- DMH:
-
Dorsal medial hypothalamus
- Drd1 :
-
Dopamine receptor D1
- DYN:
-
Dynorphin
- ECM:
-
Extracellular matrix
- ECS:
-
Extracellular space
- ERα:
-
Oestrogen receptor alpha
- FSH:
-
Follicle-stimulating hormone
- GABA:
-
Gamma-aminobutyric acid
- GLP1:
-
Glucagon-like peptide-1
- Glp1r :
-
Glucagon-like peptide-1 receptor
- GnRH:
-
Gonadotropin hormone-releasing hormone
- HFD:
-
High-fat diet
- HPA:
-
Hypothalamic-pituitary-adrenal axis
- HPG:
-
Hypothalamic-pituitary–gonadal axis
- Kiss1:
-
Kisspeptin
- Lepr:
-
Leptin receptor
- LH:
-
Luteinising hormone
- MC4R:
-
Melanocortin 4 receptor
- ME:
-
Median eminence
- mENK:
-
Methionine enkephalin
- MePO:
-
Median preoptic nucleus
- NKB:
-
Neurokinin B
- NPY:
-
Neuropeptide Y
- OV:
-
Organum vasculosum of the lamina terminalis
- OXT:
-
Oxytocin
- Pdyn :
-
Prodynorphin
- POMC:
-
Proopiomelanocortin
- PVN:
-
Paraventricular nucleus
- RCA:
-
Retrochiasmatic area
- Rprm :
-
Reprimo
- SST:
-
Somatostatin
- TH:
-
Tyrosine hydroxylase
- VMH:
-
Ventral medial hypothalamus
- VMHvl:
-
Ventrolateral division of VMH
- αMSH:
-
Alpha-melanocyte-stimulating hormone
References
Timper K, Bruning JC (2017) Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. Dis Model Mech 10(6):679–689
Zimmerman CA, Leib DE, Knight ZA (2017) Neural circuits underlying thirst and fluid homeostasis. Nat Rev Neurosci 18(8):459–469
Tran LT, Park S, Kim SK, Lee JS, Kim KW, Kwon O (2022) Hypothalamic control of energy expenditure and thermogenesis. Exp Mol Med 54(4):358–369
Gautron L, Elmquist JK, Williams KW (2015) Neural control of energy balance: translating circuits to therapies. Cell 161(1):133–145
Knepper MA, Kwon TH, Nielsen S (2015) Molecular physiology of water balance. N Engl J Med 372(14):1349–1358
Adamantidis AR, de Lecea L (2023) Sleep and the hypothalamus. Science 382(6669):405–412
Herbison AE (2016) Control of puberty onset and fertility by gonadotropin-releasing hormone neurons. Nat Rev Endocrinol 12(8):452–466
Navarro VM (2020) Metabolic regulation of kisspeptin - the link between energy balance and reproduction. Nat Rev Endocrinol 16(8):407–420
Frankiensztajn LM, Elliott E, Koren O (2020) The microbiota and the hypothalamus-pituitary-adrenocortical (HPA) axis, implications for anxiety and stress disorders. Curr Opin Neurobiol 62:76–82
Agorastos A, Chrousos GP (2022) The neuroendocrinology of stress: the stress-related continuum of chronic disease development. Mol Psychiatry 27(1):502–513
Stuber GD (2023) Neurocircuits for motivation. Science 382(6669):394–398
Mei L, Osakada T, Lin D (2023) Hypothalamic control of innate social behaviors. Science 382(6669):399–404
Chen R, Wu X, Jiang L, Zhang Y (2017) Single-cell RNA-seq reveals hypothalamic cell diversity. Cell Rep 18(13):3227–3241
Kim DW, Washington PW, Wang ZQ, Lin SH, Sun C, Ismail BT, Wang H, Jiang L, Blackshaw S (2020) The cellular and molecular landscape of hypothalamic patterning and differentiation from embryonic to late postnatal development. Nat Commun 11(1):4360
Steuernagel L, Lam BYH, Klemm P, Dowsett GKC, Bauder CA, Tadross JA, Hitschfeld TS, Del Rio MA, Chen W, de Solis AJ et al (2022) HypoMap-a unified single-cell gene expression atlas of the murine hypothalamus. Nat Metab 4(10):1402–1419
Yu H, Rubinstein M, Low MJ (2022) Developmental single-cell transcriptomics of hypothalamic POMC neurons reveal the genetic trajectories of multiple neuropeptidergic phenotypes. Elife. https://doi.org/10.7554/eLife.72883
Herb BR, Glover HJ, Bhaduri A, Colantuoni C, Bale TL, Siletti K, Hodge R, Lein E, Kriegstein AR, Doege CA, Ament SA (2023) Single-cell genomics reveals region-specific developmental trajectories underlying neuronal diversity in the human hypothalamus. Sci Adv 9(45):eadf6251
Lei Y, Liang X, Sun Y, Yao T, Gong H, Chen Z, Gao Y, Wang H, Wang R, Huang Y et al (2024) Region-specific transcriptomic responses to obesity and diabetes in macaque hypothalamus. Cell Metab 36(2):438–453
D’Agostino G, Diano S (2010) Alpha-melanocyte stimulating hormone: production and degradation. J Mol Med (Berl) 88(12):1195–1201
Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD et al (2005) Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123(3):493–505
Williams KW, Elmquist JK (2012) From neuroanatomy to behavior: central integration of peripheral signals regulating feeding behavior. Nat Neurosci 15(10):1350–1355
Lu D, Willard D, Patel IR, Kadwell S, Overton L, Kost T, Luther M, Chen W, Woychik RP, Wilkison WO et al (1994) Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature 371(6500):799–802
Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB (2008) Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci 11(9):998–1000
Atasoy D, Betley JN, Su HH, Sternson SM (2012) Deconstruction of a neural circuit for hunger. Nature 488(7410):172–177
Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ (2001) Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411(6836):480–484
Andermann ML, Lowell BB (2017) Toward a wiring diagram understanding of appetite control. Neuron 95(4):757–778
Quarta C, Fioramonti X, Cota D (2020) POMC neurons dysfunction in diet-induced metabolic disease: hallmark or mechanism of disease? Neuroscience 447:3–14
Grayson BE, Seeley RJ, Sandoval DA (2013) Wired on sugar: the role of the CNS in the regulation of glucose homeostasis. Nat Rev Neurosci 14(1):24–37
Cui H, Lopez M, Rahmouni K (2017) The cellular and molecular bases of leptin and ghrelin resistance in obesity. Nat Rev Endocrinol 13(6):338–351
Koch M, Horvath TL (2014) Molecular and cellular regulation of hypothalamic melanocortin neurons controlling food intake and energy metabolism. Mol Psychiatry 19(7):752–761
Lavoie O, Michael NJ, Caron A (2023) A critical update on the leptin-melanocortin system. J Neurochem 165(4):467–486
Munzberg H, Bjornholm M, Bates SH, Myers MG Jr (2005) Leptin receptor action and mechanisms of leptin resistance. Cell Mol Life Sci 62(6):642–652
Skorupskaite K, George JT, Anderson RA (2014) The kisspeptin-GnRH pathway in human reproductive health and disease. Hum Reprod Update 20(4):485–500
Tena-Sempere M (2017) Neuroendocrinology in 2016: neuroendocrine control of metabolism and reproduction. Nat Rev Endocrinol 13(2):67–68
Clarkson J, Herbison AE (2006) Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 147(12):5817–5825
Yip SH, Boehm U, Herbison AE, Campbell RE (2015) Conditional viral tract tracing delineates the projections of the distinct kisspeptin neuron populations to gonadotropin-releasing hormone (GnRH) neurons in the mouse. Endocrinology 156(7):2582–2594
Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA (2004) Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology 80(4):264–272
Novaira HJ, Sonko ML, Hoffman G, Koo Y, Ko C, Wolfe A, Radovick S (2014) Disrupted kisspeptin signaling in GnRH neurons leads to hypogonadotrophic hypogonadism. Mol Endocrinol 28(2):225–238
Oakley AE, Clifton DK, Steiner RA (2009) Kisspeptin signaling in the brain. Endocr Rev 30(6):713–743
Aguilera G, Liu Y (2012) The molecular physiology of CRH neurons. Front Neuroendocrinol 33(1):67–84
de Kloet ER, Joels M, Holsboer F (2005) Stress and the brain: from adaptation to disease. Nat Rev Neurosci 6(6):463–475
Zhou X, Lu Y, Zhao F, Dong J, Ma W, Zhong S, Wang M, Wang B, Zhao Y, Shi Y et al (2022) Deciphering the spatial-temporal transcriptional landscape of human hypothalamus development. Cell Stem Cell 29(2):328–343
Fenselau H, Campbell JN, Verstegen AM, Madara JC, Xu J, Shah BP, Resch JM, Yang Z, Mandelblat-Cerf Y, Livneh Y, Lowell BB (2017) A rapidly acting glutamatergic ARC–>PVH satiety circuit postsynaptically regulated by alpha-MSH. Nat Neurosci 20(1):42–51
Saucisse N, Mazier W, Simon V, Binder E, Catania C, Bellocchio L, Romanov RA, Leon S, Matias I, Zizzari P et al (2021) Functional heterogeneity of POMC neurons relies on mTORC1 signaling. Cell Rep 37(2):109800
Dicken MS, Tooker RE, Hentges ST (2012) Regulation of GABA and glutamate release from proopiomelanocortin neuron terminals in intact hypothalamic networks. J Neurosci 32(12):4042–4048
Hentges ST, Nishiyama M, Overstreet LS, Stenzel-Poore M, Williams JT, Low MJ (2004) GABA release from proopiomelanocortin neurons. J Neurosci 24(7):1578–1583
Hentges ST, Otero-Corchon V, Pennock RL, King CM, Low MJ (2009) Proopiomelanocortin expression in both GABA and glutamate neurons. J Neurosci 29(43):13684–13690
Jarvie BC, Hentges ST (2012) Expression of GABAergic and glutamatergic phenotypic markers in hypothalamic proopiomelanocortin neurons. J Comp Neurol 520(17):3863–3876
Leon S, Simon V, Lee TH, Steuernagel L, Clark S, Biglari N, Leste-Lasserre T, Dupuy N, Cannich A, Bellocchio L et al (2024) Single cell tracing of Pomc neurons reveals recruitment of “Ghost” subtypes with atypical identity in a mouse model of obesity. Nat Commun 15(1):3443
Trotta M, Bello EP, Alsina R, Tavella MB, Ferran JL, Rubinstein M, Bumaschny VF (2020) Hypothalamic Pomc expression restricted to GABAergic neurons suppresses Npy overexpression and restores food intake in obese mice. Mol Metab 37:100985
Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS (1998) Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 18(23):9996–10015
Inutsuka A, Yamanaka A (2013) The physiological role of orexin/hypocretin neurons in the regulation of sleep/wakefulness and neuroendocrine functions. Front Endocrinol (Lausanne) 4:18
Li Y, Gao XB, Sakurai T, van den Pol AN (2002) Hypocretin/Orexin excites hypocretin neurons via a local glutamate neuron-A potential mechanism for orchestrating the hypothalamic arousal system. Neuron 36(6):1169–1181
Schone C, Apergis-Schoute J, Sakurai T, Adamantidis A, Burdakov D (2014) Coreleased orexin and glutamate evoke nonredundant spike outputs and computations in histamine neurons. Cell Rep 7(3):697–704
Eyigor O, Minbay Z, Kafa IM (2012) Glutamate and orexin neurons. Vitam Horm 89:209–222
Bubser M, Fadel JR, Jackson LL, Meador-Woodruff JH, Jing D, Deutch AY (2005) Dopaminergic regulation of orexin neurons. Eur J Neurosci 21(11):2993–3001
Saito YC, Tsujino N, Abe M, Yamazaki M, Sakimura K, Sakurai T (2018) Serotonergic input to orexin neurons plays a role in maintaining wakefulness and REM sleep architecture. Front Neurosci 12:892
Ohno K, Hondo M, Sakurai T (2008) Cholinergic regulation of orexin/hypocretin neurons through M(3) muscarinic receptor in mice. J Pharmacol Sci 106(3):485–491
Williams KW, Margatho LO, Lee CE, Choi M, Lee S, Scott MM, Elias CF, Elmquist JK (2010) Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J Neurosci 30(7):2472–2479
Sohn JW, Williams KW (2012) Functional heterogeneity of arcuate nucleus pro-opiomelanocortin neurons: implications for diverging melanocortin pathways. Mol Neurobiol 45(2):225–233
Biglari N, Gaziano I, Schumacher J, Radermacher J, Paeger L, Klemm P, Chen W, Corneliussen S, Wunderlich CM, Sue M et al (2021) Functionally distinct POMC-expressing neuron subpopulations in hypothalamus revealed by intersectional targeting. Nat Neurosci 24(7):913–929
Tucker JAL, Bornath DPD, McCarthy SF, Hazell TJ (2024) Leptin and energy balance: exploring Leptin’s role in the regulation of energy intake and energy expenditure. Nutr Neurosci 27(1):87–95
van Bloemendaal L, Ten Kulve JS, la Fleur SE, Ijzerman RG, Diamant M (2014) Effects of glucagon-like peptide 1 on appetite and body weight: focus on the CNS. J Endocrinol 221(1):T1-16
Allard C, Cota D, Quarta C (2023) Poly-agonist pharmacotherapies for metabolic diseases: hopes and new challenges. Drugs. https://doi.org/10.1007/s40265-023-01982-6
Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, Lowell BB, Elmquist JK (2008) Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest 118(5):1796–1805
Dodd GT, Michael NJ, Lee-Young RS, Mangiafico SP, Pryor JT, Munder AC, Simonds SE, Bruning JC, Zhang ZY, Cowley MA et al (2018) Insulin regulates POMC neuronal plasticity to control glucose metabolism. Elife. https://doi.org/10.7554/eLife.38704
Swanson LW, Kuypers HG (1980) A direct projection from the ventromedial nucleus and retrochiasmatic area of the hypothalamus to the medulla and spinal cord of the rat. Neurosci Lett 17(3):307–312
Elias CF, Lee C, Kelly J, Aschkenasi C, Ahima RS, Couceyro PR, Kuhar MJ, Saper CB, Elmquist JK (1998) Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron 21(6):1375–1385
Horvath TL, Naftolin F, Kalra SP, Leranth C (1992) Neuropeptide-Y innervation of beta-endorphin-containing cells in the rat mediobasal hypothalamus: a light and electron microscopic double immunostaining analysis. Endocrinology 131(5):2461–2467
Baker RA, Herkenham M (1995) Arcuate nucleus neurons that project to the hypothalamic paraventricular nucleus: neuropeptidergic identity and consequences of adrenalectomy on mRNA levels in the rat. J Comp Neurol 358(4):518–530
Le Tissier P, Campos P, Lafont C, Romano N, Hodson DJ, Mollard P (2017) An updated view of hypothalamic-vascular-pituitary unit function and plasticity. Nat Rev Endocrinol 13(5):257–267
Gao Y, Tschop MH, Luquet S (2014) Hypothalamic tanycytes: gatekeepers to metabolic control. Cell Metab 19(2):173–175
Prevot V, Dehouck B, Sharif A, Ciofi P, Giacobini P, Clasadonte J (2018) The versatile tanycyte: a hypothalamic integrator of reproduction and energy metabolism. Endocr Rev 39(3):333–368
Theodosis DT, Pierre K, Cadoret MA, Allard M, Faissner A, Poulain DA (1997) Expression of high levels of the extracellular matrix glycoprotein, tenascin-C, in the normal adult hypothalamoneurohypophysial system. J Comp Neurol 379(3):386–398
Horii-Hayashi N, Sasagawa T, Nishi M (2017) Insights from extracellular matrix studies in the hypothalamus: structural variations of perineuronal nets and discovering a new perifornical area of the anterior hypothalamus. Anat Sci Int 92(1):18–24
Vargova L, Sykova E (2014) Astrocytes and extracellular matrix in extrasynaptic volume transmission. Philos Trans R Soc Lond B Biol Sci 369(1654):20130608
Akhmanova M, Osidak E, Domogatsky S, Rodin S, Domogatskaya A (2015) Physical, spatial, and molecular aspects of extracellular matrix of in vivo niches and artificial scaffolds relevant to stem cells research. Stem Cells Int 2015:167025
Mirzadeh Z, Alonge KM, Cabrales E, Herranz-Perez V, Scarlett JM, Brown JM, Hassouna R, Matsen ME, Nguyen HT, Garcia-Verdugo JM et al (2019) Perineuronal net formation during the critical period for neuronal maturation in the hypothalamic arcuate nucleus. Nat Metab 1(2):212–221
Huang H, Joffrin AM, Zhao Y, Miller GM, Zhang GC, Oka Y, Hsieh-Wilson LC (2023) Chondroitin 4-O-sulfation regulates hippocampal perineuronal nets and social memory. Proc Natl Acad Sci U S A 120(24):e2301312120
Iwakura Y, Kobayashi Y, Namba H, Nawa H, Takei N (2024) Epidermal growth factor suppresses the development of gabaergic neurons via the modulation of perineuronal net formation in the neocortex of developing rodent brains. Neurochem Res 49(5):1347–1358
Sun J, Wang X, Sun R, Xiao X, Wang Y, Peng Y, Gao Y (2023) Microglia shape AgRP neuron postnatal development via regulating perineuronal net plasticity. Mol Psychiatry. https://doi.org/10.1038/s41380-023-02326-2
Willis A, Pratt JA, Morris BJ (2022) Enzymatic degradation of cortical perineuronal nets reverses GABAergic interneuron maturation. Mol Neurobiol 59(5):2874–2893
Chen S, Xu H, Dong S, Xiao L (2022) Morpho-electric properties and diversity of oxytocin neurons in paraventricular nucleus of hypothalamus in female and male mice. J Neurosci 42(14):2885–2904
Walter MH, Abele H, Plappert CF (2021) The role of oxytocin and the effect of stress during childbirth: neurobiological basics and implications for mother and child. Front Endocrinol (Lausanne) 12:742236
Dabrowska J, Hazra R, Ahern TH, Guo JD, McDonald AJ, Mascagni F, Muller JF, Young LJ, Rainnie DG (2011) Neuroanatomical evidence for reciprocal regulation of the corticotrophin-releasing factor and oxytocin systems in the hypothalamus and the bed nucleus of the stria terminalis of the rat: Implications for balancing stress and affect. Psychoneuroendocrinology 36(9):1312–1326
Eliava M, Melchior M, Knobloch-Bollmann HS, Wahis J, da Silva GM, Tang Y, Ciobanu AC, Triana Del Rio R, Roth LC, Althammer F et al (2016) A new population of parvocellular oxytocin neurons controlling magnocellular neuron activity and inflammatory pain processing. Neuron 89(6):1291–1304
McCarthy MM (2010) How it’s made: organisational effects of hormones on the developing brain. J Neuroendocrinol 22(7):736–742
Tsukamura H, Homma T, Tomikawa J, Uenoyama Y, Maeda K (2010) Sexual differentiation of kisspeptin neurons responsible for sex difference in gonadotropin release in rats. Ann N Y Acad Sci 1200:95–103
Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M (2007) Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology 148(4):1774–1783
Simerly RB (2002) Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci 25:507–536
Morris JA, Jordan CL, Breedlove SM (2004) Sexual differentiation of the vertebrate nervous system. Nat Neurosci 7(10):1034–1039
Homma T, Sakakibara M, Yamada S, Kinoshita M, Iwata K, Tomikawa J, Kanazawa T, Matsui H, Takatsu Y, Ohtaki T et al (2009) Significance of neonatal testicular sex steroids to defeminize anteroventral periventricular kisspeptin neurons and the GnRH/LH surge system in male rats. Biol Reprod 81(6):1216–1225
Clarkson J, Herbison AE (2016) Hypothalamic control of the male neonatal testosterone surge. Philos Trans R Soc Lond B Biol Sci 371(1688):20150115
Wright CL, Schwarz JS, Dean SL, McCarthy MM (2010) Cellular mechanisms of estradiol-mediated sexual differentiation of the brain. Trends Endocrinol Metab 21(9):553–561
Bateman HL, Patisaul HB (2008) Disrupted female reproductive physiology following neonatal exposure to phytoestrogens or estrogen specific ligands is associated with decreased GnRH activation and kisspeptin fiber density in the hypothalamus. Neurotoxicology 29(6):988–997
Clarkson J, Boon WC, Simpson ER, Herbison AE (2009) Postnatal development of an estradiol-kisspeptin positive feedback mechanism implicated in puberty onset. Endocrinology 150(7):3214–3220
Semaan SJ, Murray EK, Poling MC, Dhamija S, Forger NG, Kauffman AS (2010) BAX-dependent and BAX-independent regulation of Kiss1 neuron development in mice. Endocrinology 151(12):5807–5817
Mayer C, Acosta-Martinez M, Dubois SL, Wolfe A, Radovick S, Boehm U, Levine JE (2010) Timing and completion of puberty in female mice depend on estrogen receptor alpha-signaling in kisspeptin neurons. Proc Natl Acad Sci U S A 107(52):22693–22698
van Veen JE, Kammel LG, Bunda PC, Shum M, Reid MS, Massa MG, Arneson D, Park JW, Zhang Z, Joseph AM et al (2020) Hypothalamic estrogen receptor alpha establishes a sexually dimorphic regulatory node of energy expenditure. Nat Metab 2(4):351–363
Krause WC, Rodriguez R, Gegenhuber B, Matharu N, Rodriguez AN, Padilla-Roger AM, Toma K, Herber CB, Correa SM, Duan X et al (2021) Oestrogen engages brain MC4R signalling to drive physical activity in female mice. Nature 599(7883):131–135
Correa SM, Newstrom DW, Warne JP, Flandin P, Cheung CC, Lin-Moore AT, Pierce AA, Xu AW, Rubenstein JL, Ingraham HA (2015) An estrogen-responsive module in the ventromedial hypothalamus selectively drives sex-specific activity in females. Cell Rep 10(1):62–74
Feng C, Wang Y, Zha X, Cao H, Huang S, Cao D, Zhang K, Xie T, Xu X, Liang Z, Zhang Z (2022) Cold-sensitive ventromedial hypothalamic neurons control homeostatic thermogenesis and social interaction-associated hyperthermia. Cell Metab 34(6):888–901
Shi H, Seeley RJ, Clegg DJ (2009) Sexual differences in the control of energy homeostasis. Front Neuroendocrinol 30(3):396–404
Tramunt B, Smati S, Grandgeorge N, Lenfant F, Arnal JF, Montagner A, Gourdy P (2020) Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia 63(3):453–461
Bermingham KM, Linenberg I, Hall WL, Kade K, Franks PW, Davies R, Wolf J, Hadjigeorgiou G, Asnicar F, Segata N et al (2022) Menopause is associated with postprandial metabolism, metabolic health and lifestyle: The ZOE PREDICT study. EBioMedicine 85:104303
Marlatt KL, Pitynski-Miller DR, Gavin KM, Moreau KL, Melanson EL, Santoro N, Kohrt WM (2022) Body composition and cardiometabolic health across the menopause transition. Obesity (Silver Spring) 30(1):14–27
Boldarine VT, Pedroso AP, Brandao-Teles C, LoTurco EG, Nascimento CMO, Oyama LM, Bueno AA, Martins-de-Souza D, Ribeiro EB (2020) Ovariectomy modifies lipid metabolism of retroperitoneal white fat in rats: a proteomic approach. Am J Physiol Endocrinol Metab 319(2):E427–E437
Costa-Beber LC, Goettems-Fiorin PB, Dos Santos JB, Friske PT, Frizzo MN, Heck TG, Hirsch GE, Ludwig MS (2021) Ovariectomy enhances female rats’ susceptibility to metabolic, oxidative, and heat shock response effects induced by a high-fat diet and fine particulate matter. Exp Gerontol 145:111215
Wang C, He Y, Xu P, Yang Y, Saito K, Xia Y, Yan X, Hinton A Jr, Yan C, Ding H et al (2018) TAp63 contributes to sexual dimorphism in POMC neuron functions and energy homeostasis. Nat Commun 9(1):1544
Burke LK, Doslikova B, D’Agostino G, Greenwald-Yarnell M, Georgescu T, Chianese R, Martinez de Morentin PB, Ogunnowo-Bada E, Cansell C, Valencia-Torres L et al (2016) Sex difference in physical activity, energy expenditure and obesity driven by a subpopulation of hypothalamic POMC neurons. Mol Metab 5(3):245–252
Gonzalez-Garcia I, Garcia-Clave E, Cebrian-Serrano A, Le Thuc O, Contreras RE, Xu Y, Gruber T, Schriever SC, Legutko B, Lintelmann J et al (2023) Estradiol regulates leptin sensitivity to control feeding via hypothalamic Cited1. Cell Metab 35(3):438–455
Xu AW, Ste-Marie L, Kaelin CB, Barsh GS (2007) Inactivation of signal transducer and activator of transcription 3 in proopiomelanocortin (Pomc) neurons causes decreased pomc expression, mild obesity, and defects in compensatory refeeding. Endocrinology 148(1):72–80
Xu Y, Nedungadi TP, Zhu L, Sobhani N, Irani BG, Davis KE, Zhang X, Zou F, Gent LM, Hahner LD et al (2011) Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab 14(4):453–465
Yang Y, van der Klaauw AA, Zhu L, Cacciottolo TM, He Y, Stadler LKJ, Wang C, Xu P, Saito K, Hinton A Jr et al (2019) Steroid receptor coactivator-1 modulates the function of Pomc neurons and energy homeostasis. Nat Commun 10(1):1718
Yu M, Bean JC, Liu H, He Y, Yang Y, Cai X, Yu K, Pei Z, Liu H, Tu L et al (2022) SK3 in POMC neurons plays a sexually dimorphic role in energy and glucose homeostasis. Cell Biosci 12(1):170
Mansano NDS, Vieira HR, Araujo-Lopes R, Szawka RE, Donato J Jr, Frazao R (2023) Fasting modulates GABAergic synaptic transmission to arcuate kisspeptin neurons in female mice. Endocrinology. https://doi.org/10.1210/endocr/bqad150
Han Y, He Y, Harris L, Xu Y, Wu Q (2023) Identification of a GABAergic neural circuit governing leptin signaling deficiency-induced obesity. Elife. https://doi.org/10.7554/eLife.8264
Egan OK, Inglis MA, Anderson GM (2017) Leptin signaling in AgRP neurons modulates puberty onset and adult fertility in mice. J Neurosci 37(14):3875–3886
Padilla SL, Qiu J, Nestor CC, Zhang C, Smith AW, Whiddon BB, Ronnekleiv OK, Kelly MJ, Palmiter RD (2017) AgRP to kiss1 neuron signaling links nutritional state and fertility. Proc Natl Acad Sci U S A 114(9):2413–2418
Decourt C, Connolly G, Ancel C, Inglis MA, Anderson GM (2022) Agouti-related peptide neuronal silencing overcomes delayed puberty in neonatally underfed male mice. J Neuroendocrinol 34(10):e13190
Wang L, Moenter SM (2020) Differential roles of hypothalamic AVPV and arcuate kisspeptin neurons in estradiol feedback regulation of female reproduction. Neuroendocrinology 110(3–4):172–184
Fu LY, van den Pol AN (2010) Kisspeptin directly excites anorexigenic proopiomelanocortin neurons but inhibits orexigenic neuropeptide Y cells by an indirect synaptic mechanism. J Neurosci 30(30):10205–10219
Qiu J, Rivera HM, Bosch MA, Padilla SL, Stincic TL, Palmiter RD, Kelly MJ, Ronnekleiv OK (2018) Estrogenic-dependent glutamatergic neurotransmission from kisspeptin neurons governs feeding circuits in females. Elife. https://doi.org/10.7554/eLife.35656
Narita K, Nagao K, Bannai M, Ichimaru T, Nakano S, Murata T, Higuchi T, Takahashi M (2011) Dietary deficiency of essential amino acids rapidly induces cessation of the rat estrous cycle. PLoS ONE 6(11):e28136
Alonso-Caraballo Y, Ferrario CR (2019) Effects of the estrous cycle and ovarian hormones on cue-triggered motivation and intrinsic excitability of medium spiny neurons in the nucleus accumbens core of female rats. Horm Behav 116:104583
Schneider JE, Wise JD, Benton NA, Brozek JM, Keen-Rhinehart E (2013) When do we eat? Ingestive behavior, survival, and reproductive success. Horm Behav 64(4):702–728
Olofsson LE, Pierce AA, Xu AW (2009) Functional requirement of AgRP and NPY neurons in ovarian cycle-dependent regulation of food intake. Proc Natl Acad Sci U S A 106(37):15932–15937
Smith AW, Bosch MA, Wagner EJ, Ronnekleiv OK, Kelly MJ (2013) The membrane estrogen receptor ligand STX rapidly enhances GABAergic signaling in NPY/AgRP neurons: role in mediating the anorexigenic effects of 17beta-estradiol. Am J Physiol Endocrinol Metab 305(5):E632–E640
Xu AW, Kaelin CB, Morton GJ, Ogimoto K, Stanhope K, Graham J, Baskin DG, Havel P, Schwartz MW, Barsh GS (2005) Effects of hypothalamic neurodegeneration on energy balance. PLoS Biol 3(12):e415
Takeo T, Chiba Y, Sakuma Y (1993) Suppression of the lordosis reflex of female rats by efferents of the medial preoptic area. Physiol Behav 53(5):831–838
Eckersell CB, Popper P, Micevych PE (1998) Estrogen-induced alteration of mu-opioid receptor immunoreactivity in the medial preoptic nucleus and medial amygdala. J Neurosci 18(10):3967–3976
Sinchak K, Micevych PE (2001) Progesterone blockade of estrogen activation of mu-opioid receptors regulates reproductive behavior. J Neurosci 21(15):5723–5729
Mills RH, Sohn RK, Micevych PE (2004) Estrogen-induced mu-opioid receptor internalization in the medial preoptic nucleus is mediated via neuropeptide Y-Y1 receptor activation in the arcuate nucleus of female rats. J Neurosci 24(4):947–955
Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych P (2007) Membrane estrogen receptor-alpha interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. J Neurosci 27(35):9294–9300
Sanathara NM, Moreas J, Mahavongtrakul M, Sinchak K (2014) Estradiol upregulates progesterone receptor and orphanin FQ colocalization in arcuate nucleus neurons and opioid receptor-like receptor-1 expression in proopiomelanocortin neurons that project to the medial preoptic nucleus in the female rat. Neuroendocrinology 100(2–3):103–118
Yin L, Hashikawa K, Hashikawa Y, Osakada T, Lischinsky JE, Diaz V, Lin D (2022) VMHvll(Cckar) cells dynamically control female sexual behaviors over the reproductive cycle. Neuron 110(18):3000–3017
Martinez de Morentin PB, Gonzalez-Garcia I, Martins L, Lage R, Fernandez-Mallo D, Martinez-Sanchez N, Ruiz-Pino F, Liu J, Morgan DA, Pinilla L et al (2014) Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metab 20(1):41–53
Ye H, Feng B, Wang C, Saito K, Yang Y, Ibrahimi L, Schaul S, Patel N, Saenz L, Luo P et al (2022) An estrogen-sensitive hypothalamus-midbrain neural circuit controls thermogenesis and physical activity. Sci Adv 8(3):0185
Douglass AM, Resch JM, Madara JC, Kucukdereli H, Yizhar O, Grama A, Yamagata M, Yang Z, Lowell BB (2023) Neural basis for fasting activation of the hypothalamic-pituitary-adrenal axis. Nature 620(7972):154–162
Zhang Y, Du C, Wang W, Qiao W, Li Y, Zhang Y, Sheng S, Zhou X, Zhang L, Fan H et al (2024) Glucocorticoids increase adiposity by stimulating Kruppel-like factor 9 expression in macrophages. Nat Commun 15(1):1190
Fernandez G, Cabral A, De Francesco PN, Uriarte M, Reynaldo M, Castrogiovanni D, Zubiria G, Giovambattista A, Cantel S, Denoyelle S et al (2022) GHSR controls food deprivation-induced activation of CRF neurons of the hypothalamic paraventricular nucleus in a LEAP2-dependent manner. Cell Mol Life Sci 79(5):277
Yuan Y, Wu W, Chen M, Cai F, Fan C, Shen W, Sun W, Hu J (2019) Reward inhibits paraventricular CRH neurons to relieve stress. Curr Biol 29(7):1243–1251
Kim J, Lee S, Fang YY, Shin A, Park S, Hashikawa K, Bhat S, Kim D, Sohn JW, Lin D, Suh GSB (2019) Rapid, biphasic CRF neuronal responses encode positive and negative valence. Nat Neurosci 22(4):576–585
de Kloet ER, Karst H, Joels M (2008) Corticosteroid hormones in the central stress response: quick-and-slow. Front Neuroendocrinol 29(2):268–272
Jiang Z, Chen C, Weiss GL, Fu X, Stelly CE, Sweeten BLW, Tirrell PS, Pursell I, Stevens CR, Fisher MO et al (2022) Stress-induced glucocorticoid desensitizes adrenoreceptors to gate the neuroendocrine response to somatic stress in male mice. Cell Rep 41(3):111509
Evanson NK, Tasker JG, Hill MN, Hillard CJ, Herman JP (2010) Fast feedback inhibition of the HPA axis by glucocorticoids is mediated by endocannabinoid signaling. Endocrinology 151(10):4811–4819
Ga R, Campbell EJ, Walker LC, S SCn, Muthmainah M, F SK, A MG, O’Shea MJ, He S, Dayas CV, et al (2023) A paraventricular thalamus to insular cortex glutamatergic projection gates “emotional” stress-induced binge eating in females. Neuropsychopharmacology 48(13):1931–1940
Fischer S, Breithaupt L, Wonderlich J, Westwater ML, Crosby RD, Engel SG, Thompson J, Lavender J, Wonderlich S (2017) Impact of the neural correlates of stress and cue reactivity on stress related binge eating in the natural environment. J Psychiatr Res 92:15–23
Lim MC, Parsons S, Goglio A, Fox E (2021) Anxiety, stress, and binge eating tendencies in adolescence: a prospective approach. J Eat Disord 9(1):94
Luby JL, Baram TZ, Rogers CE, Barch DM (2020) Neurodevelopmental optimization after early-life adversity: cross-species studies to elucidate sensitive periods and brain mechanisms to inform early intervention. Trends Neurosci 43(10):744–751
Gee DG (2021) Early adversity and development: parsing heterogeneity and identifying pathways of risk and resilience. Am J Psychiatry 178(11):998–1013
Molet J, Heins K, Zhuo X, Mei YT, Regev L, Baram TZ, Stern H (2016) Fragmentation and high entropy of neonatal experience predict adolescent emotional outcome. Transl Psychiatry 6(1):e702
Bolton JL, Molet J, Regev L, Chen Y, Rismanchi N, Haddad E, Yang DZ, Obenaus A, Baram TZ (2018) Anhedonia following early-life adversity involves aberrant interaction of reward and anxiety circuits and is reversed by partial silencing of amygdala corticotropin-releasing hormone gene. Biol Psychiatry 83(2):137–147
Levis SC, Bentzley BS, Molet J, Bolton JL, Perrone CR, Baram TZ, Mahler SV (2021) On the early life origins of vulnerability to opioid addiction. Mol Psychiatry 26(8):4409–4416
Treadway MT, Zald DH (2011) Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev 35(3):537–555
Gunn BG, Cunningham L, Cooper MA, Corteen NL, Seifi M, Swinny JD, Lambert JJ, Belelli D (2013) Dysfunctional astrocytic and synaptic regulation of hypothalamic glutamatergic transmission in a mouse model of early-life adversity: relevance to neurosteroids and programming of the stress response. J Neurosci 33(50):19534–19554
Bolton JL, Short AK, Othy S, Kooiker CL, Shao M, Gunn BG, Beck J, Bai X, Law SM, Savage JC et al (2022) Early stress-induced impaired microglial pruning of excitatory synapses on immature CRH-expressing neurons provokes aberrant adult stress responses. Cell Rep 38(13):110600
Short AK, Thai CW, Chen Y, Kamei N, Pham AL, Birnie MT, Bolton JL, Mortazavi A, Baram TZ (2023) Single-cell transcriptional changes in hypothalamic corticotropin-releasing factor-expressing neurons after early-life adversity inform enduring alterations in vulnerabilities to stress. Biol Psychiatry Glob Open Sci 3(1):99–109
Korosi A, Shanabrough M, McClelland S, Liu ZW, Borok E, Gao XB, Horvath TL, Baram TZ (2010) Early-life experience reduces excitation to stress-responsive hypothalamic neurons and reprograms the expression of corticotropin-releasing hormone. J Neurosci 30(2):703–713
Singh-Taylor A, Molet J, Jiang S, Korosi A, Bolton JL, Noam Y, Simeone K, Cope J, Chen Y, Mortazavi A, Baram TZ (2018) NRSF-dependent epigenetic mechanisms contribute to programming of stress-sensitive neurons by neonatal experience, promoting resilience. Mol Psychiatry 23(3):648–657
Fenoglio KA, Brunson KL, Avishai-Eliner S, Chen Y, Baram TZ (2004) Region-specific onset of handling-induced changes in corticotropin-releasing factor and glucocorticoid receptor expression. Endocrinology 145(6):2702–2706
Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ (2005) Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology 30(12):2192–2204
Shin S, You IJ, Jeong M, Bae Y, Wang XY, Cawley ML, Han A, Lim BK (2023) Early adversity promotes binge-like eating habits by remodeling a leptin-responsive lateral hypothalamus-brainstem pathway. Nat Neurosci 26(1):79–91
Attig L, Solomon G, Ferezou J, Abdennebi-Najar L, Taouis M, Gertler A, Djiane J (2008) Early postnatal leptin blockage leads to a long-term leptin resistance and susceptibility to diet-induced obesity in rats. Int J Obes (Lond) 32(7):1153–1160
McCormick CM, Kehoe P, Kovacs S (1998) Corticosterone release in response to repeated, short episodes of neonatal isolation: evidence of sensitization. Int J Dev Neurosci 16(3–4):175–185
Power EM, Iremonger KJ (2021) Plasticity of intrinsic excitability across the estrous cycle in hypothalamic CRH neurons. Sci Rep 11(1):16700
Li XF, Bowe JE, Lightman SL, O’Byrne KT (2005) Role of corticotropin-releasing factor receptor-2 in stress-induced suppression of pulsatile luteinizing hormone secretion in the rat. Endocrinology 146(1):318–322
Li XF, Bowe JE, Kinsey-Jones JS, Brain SD, Lightman SL, O’Byrne KT (2006) Differential role of corticotrophin-releasing factor receptor types 1 and 2 in stress-induced suppression of pulsatile luteinising hormone secretion in the female rat. J Neuroendocrinol 18(8):602–610
Cagampang FR, Cates PS, Sandhu S, Strutton PH, McGarvey C, Coen CW, O’Byrne KT (1997) Hypoglycaemia-induced inhibition of pulsatile luteinizing hormone secretion in female rats: role of oestradiol, endogenous opioids and the adrenal medulla. J Neuroendocrinol 9(11):867–872
Li XF, Mitchell JC, Wood S, Coen CW, Lightman SL, O’Byrne KT (2003) The effect of oestradiol and progesterone on hypoglycaemic stress-induced suppression of pulsatile luteinizing hormone release and on corticotropin-releasing hormone mRNA expression in the rat. J Neuroendocrinol 15(5):468–476
Cates PS, Li XF, O’Byrne KT (2004) The influence of 17beta-oestradiol on corticotrophin-releasing hormone induced suppression of luteinising hormone pulses and the role of CRH in hypoglycaemic stress-induced suppression of pulsatile LH secretion in the female rat. Stress 7(2):113–118
Wagenmaker ER, Moenter SM (2017) Exposure to acute psychosocial stress disrupts the luteinizing hormone surge independent of estrous cycle alterations in female mice. Endocrinology 158(8):2593–2602
Phumsatitpong C, Moenter SM (2018) Estradiol-dependent stimulation and suppression of gonadotropin-releasing hormone neuron firing activity by corticotropin-releasing hormone in female mice. Endocrinology 159(1):414–425
Sohn JW, Xu Y, Jones JE, Wickman K, Williams KW, Elmquist JK (2011) Serotonin 2C receptor activates a distinct population of arcuate pro-opiomelanocortin neurons via TRPC channels. Neuron 71(3):488–497
Lam BYH, Cimino I, Polex-Wolf J, Nicole Kohnke S, Rimmington D, Iyemere V, Heeley N, Cossetti C, Schulte R, Saraiva LR et al (2017) Heterogeneity of hypothalamic pro-opiomelanocortin-expressing neurons revealed by single-cell RNA sequencing. Mol Metab 6(5):383–392
Campbell JN, Macosko EZ, Fenselau H, Pers TH, Lyubetskaya A, Tenen D, Goldman M, Verstegen AM, Resch JM, McCarroll SA et al (2017) A molecular census of arcuate hypothalamus and median eminence cell types. Nat Neurosci 20(3):484–496
Quarta C, Claret M, Zeltser LM, Williams KW, Yeo GSH, Tschop MH, Diano S, Bruning JC, Cota D (2021) POMC neuronal heterogeneity in energy balance and beyond: an integrated view. Nat Metab 3(3):299–308
Betley JN, Xu S, Cao ZFH, Gong R, Magnus CJ, Yu Y, Sternson SM (2015) Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature 521(7551):180–185
Mandelblat-Cerf Y, Ramesh RN, Burgess CR, Patella P, Yang Z, Lowell BB, Andermann ML (2015) Arcuate hypothalamic AgRP and putative POMC neurons show opposite changes in spiking across multiple timescales. Elife. https://doi.org/10.7554/eLife.07122
Huisman C, Cho H, Brock O, Lim SJ, Youn SM, Park Y, Kim S, Lee SK, Delogu A, Lee JW (2019) Single cell transcriptome analysis of developing arcuate nucleus neurons uncovers their key developmental regulators. Nat Commun 10(1):3696
Chung D, Shum A, Caraveo G (2020) GAP-43 and BASP1 in axon regeneration: implications for the treatment of neurodegenerative diseases. Front Cell Dev Biol 8:567537
Kuperman Y, Weiss M, Dine J, Staikin K, Golani O, Ramot A, Nahum T, Kuhne C, Shemesh Y, Wurst W et al (2016) CRFR1 in AgRP neurons modulates sympathetic nervous system activity to adapt to cold stress and fasting. Cell Metab 23(6):1185–1199
Chen W, Mehlkop O, Scharn A, Nolte H, Klemm P, Henschke S, Steuernagel L, Sotelo-Hitschfeld T, Kaya E, Wunderlich CM et al (2023) Nutrient-sensing AgRP neurons relay control of liver autophagy during energy deprivation. Cell Metab 35(5):786–806
Palmiter RD (2007) Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci 30(8):375–381
Szczypka MS, Rainey MA, Kim DS, Alaynick WA, Marck BT, Matsumoto AM, Palmiter RD (1999) Feeding behavior in dopamine-deficient mice. Proc Natl Acad Sci U S A 96(21):12138–12143
Zhang Q, Tang Q, Purohit NM, Davenport JB, Brennan C, Patel RK, Godschall E, Zwiefel LS, Spano A, Campbell JN, Guler AD (2022) Food-induced dopamine signaling in AgRP neurons promotes feeding. Cell Rep 41(9):111718
Alhadeff AL, Goldstein N, Park O, Klima ML, Vargas A, Betley JN (2019) Natural and drug rewards engage distinct pathways that converge on coordinated hypothalamic and reward circuits. Neuron 103(5):891–908
Mazzone CM, Liang-Guallpa J, Li C, Wolcott NS, Boone MH, Southern M, Kobzar NP, Salgado IA, Reddy DM, Sun F et al (2020) High-fat food biases hypothalamic and mesolimbic expression of consummatory drives. Nat Neurosci 23(10):1253–1266
Reichenbach A, Clarke RE, Stark R, Lockie SH, Mequinion M, Dempsey H, Rawlinson S, Reed F, Sepehrizadeh T, DeVeer M et al (2022) Metabolic sensing in AgRP neurons integrates homeostatic state with dopamine signalling in the striatum. Elife. https://doi.org/10.7554/eLife.72668
Kumar D, Candlish M, Periasamy V, Avcu N, Mayer C, Boehm U (2015) Specialized subpopulations of kisspeptin neurons communicate with GnRH neurons in female mice. Endocrinology 156(1):32–38
Matsuda F, Ohkura S, Magata F, Munetomo A, Chen J, Sato M, Inoue N, Uenoyama Y, Tsukamura H (2019) Role of kisspeptin neurons as a GnRH surge generator: comparative aspects in rodents and non-rodent mammals. J Obstet Gynaecol Res 45(12):2318–2329
Moore AM, Coolen LM, Lehman MN (2022) In vivo imaging of the GnRH pulse generator reveals a temporal order of neuronal activation and synchronization during each pulse. Proc Natl Acad Sci U S A. https://doi.org/10.1073/pnas.2117767119
Porteous R, Petersen SL, Yeo SH, Bhattarai JP, Ciofi P, de Tassigny XD, Colledge WH, Caraty A, Herbison AE (2011) Kisspeptin neurons co-express met-enkephalin and galanin in the rostral periventricular region of the female mouse hypothalamus. J Comp Neurol 519(17):3456–3469
Manchishi S, Prater M, Colledge WH (2024) Transcriptional profiling of Kiss1 neurons from arcuate and rostral periventricular hypothalamic regions in female mice. Reproduction. https://doi.org/10.1530/REP-23-0342
Cheng G, Coolen LM, Padmanabhan V, Goodman RL, Lehman MN (2010) The kisspeptin/neurokinin B/dynorphin (KNDy) cell population of the arcuate nucleus: sex differences and effects of prenatal testosterone in sheep. Endocrinology 151(1):301–311
Zhou S, Zang S, Hu Y, Shen Y, Li H, Chen W, Li P, Shen Y (2022) Transcriptome-scale spatial gene expression in rat arcuate nucleus during puberty. Cell Biosci 12(1):8
Clarkson J, Herbison AE (2011) Dual phenotype kisspeptin-dopamine neurones of the rostral periventricular area of the third ventricle project to gonadotrophin-releasing hormone neurones. J Neuroendocrinol 23(4):293–301
Stephens SBZ, Rouse ML, Tolson KP, Liaw RB, Parra RA, Chahal N, Kauffman AS (2017) Effects of Selective Deletion of Tyrosine Hydroxylase from Kisspeptin Cells on Puberty and Reproduction in Male and Female Mice. eNeuro. https://doi.org/10.1523/ENEURO.0150-17.201
Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE (2005) Morphologic evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence. J Comp Neurol 489(3):372–386
Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Imamoglu S et al (2009) TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nat Genet 41(3):354–358
Uenoyama Y, Tsuchida H, Nagae M, Inoue N, Tsukamura H (2022) Opioidergic pathways and kisspeptin in the regulation of female reproduction in mammals. Front Neurosci 16:958377
Nakamura S, Watanabe Y, Goto T, Ikegami K, Inoue N, Uenoyama Y, Tsukamura H (2022) Kisspeptin neurons as a key player bridging the endocrine system and sexual behavior in mammals. Front Neuroendocrinol 64:100952
Ramos-Pittol JM, Fernandes-Freitas I, Milona A, Manchishi SM, Rainbow K, Lam BYH, Tadross JA, Beucher A, Colledge WH, Cebola I et al (2023) Dax1 modulates ERalpha-dependent hypothalamic estrogen sensing in female mice. Nat Commun 14(1):3076
Smith JT (2013) Sex steroid regulation of kisspeptin circuits. Adv Exp Med Biol 784:275–295
Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA (2005) Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146(9):3686–3692
Gocz B, Takacs S, Skrapits K, Rumpler E, Solymosi N, Poliska S, Colledge WH, Hrabovszky E, Sarvari M (2022) Estrogen differentially regulates transcriptional landscapes of preoptic and arcuate kisspeptin neuron populations. Front Endocrinol (Lausanne) 13:960769
Campbell RE, Coolen LM, Hoffman GE, Hrabovszky E (2022) Highlights of neuroanatomical discoveries of the mammalian gonadotropin-releasing hormone system. J Neuroendocrinol 34(5):e13115
Shan Y, Saadi H, Wray S (2020) Heterogeneous origin of gonadotropin releasing hormone-1 neurons in mouse embryos detected by islet-1/2 expression. Front Cell Dev Biol 8:35
Duittoz AH, Tillet Y, Geller S (2022) The great migration: How glial cells could regulate GnRH neuron development and shape adult reproductive life. J Chem Neuroanat 125:102149
Pellegrino G, Martin M, Allet C, Lhomme T, Geller S, Franssen D, Mansuy V, Manfredi-Lozano M, Coutteau-Robles A, Delli V et al (2021) GnRH neurons recruit astrocytes in infancy to facilitate network integration and sexual maturation. Nat Neurosci 24(12):1660–1672
Cannarella R, Paganoni AJJ, Cicolari S, Oleari R, Condorelli RA, La Vignera S, Cariboni A, Calogero AE, Magni P (2021) Anti-mullerian hormone, growth hormone, and insulin-like growth factor 1 modulate the migratory and secretory patterns of GnRH neurons. Int J Mol Sci 22(5):2445
Paganoni AJJ, Cannarella R, Oleari R, Amoruso F, Antal R, Ruzza M, Olivieri C, Condorelli RA, La Vignera S, Tolaj F et al (2023) Insulin-like growth factor 1, growth hormone, and anti-mullerian hormone receptors are differentially expressed during gnrh neuron development. Int J Mol Sci 24(17):13073
Wierman ME, Kiseljak-Vassiliades K, Tobet S (2011) Gonadotropin-releasing hormone (GnRH) neuron migration: initiation, maintenance and cessation as critical steps to ensure normal reproductive function. Front Neuroendocrinol 32(1):43–52
Levine JE, Norman RL, Gliessman PM, Oyama TT, Bangsberg DR, Spies HG (1985) In vivo gonadotropin-releasing hormone release and serum luteinizing hormone measurements in ovariectomized, estrogen-treated rhesus macaques. Endocrinology 117(2):711–721
Moffitt JR, Bambah-Mukku D, Eichhorn SW, Vaughn E, Shekhar K, Perez JD, Rubinstein ND, Hao J, Regev A, Dulac C, Zhuang X (2018) Molecular, spatial, and functional single-cell profiling of the hypothalamic preoptic region. Science. https://doi.org/10.1126/science.aau5324
Bardoczi Z, Wilheim T, Skrapits K, Hrabovszky E, Racz G, Matolcsy A, Liposits Z, Sliwowska JH, Dobolyi A, Kallo I (2018) GnRH neurons provide direct input to hypothalamic tyrosine hydroxylase immunoreactive neurons which is maintained during lactation. Front Endocrinol (Lausanne) 9:685
Mitchell V, Loyens A, Spergel DJ, Flactif M, Poulain P, Tramu G, Beauvillain JC (2003) A confocal microscopic study of gonadotropin-releasing hormone (GnRH) neuron inputs to dopaminergic neurons containing estrogen receptor alpha in the arcuate nucleus of GnRH-green fluorescent protein transgenic mice. Neuroendocrinology 77(3):198–207
Romanov RA, Alpar A, Hokfelt T, Harkany T (2017) Molecular diversity of corticotropin-releasing hormone mRNA-containing neurons in the hypothalamus. J Endocrinol 232(3):R161–R172
Berkhout JB, Poormoghadam D, Yi C, Kalsbeek A, Meijer OC, Mahfouz A (2024) An integrated single-cell RNA-seq atlas of the mouse hypothalamic paraventricular nucleus links transcriptomic and functional types. J Neuroendocrinol 36(2):e13367
Jeanneteau FD, Lambert WM, Ismaili N, Bath KG, Lee FS, Garabedian MJ, Chao MV (2012) BDNF and glucocorticoids regulate corticotrophin-releasing hormone (CRH) homeostasis in the hypothalamus. Proc Natl Acad Sci U S A 109(4):1305–1310
Gillies GE, Linton EA, Lowry PJ (1982) Corticotropin releasing activity of the new CRF is potentiated several times by vasopressin. Nature 299(5881):355–357
Itoi K, Helmreich DL, Lopez-Figueroa MO, Watson SJ (1999) Differential regulation of corticotropin-releasing hormone and vasopressin gene transcription in the hypothalamus by norepinephrine. J Neurosci 19(13):5464–5472
Erkut ZA, Pool C, Swaab DF (1998) Glucocorticoids suppress corticotropin-releasing hormone and vasopressin expression in human hypothalamic neurons. J Clin Endocrinol Metab 83(6):2066–2073
Romanov RA, Alpar A, Zhang MD, Zeisel A, Calas A, Landry M, Fuszard M, Shirran SL, Schnell R, Dobolyi A et al (2015) A secretagogin locus of the mammalian hypothalamus controls stress hormone release. EMBO J 34(1):36–54
de Kloet AD, Wang L, Pitra S, Hiller H, Smith JA, Tan Y, Nguyen D, Cahill KM, Sumners C, Stern JE, Krause EG (2017) A unique, “angiotensin-sensitive” neuronal population coordinates neuroendocrine, cardiovascular, and behavioral responses to stress. J Neurosci 37(13):3478–3490
Clasadonte J, Prevot V (2018) The special relationship: glia-neuron interactions in the neuroendocrine hypothalamus. Nat Rev Endocrinol 14(1):25–44
Kohnke S, Buller S, Nuzzaci D, Ridley K, Lam B, Pivonkova H, Bentsen MA, Alonge KM, Zhao C, Tadross J et al (2021) Nutritional regulation of oligodendrocyte differentiation regulates perineuronal net remodeling in the median eminence. Cell Rep 36(2):109362
Garcia-Caceres C, Balland E, Prevot V, Luquet S, Woods SC, Koch M, Horvath TL, Yi CX, Chowen JA, Verkhratsky A et al (2019) Role of astrocytes, microglia, and tanycytes in brain control of systemic metabolism. Nat Neurosci 22(1):7–14
Xu Y, Yang D, Wang L, Krol E, Mazidi M, Li L, Huang Y, Niu C, Liu X, Lam SM et al (2023) Maternal high fat diet in lactation impacts hypothalamic neurogenesis and neurotrophic development, leading to later life susceptibility to obesity in male but not female mice. Adv Sci (Weinh) 10(35):e2305472
Mainardi M, Scabia G, Vottari T, Santini F, Pinchera A, Maffei L, Pizzorusso T, Maffei M (2010) A sensitive period for environmental regulation of eating behavior and leptin sensitivity. Proc Natl Acad Sci U S A 107(38):16673–16678
Patterson CM, Dunn-Meynell AA, Levin BE (2008) Three weeks of early-onset exercise prolongs obesity resistance in DIO rats after exercise cessation. Am J Physiol Regul Integr Comp Physiol 294(2):R290-301
He Z, Gao Y, Alhadeff AL, Castorena CM, Huang Y, Lieu L, Afrin S, Sun J, Betley JN, Guo H, Williams KW (2018) Cellular and synaptic reorganization of arcuate NPY/AgRP and POMC neurons after exercise. Mol Metab 18:107–119
Mainardi M, Pizzorusso T, Maffei M (2013) Environment, leptin sensitivity, and hypothalamic plasticity. Neural Plast 2013:438072
Scabia G, Barone I, Mainardi M, Ceccarini G, Scali M, Buzzigoli E, Dattilo A, Vitti P, Gastaldelli A, Santini F et al (2018) The antidepressant fluoxetine acts on energy balance and leptin sensitivity via BDNF. Sci Rep 8(1):1781
Barone I, Melani R, Mainardi M, Scabia G, Scali M, Dattilo A, Ceccarini G, Vitti P, Santini F, Maffei L et al (2018) Fluoxetine Modulates the Activity of Hypothalamic POMC Neurons via mTOR Signaling. Mol Neurobiol 55(12):9267–9279
Acknowledgements
CQ acknowledges INSERM, the European Research Council (ERCcog project 101124230 — Ghostbuster), the University of Bordeaux, Agencie Nationale Recherche (ANR-20-CE14-0046), French Societies of Endocrinology, Nutrition, and Diabetes (SFE, SFN, and SFD), Fyssen Foundation, and Institut Benjamin Delessert.
Author information
Authors and Affiliations
Contributions
THL, JCN, CQ wrote, revised, and amended the manuscript, JCN designed the graphic. CQ conceptualised the work and the ideas. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This study does not contain any studies with animals performed by any of the authors.
Informed consent
For this type of study formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, T.H., Nicolas, JC. & Quarta, C. Molecular and functional mapping of the neuroendocrine hypothalamus: a new era begins. J Endocrinol Invest (2024). https://doi.org/10.1007/s40618-024-02411-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40618-024-02411-5