Abstract
Purpose
Aging is associated with changes in glucose homeostasis related to both decreased insulin secretion and/or impaired insulin action, contributing to the high prevalence of type 2 diabetes (T2D) in the elderly population. Additionally, studies are showing that chronically high levels of circulating insulin can also lead to insulin resistance. In contrast, physical exercise has been a strategy used to improve insulin sensitivity and metabolic health. However, the molecular alterations resulting from the effects of physical exercise in the liver on age-related hyperinsulinemia conditions are not yet fully established. This study aimed to investigate the effects of 7 days of aerobic exercise on hepatic metabolism in aged hyperinsulinemic rats (i.e., Wistar and F344) and in Slc2a4+/− mice (hyperglycemic and hyperinsulinemic mice).
Results
Both aged models showed alterations in insulin and glucose tolerance, which were associated with essential changes in hepatic fat metabolism (lipogenesis, gluconeogenesis, and inflammation). In contrast, 7 days of physical exercise was efficient in improving whole-body glucose and insulin sensitivity, and hepatic metabolism. The Slc2a4+/− mice presented significant metabolic impairments (insulin resistance and hepatic fat accumulation) that were improved by short-term exercise training. In this scenario, high circulating insulin may be an important contributor to age-related insulin resistance and hepatic disarrangements in some specific conditions.
Conclusion
In conclusion, our data demonstrated that short-term aerobic exercise was able to control mechanisms related to hepatic fat accumulation and insulin sensitivity in aged rodents. These effects could contribute to late-life metabolic health and prevent the development/progression of age-related T2D.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the increase in life expectancy in recent decades, metabolic alterations in late life are emerging [1]. A common factor in aged individuals predisposed to type 2 diabetes (T2D) is insulin resistance [2]. A sedentary lifestyle, age-related sarcopenia, high caloric diets, and an obesogenic lifestyle are factors positively correlated with insulin resistance and T2D development [3]. Moreover, there is a close relationship between aging and metabolic liver complications [4]. This metabolic stress in glucose and fat metabolism is described to increase the circulating insulin levels as a compensatory mechanism to obtain whole-body homeostasis [5]. However, this model fails to explain the hyperinsulinemia presented in some individuals before the presence of insulin resistance [6, 7]. In addition, chronically high levels of circulating insulin can lead to insulin resistance and are associated with metabolic disturbances associated with aging, obesity, T2D, cardiovascular diseases, and cancer [7,8,9,10,11]. Nevertheless, the impacts of hyperinsulinemia at the molecular level on liver metabolism are not fully established.
The concentration of plasma insulin is regulated by its secretion by pancreatic islets and clearance (mainly by the liver, 50–80%) [12]. Thus, defects in insulin secretion/clearance control may appear in response to genetic and environmental factors, as well as aging [13,14,15]. The liver is a critical organ in controlling glucose homeostasis and fat metabolism, with an essential role of insulin that inhibits gluconeogenesis and stimulates glycogen synthesis and de novo lipogenesis (DNL) [16]. In an insulin-resistant state, this inhibition in gluconeogenesis is deficient, contributing to DNL, resulting in hyperglycemia and hepatic fat accumulation [16]. Non-alcoholic fatty liver disease (NAFLD) or metabolic-associated fatty liver disease (MAFLD) are both characterized by hepatic fat accumulation, showing a positive correlation with metabolic syndrome and a high frequency in aged individuals [4]. Interestingly, studies have shown that hyperinsulinemia and glucose intolerance play roles in developing hepatic steatosis, even in normal body weight patients [17]. Thus, strategies to improve metabolic health and liver function could be essential to prevent T2D development and the progression of NAFLD to non-alcoholic steatohepatitis (NASH) and cirrhosis.
Physical exercise is an effective strategy to improve whole-body glucose homeostasis, including liver function and hepatic metabolism [18]. In addition, individuals with critical hepatic disturbances show significant improvements when submitted to a physical exercise protocol, [18]. This beneficial effect is mediated by the indirect impact on whole-body glucose and fat metabolism, but also by the direct effect on the liver tissue, controlling the hepatic insulin signaling pathway, gluconeogenic enzymes, glycogen synthesis, and fatty acid metabolization [19, 20].
Insulin triggers an intracellular signal through the autophosphorylation of its membrane receptor (insulin receptor) and subsequent cytoplasmic protein phosphorylation (Insulin receptor substrate—IRS, phosphoinositide 3-kinase—PI3K, protein kinase B—PKB/Akt) [21]. However, chronic insulin exposure or hyperinsulinemia can promote hepatocyte senescence [22, 23]. On the other hand, cellular senescence is also an inductor of hepatic steatosis [24]. Cellular senescence is caused by a variety of stressors and could be one of the contributors to age-related disease, being characterized by irreversible proliferative cell arrest, which is mediated by p53, p21, and p16 activation [25]. These senescent cells have increased secretion of proinflammatory markers and senescence-associated secretory phenotype (SASP) mediating tissue dysfunction [25]. Interestingly, cellular senescence can be targeted using senolytic drugs (Dasatinib + Quercetin) in the context of hepatocyte dysfunction [22, 24], but the effects of physical exercise in this context are poorly explored. Then signaling pathways that are altered in the context of aging, hyperinsulinemia, and NAFLD may be potential targets to explore the effects of physical exercise.
In summary, we speculate that a few physical exercise training sessions would act on molecular mechanisms related to cellular senescence, hepatic fat accumulation, and glucose homeostasis in aged rodents with metabolic impairments. Moreover, this exercise training protocol could contribute to the understanding of mechanisms mediated directly by physical exercise, and not due to alterations in body weight and adiposity. Thus, the main objective of this study was to investigate the effects of 7 days (short-term) of aerobic physical exercise on hepatic metabolism in hyperinsulinemic aged rats (Wistar and F344) and in Slc2a4+/− mice (hyperglycemic and hyperinsulinemic mice).
Results
Aged F344 and Wistar rats are hyperinsulinemic and glucose intolerant independently of body weight gain, but physical exercise counteracts this
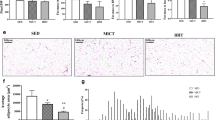
To access the effects of aging dependent or not on increased adiposity during aging, we looked at two aging models that showed different body weight patterns during development. First, a higher weight gain was observed during 17 months of development in Wistar rats than in F344 rats, where the Δbody weight between the 1st and 17th months was significantly higher in Wistar rats (Figure S1A–C). After confirming these morphological differences between the two species (Figure S1E and I), the aged animals were submitted to a short-term swimming physical exercise protocol, which decreased fasting glucose in F344 rats compared to aged sedentary animals and protected them from the higher hyperinsulinemia and HOMA-IR observed in aged F344 rats, independent of changes in the final body weight (Fig. 1a–d). Positive effects of physical exercise were also observed in the glucose and insulin tolerance tests compared to sedentary-aged rats (Fig. 1e, f). When the insulin signaling pathway was evaluated in the liver of F344 rats, aged animals showed a significant reduction in pIRY972, pIRS1Y612, pAktS473, and pGSK3βS9 compared to the young group (Fig. 1g). On the other hand, physical exercise restored hepatic pIRS1Y612, pAktS473, and pGSK3βS9 compared to sedentary-aged rats (Fig. 1h).
Physiological and metabolic parameters in F344 and Wistar rats. a Final body weight of F344 rats. b Fasting glucose. c Fasting insulin. d HOMA-IR index. e Glucose tolerance test curve (GTT) and area under the curve (AUC). f Insulin tolerance test curve (ITT) and area under the curve (AUC). g Insulin-signaling pathway proteins (pIRY972, pIRS1Y612, pAktS473, pGSK3βS9) in the hepatic tissue of young (n = 4) and aged (n = 5) F344 rats. h Insulin-signaling pathway proteins in the hepatic tissue of aged (n = 5) and aged + exercise (n = 5) F344 rats. i Final body weight of Wistar rats. j Fasting glucose. k Fasting insulin. l HOMA-IR index. m Glucose tolerance test curve (GTT) and area under the curve (AUC). n Insulin tolerance test curve (ITT) and area under the curve (AUC). o Insulin-signaling pathway proteins (pIRY972, pIRS1Y612, pAktS473, pGSK3βS9) in the hepatic tissue of young (n = 4) and aged (n = 5) Wistar rats. p Insulin-signaling pathway proteins in the hepatic tissue of aged (n = 5) and aged + exercise (n = 5) Wistar rats. In the physiological parameters and metabolic tests (a–f, i–n), n = 7 was used. Data were analyzed by one-way ANOVA followed by Tukey’s test (a–f, i–n), two-way ANOVA followed by Sidak's test (e, f, n, o), and unpaired two-tailed Student’s t test (g, h, o, p). *p < 0.05 versus the young group. #p < 0.05 versus aged group. ap < 0.05 young versus aged group. bp < 0.05 aged versus aged + exe group. cp < 0.05 young versus aged + exe group
The same metabolic characteristics were observed in Wistar rats without changing the fasting glucose levels (Fig. 1i, j). On the other hand, sedentary-aged Wistar rats demonstrated higher fasting insulin and HOMA-IR but impaired glucose and insulin tolerance compared to young rats (Fig. 1k–n). In contrast, exercised aged Wistar rats presented improvements in these parameters, with decreased fasting insulin levels and HOMA-IR, and improved glucose and insulin tolerance, with no change in body weight (Fig. 1k–n). Regarding hepatic insulin-related proteins, aged Wistar rats showed decreased pAktS473 compared to young rats but increased pAktS473 and pGSK3βS9 in response to physical exercise compared to sedentary-aged rats (Fig. 1o, p). In summary, both aging models demonstrated significant metabolic impairments and hyperinsulinemia, independently of the specific morphological conditions of each one.
Hepatic lipogenesis signals are increased in F344 and Wistar-aged rats and attenuated by physical exercise
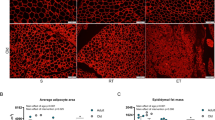
Despite the lack of crucial changes in the histological sections among the F344 groups, aged F344 rats displayed a tendency to accumulate TG (p = 0.06) in hepatic tissue and show higher circulating TG and cholesterol compared to young rats (Fig. 2a–c). Physical exercise decreased hepatic and circulating TG and cholesterol compared to sedentary-aged rats (Fig. 2a–c). These data were accompanied by impaired mitochondrial activity in response to ADP, succinate, and FCCP in sedentary-aged rats compared to young rats (Fig. 2d). These changes in fat metabolism were observed in mRNA levels of Cd36, Srebp1, Fas, Cpt1α, and Pparα. Aging increased the mRNA levels of Cd36 and Fas and showed a tendency to increase Srebp1 (p = 0.07) compared to the young group (Fig. 2e). At the same time, physical exercise decreased Fas levels and tended to decrease Srebp1 (p = 0.07) compared to the sedentary-aged group (Fig. 2e). Physical exercise also increased Cpt1α mRNA and tended to increase Pparα compared to aged F344 rats (Fig. 2e). In the protein level, decreased pAMPKT172, pACCS79 and increased SREBP1 were observed in the liver of aged F344 rats compared to young animals (Fig. 2f). Physical exercise was able to increase pACCS79 and decrease FAS content compared to sedentary-aged rats , with a trend to decrease in SPREBP1 (p = 0.06) (Fig. 2g). When comparing the liver sections from Wistar rats, visible lipid accumulation in the aged group was observed compared to young and aged exercised groups, which was accompanied by higher hepatic TG and cholesterol in the aged animals (Fig. 2h–j). Sedentary-aged Wistar rats showed increased serum TG and cholesterol compared to young rats. Furthermore, physical exercise decreased serum TG levels in the aged group, with improved hepatic mitochondrial activity in response to FCCP and rotenone (Fig. 2j, k). The mRNA levels of Cd36 and Fas were increased in sedentary aged Wistar rats, and physical exercise restored these values to lower levels (Fig. 2l). Exercised aged Wistar rats demonstrated higher Cpt1α mRNA content than sedentary-aged rats , with no alterations in Srebp1 and Pparα levels (Fig. 2l). Hepatic pACCS79 was attenuated in aged compared to young Wistar rats, but physical exercise increased its phosphorylation compared to sedentary-aged rats (Fig. 2m, n). Moreover, SREBP1 and FAS content were reduced in response to physical exercise in aged rats (Fig. 2n).
Hepatic fat metabolism in F344 and Wistar rats. a Representative images of hematoxylin–eosin (H&E, × 10) and Oil Red O (ORO, × 40) staining in the liver of F344 groups. b Hepatic TG and cholesterol. c Seric TG and cholesterol. d Liver mitochondrial respiration (n = 4, 5, 5). e Fat metabolism-related proteins (pAMPKT172, pACCS79, SREBP1, FAS) in the liver tissue of young (n = 4) and aged (n = 5) F344 rats. f Fat metabolism-related proteins (pAMPKT172, pACCS79, SREBP1, FAS) in the liver tissue of aged (n = 5) and aged + exe (n = 5) F344 rats. g Fat metabolism-related genes (Cd36, Srebp1, Fas, Cpt1α, and Pparα) in the liver tissue of young (n = 5), aged (n = 6) and aged + exe (n = 6) F344 rats. h Representative images of H&E and ORO staining in the liver of Wistar rats. i Hepatic TG and cholesterol. j Seric TG and cholesterol. k Liver mitochondrial respiration (n = 4, 5, and 5). l Fat metabolism-related proteins (pAMPKT172, pACCS79, SREBP1, FAS) in the liver tissue of young (n = 4) and aged (n = 5) Wistar rats. m Fat metabolism-related proteins (pAMPKT172, pACCS79, SREBP1, FAS) in the liver tissue of aged (n = 5) and aged + exe (n = 5) F344 rats. n Fat metabolism-related genes (Cd36, Srebp1, Fas, Cpt1α, and Pparα) in the liver tissue of young (n = 5), aged (n = 6), and aged + exe (n = 6) Wistar rats. In graphs b, c, i, j, n = 7 was used. Data were analyzed by one-way ANOVA followed by Tukey’s test (b–e, i–l) and unpaired two-tailed Student’s t test (f, g, m, n). *p < 0.05 versus the young group. #p < 0.05 versus aged group. The magnification bar (a, h) represents 100 µm (× 10) and 50 µm (× 40)
Physical exercise regulates hepatic gluconeogenesis, inflammation, and senescent markers in aged F344 and Wistar rats
In response to pyruvate, the aged exercised F344 rats showed a lower AUC than aged sedentary rats (Fig. 3a). Besides the lack of difference between young and aged F344 rats in the pyruvate tolerance test, the aged sedentary group showed higher mRNA levels of hepatic G6pase, Pepck, and Pc, while physical exercise suppressed these gluconeogenic genes (Fig. 3b). On the other hand, the aged Wistar rats demonstrated higher glycemia during the pyruvate tolerance test than the young group, accompanied by increased hepatic mRNA levels of G6pase, Pepck, and Pc (Fig. 3k, l). When exercised, aged Wistar rats demonstrated attenuation of the glycemic response after pyruvate injection and decreased hepatic G6pase, Pepck, and Pc genes compared to aged sedentary rats (Fig. 3k, l). Regarding some markers of hepatic damage and inflammation, aged F344 rats showed a trend (p = 0.06) to increase and aged Wistar showed higher collagen deposition in the liver compared to their controls (Fig. 3c, d, m, n). The F344 rats showed attenuated ALT and AST serum levels when exercised compared to aged sedentary F344 rats (Fig. 3e). On the other hand, aged Wistar rats showed higher ALT serum levels than young animals, but physical exercise attenuated this parameter compared to aged sedentary animals (Fig. 3o). This was accompanied by significant modulations in inflammatory markers (Nfκb, Tnfα, and Ikk) in the hepatic tissue of F344 and Wistar rats (Fig. 3f, p). In addition, both aged F344 and Wistar rats showed higher SA-β-Gal activity in the liver compared to the young group, which was downregulated by physical exercise (Fig. 3h, r). This fact was observed in the presence of a trend to modulate specific senescent markers (p21, p53, and p16) and SASP markers (Ccl2, Ccl5, and Cxcl12) in the liver of aged F344 rats, but a more pronounced and significant effect in the liver of aged Wistar rats (Fig. 3i, j, s, t). Conversely, physical exercise was able to protect the accumulation of these genes in the liver of aged Wistar rats (Fig. 3s, t).
Hepatic gluconeogenesis, inflammation, and senescent markers. a Pyruvate tolerance test curve (PTT) and area under the curve (AUC) in F344 rats. b Gluconeogenic genes (G6pase, Pepck, and Pc) in the liver of F344 rats. c Representative Masson’s trichrome images. d Collagen area quantification (n = 3). e Seric ALT and AST. f Inflammatory genes (Nfκb, Tnfα, and Ikk) in the hepatic tissue. g Representative SA-β-Gal staining in the liver sections. h SA-β-Gal staining area quantification. i Senescent markers (p21, p53, p16) in the hepatic tissue. j SASP markers (Ccl2, Ccl5, Cxcl12) in the hepatic tissue. k Pyruvate tolerance test curve (PTT) and area under the curve (AUC) in Wistar rats. l Gluconeogenic genes (G6pase, Pepck, and Pc) in the liver of Wistar rats. m Representative Masson’s trichrome images. n Collagen area quantification (n = 3). o Seric ALT and AST. p Inflammatory genes (Nfκb, Tnfα, and Ikk) in the hepatic tissue. q Representative SA-β-Gal staining in the liver sections. r SA-β-Gal staining area quantification. s Senescent markers (p21, p53, p16) in the hepatic tissue. t SASP markers (Ccl2, Ccl5, Cxcl12) in the hepatic tissue. The magnification bar in the histological images represents 50 µm (× 40). In graphs a, b, f, i, j, k, l, p, s, and t, n = 5, 6, and 6 were used. In graphs e, h, o, r, n = 7 was used. Data were analyzed by one-way ANOVA followed by Tukey’s test (a–t), and two-way ANOVA followed by Sidak’s test (a, k). *p < 0.05 versus the young group. #p < 0.05 versus aged group. ap < 0.05 young versus aged group. bp < 0.05 aged versus aged + exe group. ap < 0.05 young versus aged group. bp < 0.05 aged versus aged + exe group
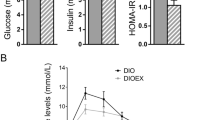
Physical exercise controls hyperglycemia, hyperinsulinemia, and hepatic metabolism in Slc2a4 +/− mice
Since we observed a hyperinsulinemic state in F344 and Wistar rats and based on previous investigations [26], we hypothesized that fasting hyperglycemia and hyperinsulinemia might be one crucial effector for insulin resistance, affecting the hepatic metabolism. We then generated whole-body Slc2a4 heterozygous mice (Slc2a+/−) with a partial deletion of the GLUT4 transporter, that presents hyperglycemia and hyperinsulinemia without body weight alterations and under a chow diet feeding (Fig. 4a–d). These mice are glucose and insulin intolerant and have impaired pAktS473 and pGSK3βS9 in the hepatic tissue compared to Wild Type (WT) mice (Fig. 4e–g). Interestingly, physical exercise in these mice decreased fasting glucose and insulin levels, improved glucose and insulin tolerance, and increased hepatic pAktS473 and pGSK3βS9 compared to Slc2a+/−, despite changes in body weight (Fig. 4A–H). The Slc2a+/− sedentary mice showed higher hepatic TG content, ALT, and AST, and a tendency to higher hepatic cholesterol compared to WT. However, when exercised, Slc2a+/− mice presented a decrease in all these parameters (Fig. 4i–k). Decreased pAMPKT172, pACCS79, and increased SREBP1 and FAS were also observed in the liver of Slc2a+/− sedentary mice compared to WT (Fig. 4l). In contrast, physical exercise tended to increase pAMPKT172 (p = 0.06), upregulate pACCS79, and downregulate SREBP1 and FAS in the hepatic tissue of Slc2a+/− mice (Fig. 4m).
Physiological and metabolic parameters and hepatic fat metabolism in Slc2a4+/− mice. a Body weight in WT, Slc2a4+/− and Slc2a4+/− + Exe groups. b Fasting glucose. c Fasting insulin. d HOMA-IR. e Glucose tolerance test curve (GTT) and area under the curve (AUC). f Insulin tolerance test curve (ITT) and area under the curve (AUC). g Insulin-signaling pathway proteins (pAktS473, pGSK3βS9) in the hepatic tissue of WT (n = 4) and Slc2a4+/− (n = 5) mice. h Insulin-signaling pathway proteins (pAktS473, pGSK3βS9) in the hepatic tissue of Slc2a4+/− (n = 5) and Slc2a4+/− + Exe (n = 5) mice. i Representative images of hematoxylin–eosin (H&E) and Oil Red O (ORO) staining in the liver of WT, Slc2a4+/− and Slc2a4+/− + Exe. The magnification bar represents 100 µm (× 10) and 50 µm (× 40). j Hepatic TG and cholesterol. k Seric ALT and AST. l Fat metabolism-related proteins (pAMPKT172/pACCS79/SREBP1/FAS) in the liver tissue of WT (n = 4) and Slc2a4+/− (n = 5) mice. m Fat metabolism-related proteins (pAMPKT172/pACCS79/SREBP1/FAS) in the liver tissue of Slc2a4+/− (n = 5) and Slc2a4+/− + Exe (n = 5) mice. N. Proposed model for the positive effects of physical exercise in controlling age-related hyperinsulinemia and hepatic metabolism. In graphs A–F and J–K, n = 5 was used. Data were analyzed by one-way ANOVA followed by Tukey's test (A-F, J, K), two-way ANOVA followed by Sidak's test (E, F), and unpaired two-tailed Student's t test (G, H, L, M). * p < 0.05 versus WT group. # p < 0.05 versus Slc2a4+/− group. a p < 0.05 WT versus Slc2a4+/− group. b p < 0.05 Slc2a4+/− versus Slc2a4+/− + Exe group
Because we observed alterations in the hepatic metabolism of hyperinsulinemic and aged rats, we also confirmed the effects of prolonged insulin treatment in hepatocytes (Figure S2A). HepG2 cells were treated with insulin (100 nM for 24 h) and pIRS1Y612, pAktS473, SREBP1, and FAS proteins were evaluated after another insulin stimulus (20 nM for 15 min). The cells exposed to high insulin showed impaired pIRS1Y612 and pAktS473 but also increased SREBP1 and FAS content (Figure S2B). Moreover, in the presence of palmitate and high insulin, these cells presented higher intracellular fat accumulation compared to control cells (Figure S2C–E). Regarding hepatic glucose production, the prolonged insulin treatment increased mRNA levels of G6pase in the basal state and after cAMP addition, as well as higher mRNA levels of Pepck and Pc in response to cAMP and cAMP + Insulin (Figure S2F, G). These data were accompanied by higher glucose output from the hepatocytes in response to cAMP and a tendency to increase glucose production in the basal state (p = 0.07) and after using cAMP + Insulin (p = 0.06) (Figure S2H).
ALT and AST are positively correlated with fasting insulin in women subjects of different ages
Corroborating the data obtained from the animal models, the serum AST levels in weight-matched (57–62 kg) women (Fig. 5a–c), with distinct ages (20–29: n = 13; 30–39: n = 12; 40–49: n = 9; 50–59: n = 13; 60–73: n = 10) were positively correlated (R = 0.322, p = 0.014) with the fasting glucose levels (Fig. 5d), and both serum ALT and AST levels were positively correlated (R = 0.304, p = 0.021; R = 0.262, p = 0.048) with the fasting insulin levels (Fig. 5e). On the other hand, there were no significant correlations of ALT and AST with age, body weight, or % fat mass (Fig. 5f–h). Then increased circulating insulin levels may be correlated with hepatic alterations in humans, independent of body weight changes.
Human parameters correlations with serum ALT and AST. a Schematic figure of the serum sample collection from weight-matched women subjects. b Number of individuals by age (20–29, 30–39, 40–49, 50–59, and 60–73). c Body weight of the individuals. d–h. Pearson correlations of ALT and AST serum enzyme levels in human samples with fasting glucose (d), fasting insulin (e), age (f), body weight (g), and % fat mass (h). A number of 57 individuals was utilized
Discussion
Hyperinsulinemia is positively correlated with NAFLD, insulin resistance, and T2D development. Whether hyperinsulinemia is a primary effector or a consequence of insulin resistance may depend on each individual, since epigenetic mechanisms could lead to hyperinsulinemia before alterations in basal glucose levels [9]. Understanding this process may be essential to avoid insulin resistance development during aging. This study observed that aging associated or not with increased adiposity leads to hyperinsulinemia and negative hepatic modulations. A genetic model of hyperglycemia and hyperinsulinemia also displayed hepatic fat accumulation and liver insulin resistance. However, when these rodent models were exercised, positive effects were observed on whole-body glucose homeostasis and hepatic metabolism.
Insulin is a crucial hormone involved in the control and metabolization of metabolic substrates, and defects in insulin signaling may contribute to hyperglycemia and lipogenesis. The cause of insulin resistance could be attributed to environmental, dietary, and genetic factors, etc. Furthermore, changes in the beta-cell function and structure may be compromised in response to aging, leading to compensatory insulin secretion and hyperplasia of this endocrine organ [27, 28]. However, the continuous delivery of insulin induces its desensitization [29,30,31]. Therefore, hyperinsulinemia may act as a prior effector for insulin resistance development in some cases. In non-diabetic obese individuals, insulin hypersecretion is more prevalent than insulin resistance [32]. Moreover, in mice fed with a high-fat diet, hyperinsulinemia is necessary to induce body and hepatic fat accumulations [33]. The main factor involved in the control of insulin levels is the secretion and clearance of this hormone, a mechanism that could be affected by aging. However, data on the control of insulin levels with aging are still inconclusive [34,35,36]. Therefore, the homeostatic control of pulsatile insulin secretion is essential to provide the metabolic effects of insulin, and sustained insulin secretion may contribute to insulin resistance and appear before changes in glycemia. In this study, age-related hyperinsulinemia was associated with hepatic metabolic disturbances, but physical exercise counteracted the negative effect of prolonged high insulin exposure.
In the Relationship between Insulin Sensitivity and Cardiovascular disease (RISC) study, the subjects with hypersecretion of insulin showed a tendency to be older, with higher fat mass, a harmed lipid profile, and hepatic insulin resistance [37]. In animal models, high insulin circulating levels were observed in F344 and Wistar-aged rats compared to young rats, accompanied by glucose intolerance and decreased hepatic insulin sensitivity. These findings occurred without changes in the fasting glucose levels, but with a visible discrepancy in the morphology of these rat models [26] since Wistar rats have increased body fat accumulation with aging. In contrast, physical exercise efficiently controlled hyperinsulinemia, glucose and insulin tolerance, and hepatic insulin sensitivity.
Due to the positive correlation between hyperinsulinemia and NAFLD [17, 38,39,40], we observed aging-induced hyperinsulinemia effects in the hepatic tissue. Aging caused significant hepatic changes in F344 and Wistar rats; however, we did not observe a pattern of molecular alterations in these models. In aged F344 rats, the liver contents of pAMPKT172 and pACCS79 were reduced, while SREBP1 was increased compared to young rats. Also, the amount of hepatic TG tended to be higher (p = 0.06) with no changes in the cholesterol. In contrast, serum TG and cholesterol were higher in aged F344 rats. When we analyzed Wistar rats, aging increased hepatic and serum TG and cholesterol, with an impairment only in the pACCS79. These divergences may be related to the difference in the genetic background in F344 (isogenic) and Wistar (heterogeneous) rats and distinct proteins involved in hepatic fat accumulation, which are dysregulated by aging. The positive effects of physical exercise were observed in the attenuation of some lipogenic markers (SREBP1 and FAS) and upregulation of lipid oxidation markers (pACCS79, Cpt1α, and Pparα), resulting in better control of lipid metabolism and hepatic fat accumulation. To establish causality and to study the direct effects of prolonged insulin treatment in hepatocytes, we incubated HepG2 cells with high insulin. It was found with impaired pIRS1Y612 and pAktS473. Also, there was an increase in lipogenic proteins (SREBP and FAS) and higher intracellular lipid accumulation in this in vitro model of hyperinsulinemia. Moreover, in response to hyperinsulinemia, HepG2 cells showed increased gluconeogenic genes (G6pase, Pepck, and Pc) and increased glucose output.
Regarding hepatic glucose production, aged F344 rats presented normal pyruvate tolerance but increased hepatic mRNA for gluconeogenic markers (G6pase, Pepck, and Pc). On the other hand, aged Wistar rats presented pyruvate intolerance and increased hepatic gluconeogenic markers, suggesting elevated gluconeogenesis in the liver of these animals. In F344, a few regions of collagen deposition in the liver with aging and increased Nfκb and Tnfα mRNA were observed compared to young F344 rats, with no changes in the ALT and AST enzymes. In the Wistar rats, collagen in the liver sections with aging was more severe, accompanied by increased Nfκb, Tnfα, and Ikk mRNA, as well as ALT. Thus, it is understandable that aging associated with hyperinsulinemia and increased body adiposity may worsen metabolic health and hepatic metabolism. Furthermore, necessary molecular signatures were observed in the liver of F344 rats, which are leaner than Wistar rats when old. Nevertheless, both aged models submitted to physical exercise presented improved pyruvate tolerance, gluconeogenic markers, and liver inflammation, demonstrating that physical exercise is essential to improve hepatic metabolism, independently of the body adiposity.
To further investigate the role of hyperinsulinemia in hepatic metabolism, we used Slc2a4+/− mice that display hyperglycemia and hyperinsulinemia. These mice presented glucose and insulin intolerance, accompanied by decreased insulin sensitivity in the liver. We also observed increased hepatic TG, ALT, and AST, decreased pAMPKT172 and pACCS79, and increased SREBP1 and FAS in the liver of Slc2a4+/− mice compared to WT. When submitted to physical exercise, all these metabolic and molecular signatures were negated, confirming the importance of physical exercise in the control of hyperinsulinemia and its consequences.
The role of physical exercise in the control of hepatic fat accumulation is well established [18]. However, the effectors responsible for initiating the signal to a lipogenic program in the liver are still debated. The efficiency of hepatic insulin signaling may be essential to maintain whole-body homeostasis. Liver-specific insulin receptor knockout (LIRKO) mice have age-related liver dysfunction and hepatic insulin resistance [41]. In addition, when submitted to a high-dose hyperinsulinemic-euglycemic clamp, LIRKO mice showed extrahepatic (muscle and fat) insulin resistance [42]. Other factors such as high caloric intake and aging contribute to hepatic insulin resistance mediated by pro-inflammatory cytokines secreted by adipose tissue [43, 44] that is also counteracted by a physical exercise program [45]. There are essential mechanisms involved in this regulation that has already been well described and recently revised [3]. The translation of these results to humans is observed in cohort studies where hyperinsulinemia was correlated with NAFLD. Studies involving patients without diabetes or with unchanged transaminases observed a positive association between hyperinsulinemia and NAFLD [38, 39]. Insulin levels correlate with hepatic lipid accumulation, where NAFLD individuals showed higher intrahepatic insulin levels than healthy controls, suggesting lower hepatic insulin clearance in NAFLD [40].
Several recent works have shown hyperinsulinemia as an inductor of cellular senescence and the contribution of this scenario to hepatic steatosis [22, 24]. Interestingly, these aging-associated metabolic disorders could be partially corrected by changes in the feeding pattern (dietary restriction) or senolytic drugs (Dasatanib + Quercetin). Here, we have shown that short-term physical exercise was effective in decreasing markers of cell senescence and SASP in the liver of old rats. Future works should investigate the combination of senolytic therapies and exercise to evaluate the safety and efficiency to decrease biological age and promote positive metabolic effects. Besides the environmental factors (diet, smoke exposure, inactivity, etc.), the genetic factors predisposing hyperinsulinemia in humans may also play an expressive role in the progression of type 2 diabetes and hepatic fat accumulation [46, 47].
In this scenario, patterns of liver transcriptome and the use of high-throughput genome-wide association studies (GWAS) in patients with hyperinsulinemia will provide a better understanding of the epigenetic regulations related to this phenotype [48]. Identifying early indicators of type 2 diabetes could be crucial for determining insulin resistance and T2D development. Therefore, here we demonstrate in a singular way that short-term physical training was able to reduce the deleterious effects of aging and hyperinsulinemia on hepatic metabolism in rodents. It is important to emphasize that previous works investigating the role of physical exercise in this scenario may have confounding factors, such as experimental diets and changes in body weight and adiposity [49,50,51]. Moreover, it is important to highlight that we found physiological and hepatic disturbances in both aged models (F344 and Wistar), which have distinct adiposity profiles with aging. These differences in adiposity with aging could be related to specific hormonal changes, molecular signatures, or genetic variations that are responsive to the presence of senescent cells [52, 53]. This fact highlights the role of aging in the induction of insulin resistance, hyperinsulinemia, and hepatic damage independently of body fat accumulation. Conversely, short-term physical exercise was effective in restoring insulin sensitivity and glucose homeostasis, as well as improving hepatic metabolism.
Conclusion
In summary, we conclude that short-term aerobic exercise training is an effective strategy to control age-related hyperinsulinemia in animal models, improving hepatic metabolic pathways (insulin sensitivity, lipogenesis, gluconeogenesis, inflammation, and cellular senescence) (Fig. 4n). Further investigations aiming at other physical exercise programs in aged individuals will contribute to the field, providing new evidence about the role of hyperinsulinemia in the development of hepatic fat accumulation and T2D. Thus, physical exercise practice must be debated as an essential strategy with low cost to improve metabolic health in late life, preventing diseases potentiated by insulin resistance.
Experimental procedures
Experimental animals
Male Wistar and F344 rats were obtained from the Central Animal Breeding Center of the University of Campinas. Rats of 17 months of age were considered for the aged groups, which corresponds to 60 years of age in humans [54]. The animals were distributed into (1) young, 5-month-old sedentary rats; (2) aged, 17-month-old sedentary rats; (3) aged + exe, 17-month-old exercised rats. A pair of TALENs targeting exon 4 of the mSlc2a4 gene (NM_009204.2) was used to generate the Slc2a4 knockout mice (Cyagen®). Heterozygous Slc2a4 mice (Slc2a4+/−) were obtained by crossing Slc2a4−/− females (homozygous) with Wild-Type males. The Indels (insertions/deletions) were confirmed by DNA extracted from the tail, followed by PCR amplification and DNA Sanger sequencing (Figure S3). The following reverse primer was utilized: mSlc2a4-R: 5′-GCCGAGGATAGCTGCATATTCCA-3′; product size: 466 bp; annealing temperature: 59 °C. The male Slc2a4+/− mice were distributed into (1) Wild-Type (WT), 8-month-old sedentary mice; (2) Slc2a4+/−, 8-month-old sedentary mice, (3) Slc2a4+/−, 8-month-old exercised mice. All the animals were conditioned in cages with free access to water and a standard diet (Nuvilab®) at 22 °C. The Ethics Committee of UNICAMP approved the experimental procedures (4531-1, 5107-1, 5003-1).
Short-term swimming exercise
The Wistar and F344 rats were submitted to an aerobic short-term swimming exercise in plastic barrels (45 cm diameter, ~ 34 °C). Short-term physical exercise protocols provide a better understanding of the effects of physical exercise with a minimum change in body weight and adiposity observed in the experimental groups. Therefore, the rats were adapted to the swimming exercise for 5 days (10 min/day) and submitted to 7 days of swimming exercise as previously reported [55]. The physical exercise sessions were composed of 4 bouts of 30 min, separated by 10 min of rest (2 h of physical exercise). In both models, an attached load of 1.5% g of body weight was utilized in the 2nd, 3rd, and 4th bouts to normalize the differences in the body weight between Wistar and F344 rats. The physiological tests and tissue extraction were performed 16 h after the physical exercise session, with a 6 h fasting period, as detailed in Figure S4.
Short-term treadmill exercise
The Slc2a4+/− mice were submitted to the short-term treadmill exercise. First, mice were adapted to the treadmill (AVS Projetos®, São Carlos, Brazil) for 5 days at 10 m/min for 10 min, following the incremental load test (ILT). The ILT was performed at the intensity of 3 m/min with increments of 3 m/min every 3 min until the animal’s exhaustion. After identifying each mouse’s exhaustion, the exhaustion velocity (EV) was utilized to prescribe the physical exercise intensity. The Slc2a4+/−mice were submitted to 7 days of treadmill exercise with 60% of the EV for 60 min. The physiological tests and tissue extraction were performed 16 h after the physical exercise session, with a 6 h fasting period, as detailed in Figure S4.
Serum analysis
Sixteen hours after the physical exercise sessions, blood was collected from the tail to obtain the serum and stored at − 80 °C. Triglycerides (TG), cholesterol, alanine transaminase (ALT), and aspartate transaminase (AST) were measured using commercial colorimetric kits (Laborlab®, São Paulo, Brazil). β-Hydroxybutyrate levels were measured using a colorimetric kit (Cayman Chemical®, Ann Arbor, MI, USA). Insulin circulating levels were evaluated by enzyme-linked immunosorbent assay (RayBiotech®, Norcross, GA; #ELM-Insulin). The Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) index was calculated using the fasting glucose and insulin levels from the same moment [fasting plasma glucose (mmol/L) × fasting plasma insulin (μU/mL)/22.5].
Insulin tolerance test (ITT), glucose tolerance test (GTT), and pyruvate tolerance test (PTT)
With a 6 h fasting period and 16 h after the physical exercise sessions, tail blood was collected to obtain the fasting glycemia and 0’ of the GTT (Accu-Chek glucometer, Roche Diagnostics®). After determining the basal glucose values, a 25% glucose solution was intraperitoneally injected at 2 g/kg, followed by glucose measurements at 30′, 60′, and 120′ after injection.
The ITT was performed in the same condition (6 h fasting period and 16 h after physical exercise sessions) with an intraperitoneal injection of 0.3% insulin (Humulin R; Lilly®, Indianapolis, IN, USA) at 1.5 U/kg. The glucose values were obtained at 10′, 15′, 20′, 25’, and 30’ after the insulin stimuli.
The PTT was also performed after 6 h of fasting and 16 h after physical exercise sessions. Sodium pyruvate (25%) was intraperitoneally injected at 1.5 g/Kg after determining the basal glucose values. Glycemia was measured 30′, 60′, and 120′ after pyruvate injection. After all of these tests, the animals were returned to the cage and monitored for the rest of the day.
Tissue extraction and molecular analysis (immunoblotting and RT-qPCR)
Sixteen hours after the last physical exercise session (6 h fasted) and anesthesia (90 mg/kg ketamine chloralhydrate and 10 mg/kg xylazine), the hepatic tissue was removed and stored (− 80 °C) for future analysis. To evaluate the insulin pathway-related proteins, insulin was intraperitoneally injected (10 U/kg) 10 min before the tissue extraction. The adipose tissue from different regions (ingWAT, pgWAT, retroWAT, meseWAT, and BAT), gastrocnemius skeletal muscle (Gastro.), heart, spleen, and kidney were removed for tissue weighting.
The immunoblotting analysis was performed by hepatic tissue homogenization with protein extraction buffer using TissueLyser II (Qiagen®). Next, the lysed samples were centrifuged at 12,000 rpm at 4 °C for 15 min, and the supernatant was obtained to measure the total protein sample content (bicinchoninic acid method). Approximately 30 µg of each sample with Laemmli 6 × were submitted to SDS–PAGE to separate the molecular weights. The gels were transferred to nitrocellulose membranes and blocked with 5% milk (50 min, room temperature). The protein loading and transfer were checked with Ponceau S-Stain. The Ponceau was washed and the membranes were incubated with the following primary antibodies (4 °C overnight): pIRβ (Y972) #GTX25678 from GeneTex®; pIRS1 (Y612) #sc-17195, IRS1 #sc-559, FAS #sc-48357 from Santa Cruz Biotechnology®; SREBP-1/2 #bs-1402R from Bioss®; IRβ #3025, pAkt (S473) #4060, Akt #4685, pGSK3β (S9) #9322, GSK3β #5676, pAMPKα (T172) #50081, AMPKα #2532, pACC (S79) #3661, ACC #3662, β-actin #3700, GAPDH #2118, vinculin #4650, α-tubulin #2144 from Cell Signaling Techonology®. After incubation with a specific primary antibody, the membranes were incubated with respective secondary antibodies (rabbit or mouse, 50 min at room temperature) and labeled with chemiluminescence reagent (ECL), and acquired with G:Box XR5 (Syngene®, Frederick, MD, USA). Densitometry was measured with UN-SCAN-IT gel 6.1® software.
In the quantitative real-time polymerase chain reaction (RT-qPCR) analysis, the complementary DNA (cDNA) was obtained from hepatic RNA. A hepatic tissue fragment (~ 30 mg) was lysed in Trizol (Life Technologies®, USA) and submitted to the RNA extraction protocol. cDNA (2 µg) was synthesized using High-Capacity cDNA Reverse Transcription Kits (Applied Biosystems®, Forest City, CA) and subjected to RT-qPCR using 100 ng cDNA, 150 nM primers, and iTaq Universal SYBR Green Supermix (Bio-Rad®, Hercules, CA, USA) in the 7500 Fast Real-Time PCR System (Applied Biosystems®, Forest City, CA). The ΔΔCt was evaluated for normalizing the genes by a housekeeping gene (Actb). The primer sequences are listed in Table S1.
Histological analysis and hepatic fat measurement
In the tissue extraction, small fragments of hepatic and adipose tissues were fixed in 4% PFA solution for 24 h, dehydrated, embedded in paraffin, and sliced (10 µM) in a microtome (Leica®, RM2145). The slides were hydrated and stained with hematoxylin–eosin (H&E) or Masson’s trichrome. The images were acquired and the blue area (collagen) was quantified using ImageJ software. Another fragment of hepatic tissue was frozen in pre-cooled isopentane followed by cryopreservation at − 80 °C. These fragments were cut (10 µM) in a cryostat (Leica®, CM1850) and placed in adhesion slides. The slides were stained with Oil Red O solution (ORO, Sigma Aldrich®, St. Louis, MO) for 25 min and with hematoxylin for 2 min, followed by 30 min of washing. The slides were sealed using gelatin:glycerin solution and the images were obtained. Another set of slides was fixed with 0.2% glutaraldehyde in PBS for 10 min, washed, and incubated with the senescence-associated expression of β-galactosidase (SA-β-Gal) staining solution overnight at 37 °C (ab65351, Abcam®). The slides were washed with PBS and counterstained with hematoxylin, washed again, and sealed. All the images were acquired in an optical microscope (LAB2000, LABORANA®, São Paulo, Brazil) with Moticam Pro 282B 5.0 megapixels (Motic®, Hong Kong, China).
The hepatic TG and cholesterol were measured with a calorimetric kit (Laborlab®, São Paulo, Brazil) by previous lipid extraction in the liver tissue using the Folch method (56).
High-resolution respirometry
The hepatic oxygen consumption was measured using Oxygraph-2k (Oroboros Instruments®, Innsbruck, Austria) as described before [57]. Approximately 50 mg of liver tissue was homogenized in 500 µL of mitochondrial respiration medium (MIR) using pestles. The samples were added to the equipment chambers at 37 °C. After that, malate (0.5 mM), glutamate (10 mM), and pyruvate (5 mM) were injected first, followed by ADP (2.5 mM) to measure complex I-dependent respiration. Succinate (10 mM) was added to evaluate the combined Complex I and II respiration. The mitochondrial coupling was analyzed by adding ATP synthase inhibitor oligomycin (2.5 mM) and mitochondrial uncoupling by adding FCCP (0.5 mM). Finally, the Complex II-mediated respiration was measured by adding Complex I inhibitor rotenone (0.5 µM).
Statistical analysis
To analyze the significant differences among the groups, the distribution of the data was evaluated by the Shapiro–Wilk test. The comparison between the two groups was analyzed using the Student’s t test or Mann–Whitney test. Comparing three groups was analyzed by one-way ANOVA followed by Tukey’s post-hoc test for normal distribution. In the time-dependent data (GTT, ITT, PTT), two-way ANOVA followed by Sidak’s post-hoc was adopted using simple effect within rows analysis. The statistical significance was assumed when p < 0.05. The graphics illustrate the mean ± standard error of the mean (SEM). The GraphPad Prism 8.0.1® software was utilized for data statistical analysis and graphing.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Smith HJ, Sharma A, Mair WB (2020) Metabolic communication and healthy aging: where should we focus our energy? Dev Cell. https://doi.org/10.1016/j.devcel.2020.06.011
Park MH, Kim DH, Lee EK et al (2014) Age-related inflammation and insulin resistance: a review of their intricate interdependency. Arch Pharm Res 37(12):1507–1514. https://doi.org/10.1007/s12272-014-0474-6
Roden M, Shulman GI (2019) The integrative biology of type 2 diabetes. Nature 576(7785):51–60. https://doi.org/10.1038/s41586-019-1797-8
Sheedfar F, Di BS, Koonen D, Vinciguerra M (2013) Liver diseases and aging: friends or foes? Aging Cell 12(6):950–954. https://doi.org/10.1111/acel.12128
Kahn BB (1998) Type 2 diabetes: when insulin secretion fails to compensate for insulin resistance. Cell 92(5):593–596
Corkey BE (2012) Banting lecture 2011: hyperinsulinemia: cause or consequence? Diabetes 61(1):4–13. https://doi.org/10.2337/db11-1483
Janssen JAMJL (2021) Hyperinsulinemia and its pivotal role in aging, obesity, type 2 diabetes, cardiovascular disease and cancer. Int J Mol Sci 22(15):7797. https://doi.org/10.3390/ijms22157797
Dankner R, Chetrit A, Shanik MH, Raz I, Roth J (2012) Basal state hyperinsulinemia in healthy normoglycemic adults heralds dysglycemia after more than two decades of follow up. Diabetes Metab Res Rev. https://doi.org/10.1002/dmrr.2322
Thomas DD, Corkey BE, Istfan NW, Apovian CM (2019) Hyperinsulinemia: an early indicator of metabolic dysfunction. J Endocr Soc 3(9):1727–1747. https://doi.org/10.1210/js.2019-00065
Cen HH, Hussein B, Botezelli JD et al (2022) Human and mouse muscle transcriptomic analyses identify insulin receptor mRNA downregulation in hyperinsulinemia-associated insulin resistance. FASEB J. https://doi.org/10.1096/fj.202100497RR
Li Q, Hagberg CE, Silva Cascales H et al (2021) Obesity and hyperinsulinemia drive adipocytes to activate a cell cycle program and senesce. Nat Med 27(11):1941–1953. https://doi.org/10.1038/s41591-021-01501-8
Duckworth WC, Bennett RG, Hamel FG (1998) Insulin degradation: progress and potential*. Endocr Rev. https://doi.org/10.1210/edrv.19.5.0349
Fink RI, Revers RR, Kolterman OG, Olefsky JM (1985) The metabolic clearance of insulin and the feedback inhibition of insulin secretion are altered with aging. Diabetes. https://doi.org/10.2337/diab.34.3.275
Chang AM, Halter JB (2003) Aging and insulin secretion. Am J Physiol Metab 284(1):E7–E12. https://doi.org/10.1152/ajpendo.00366.2002
Geloneze B, de Oliveira MS, Vasques ACJ, Novaes FS, Pareja JC, Tambascia MA (2014) Impaired incretin secretion and pancreatic dysfunction with older age and diabetes. Metabolism 63(7):922–929. https://doi.org/10.1016/j.metabol.2014.04.004
Samuel VT, Shulman GI (2018) Nonalcoholic fatty liver disease as a nexus of metabolic and hepatic diseases. Cell Metab 27(1):22–41. https://doi.org/10.1016/j.cmet.2017.08.002
Lee JH, Rhee PL, Lee JK et al (1998) Role of hyperinsulinemia and glucose intolerance in the pathogenesis of nonalcoholic fatty liver in patients with normal body weight. Korean J Intern Med. https://doi.org/10.3904/kjim.1998.13.1.10
Thyfault JP, Scott RR (2020) Exercise combats hepatic steatosis: potential mechanisms and clinical implications. Diabetes. https://doi.org/10.2337/dbi18-0043
Muñoz VR, Gaspar RC, Crisol BM et al (2017) Physical exercise reduces pyruvate carboxylase (PCB) and contributes to hyperglycemia reduction in obese mice. J Physiol Sci. https://doi.org/10.1007/s12576-017-0559-3
Muñoz VRVR, Gaspar RCRC, Kuga GKGK et al (2018) Exercise decreases CLK2 in the liver of obese mice and prevents hepatic fat accumulation. J Cell Biochem 119(7):5885–5892. https://doi.org/10.1002/jcb.26780
Batista TM, Haider N, Kahn CR (2021) Defining the underlying defect in insulin action in type 2 diabetes. Diabetologia. https://doi.org/10.1007/s00125-021-05415-5
Baboota RK, Spinelli R, Erlandsson MC et al (2022) Chronic hyperinsulinemia promotes human hepatocyte senescence. Mol Metab 64:101558. https://doi.org/10.1016/j.molmet.2022.101558
Meijnikman AS, van Olden CC, Aydin Ö et al (2022) Hyperinsulinemia is highly associated with markers of hepatocytic senescence in two independent cohorts. Diabetes 71(9):1929–1936. https://doi.org/10.2337/db21-1076
Ogrodnik M, Miwa S, Tchkonia T et al (2017) Cellular senescence drives age-dependent hepatic steatosis. Nat Commun 8(1):15691. https://doi.org/10.1038/ncomms15691
Kumari R, Jat P (2021) Mechanisms of cellular senescence: cell cycle arrest and senescence associated secretory phenotype. Front cell Dev Biol 9:645593. https://doi.org/10.3389/fcell.2021.645593
Muñoz VR, Gaspar RC, Kuga GK et al (2018) The effects of aging on rho-kinase and insulin signaling in skeletal muscle and white adipose tissue of rats. J Gerontol Ser A. https://doi.org/10.1093/gerona/gly293
Reaven E, Wright D, Mondon CE, Solomon R, Ho H, Reaven GM (1983) Effect of age and diet on insulin secretion and insulin action in the rat. Diabetes 32(2):175–180. https://doi.org/10.2337/diab.32.2.175
Reaven E, Curry D, Moore J, Reaven G (1983) Effect of age and environmental factors on insulin release from the perfused pancreas of the rat. J Clin Investig 71(2):345–350. https://doi.org/10.1172/JCI110775
Shanik MH, Xu Y, Skrha J, Dankner R, Zick Y, Roth J (2008) Insulin resistance and hyperinsulinemia: is hyperinsulinemia the cart or the horse? Diabetes Care. https://doi.org/10.2337/dc08-s264
Marangou AG, Weber KM, Boston RC et al (1986) Metabolic consequences of prolonged hyperinsulinemia in humans. Evidence for induction of insulin insensitivity. Diabetes. https://doi.org/10.2337/diab.35.12.1383
Ward GM, Walters JM, Aitken PM, Best JD, Alford FP (1990) Effects of prolonged pulsatile hyperinsulinemia in humans. Enhancement of insulin sensitivity. Diabetes. https://doi.org/10.2337/diab.39.4.501
Ferrannini E, Natali A, Bell P, Cavallo-Perin P, Lalic N, Mingrone G (1997) Insulin resistance and hypersecretion in obesity. J Clin Investig. https://doi.org/10.1172/JCI119628
Mehran AE, Templeman NM, Brigidi GS et al (2012) Hyperinsulinemia drives diet-induced obesity independently of brain insulin production. Cell Metab 16(6):723–737. https://doi.org/10.1016/j.cmet.2012.10.019
Kurauti MA, Ferreira SM, Soares GM et al (2019) Hyperinsulinemia is associated with increasing insulin secretion but not with decreasing insulin clearance in an age-related metabolic dysfunction mice model. J Cell Physiol. https://doi.org/10.1002/jcp.27667
Gumbiner B, Polonsky KS, Beltz WF, Wallace P, Brechtel G, Fink RI (1989) Effects of aging on insulin secretion. Diabetes. https://doi.org/10.2337/diab.38.12.1549
Marmentini C, Soares GM, Bronczek GA et al (2021) Aging reduces insulin clearance in mice. Front Endocrinol (Lausanne). https://doi.org/10.3389/fendo.2021.679492
Tricò D, Natali A, Arslanian S, Mari A, Ferrannini E (2018) Identification, pathophysiology, and clinical implications of primary insulin hypersecretion in nondiabetic adults and adolescents. JCI Insight. https://doi.org/10.1172/jci.insight.124912
Rhee EJ, Lee WY, Cho YK, Kim BI, Sung KC (2011) Hyperinsulinemia and the development of nonalcoholic fatty liver disease in nondiabetic adults. Am J Med. https://doi.org/10.1016/j.amjmed.2010.08.012
Ardigò D, Numeroso F, Valtueña S et al (2005) Hyperinsulinemia predicts hepatic fat content in healthy individuals with normal transaminase concentrations. Metabolism. https://doi.org/10.1016/j.metabol.2005.05.027
Mehta SR, Godsland IF, Thomas EL et al (2012) Intrahepatic insulin exposure, intrahepatocellular lipid and regional body fat in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. https://doi.org/10.1210/jc.2011-2430
Michael MD, Kulkarni RN, Postic C et al (2000) Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell 6(1):87–97. https://doi.org/10.1016/S1097-2765(05)00015-8
Fisher SJ, Kahn CR (2003) Insulin signaling is required for insulin’s direct and indirect action on hepatic glucose production. J Clin Investig 111(4):463–468. https://doi.org/10.1172/JCI16426
Barzilai N, Ferrucci L (2012) Insulin resistance and aging: a cause or a protective response? J Gerontol Ser A Biol Sci Med Sci. https://doi.org/10.1093/gerona/gls145
Hotamisligil GS (2006) Inflammation and metabolic disorders. Nature 444(7121):860–867. https://doi.org/10.1038/nature05485
Petersen AMW, Pedersen BK (2005) The anti-inflammatory effect of exercise. J Appl Physiol 98(4):1154–1162. https://doi.org/10.1152/japplphysiol.00164.2004
Maude H, Sanchez-Cabanillas C, Cebola I (2021) Epigenetics of hepatic insulin resistance. Front Endocrinol (Lausanne). https://doi.org/10.3389/fendo.2021.681356
Spinelli R, Parrillo L, Longo M et al (2020) Molecular basis of ageing in chronic metabolic diseases. J Endocrinol Investig 43(10):1373–1389. https://doi.org/10.1007/s40618-020-01255-z
Doria A, Patti M-E, Kahn CR (2008) The emerging genetic architecture of type 2 diabetes. Cell Metab 8(3):186–200. https://doi.org/10.1016/j.cmet.2008.08.006
Hoene M, Kappler L, Kollipara L et al (2021) Exercise prevents fatty liver by modifying the compensatory response of mitochondrial metabolism to excess substrate availability. Mol Metab 54:101359. https://doi.org/10.1016/j.molmet.2021.101359
Fredrickson G, Barrow F, Dietsche K et al (2021) Exercise of high intensity ameliorates hepatic inflammation and the progression of NASH. Mol Metab 53:101270. https://doi.org/10.1016/j.molmet.2021.101270
Yang J, Sáinz N, Félix-Soriano E et al (2021) Effects of long-term DHA supplementation and physical exercise on non-alcoholic fatty liver development in obese aged female mice. Nutrients 13(2):501. https://doi.org/10.3390/nu13020501
Gustafson B, Nerstedt A, Smith U (2019) Reduced subcutaneous adipogenesis in human hypertrophic obesity is linked to senescent precursor cells. Nat Commun 10(1):2757. https://doi.org/10.1038/s41467-019-10688-x
Spinelli R, Florese P, Parrillo L et al (2022) ZMAT3 hypomethylation contributes to early senescence of preadipocytes from healthy first-degree relatives of type 2 diabetics. Aging Cell. https://doi.org/10.1111/acel.13557
Dutta S, Sengupta P (2016) Men and mice: relating their ages. Life Sci. https://doi.org/10.1016/j.lfs.2015.10.025
Muñoz VR, Gaspar RC, Kuga GK et al (2018) Exercise increases rho-kinase activity and insulin signaling in skeletal muscle. J Cell Physiol 233(6):4791–4800. https://doi.org/10.1002/jcp.26278
Folch J, Lees M, Stanley GHS (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226(1):497–509. https://doi.org/10.1016/S0021-9258(18)64849-5
Braga RR, Crisol BM, Brícola RS et al (2021) Exercise alters the mitochondrial proteostasis and induces the mitonuclear imbalance and UPRmt in the hypothalamus of mice. Sci Rep. https://doi.org/10.1038/s41598-021-82352-8
Funding
This work was supported by FAEPEX, the National Council for Scientific and Technological Development (CNPq; case numbers 303571/2018-7; 140285/2016-4; 442542/2014-3 and 306535/2017-3), the Coordination for the Improvement of Higher Education Personnel (CAPES; finance code 001), and the São Paulo Research Foundation (FAPESP; case numbers 2015/26000-2, 2016/18488-8, 2018/20872-6, 2019/11820-5, 2020/13443-1 and 2021/08692-5).
Author information
Authors and Affiliations
Contributions
VRM and JRP wrote the paper and were ultimately responsible for the experiments in this study. VRM, RCG, RLD, RFLV, GCA, BMC, designed and performed experiments with animals. MCSM and FMS designed and performed the cell culture experiments. FMS, ASRS, CRC, ERR, DEC, JCST, ILPB, and JRP contributed to the discussion and laboratory support. All the authors have read and approved this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors of this study have no competing interests to declare.
Ethical approval
All the interventions involving humans were performed after signing of the consent term and approval from the Ethics Committee of the University of Campinas (n°: 1.967.450/2017).
Informed consent
In the human study, informed consent was obtained from all participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Muñoz, V.R., Gaspar, R.C., Mancini, M.C.S. et al. Short-term physical exercise controls age-related hyperinsulinemia and improves hepatic metabolism in aged rodents. J Endocrinol Invest 46, 815–827 (2023). https://doi.org/10.1007/s40618-022-01947-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-022-01947-8