Abstract
Context and purpose
Hypocalcemia and low parathyroid hormone levels have been commonly suggested as factors able to induce central nervous system disturbances. However, evidences on the occurrence of cognitive impairment are limited or underestimated. The aim of this review is, therefore, to systematically summarize the available evidence concerning the occurrence of cognitive impairment among subjects suffering from idiopathic or secondary hypoparathyroidism.
Methods
A systematic selection of the available literature was performed by searching the online databases PubMed, Scopus and Web of Knowledge.
Results
The present systematic review included sixteen case report articles and one cross-sectional controlled study. Case reports were the most representative literature sources and involved ten women and seven men. The presence of cognitive impairment was mostly discussed in association with idiopathic hypoparathyroidism (HPT); five articles described the occurrence of cognitive impairment following postsurgical HPT. The case-controlled study reported a significant presence of peculiar cognitive deficits (e.g. reduced inhibitory control, impairment in visuo-spatial functioning among, and psychomotor retardation) among HPT subjects compared to healthy controls, with serum total calcium and its product with phosphorus as independent predictors of neuropsychological dysfunctions.
Conclusion
Even though mostly based on single case reports, the presence of neuropsychological dysfunctions in the context of HPT appears to be a consistent core finding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The term cognitive functioning refers to a wide range of multiple mental abilities, which commonly include learning, memory, reasoning, problem-solving, decision-making, and attention processes [1]. Cognitive functioning follows different and heterogeneous trajectories along the entire lifespan, which substantially explain the individual differences in the processes of adaptation to cognitive aging [2]. A commonly shared assumption is that the variance in cognitive functioning proportionally increases with age; consistently, the presence of a general age-related progressive cognitive decline has been supported by several clinical evidences, which acknowledge processing speed, delayed recall, working memory and attention as the most impaired domains [3, 4].
The evaluation and the monitoring of cognitive functioning over time represent an established routine in clinical practice, since the progression of cognitive impairment is considered a relevant key factor leading to dementia. In this context, nosographic constructs, such as subjective cognitive decline (SCD) and mild cognitive impairment (MCI), and their evolution, have been broadly investigated as features of neurodegenerative disorders, or in association to several comorbid conditions, such as heart failure [5], diabetes [6], kidney failure [7], or bone-related chronic medical conditions [8].
Due to the lack of a resolutive pharmacological approach to the progression of cognitive decline towards dementia, the investigation of potential modifiable risk factors related to cognitive impairment has gained interest increasingly over the past years. In this perspective, dysfunctions of parathyroid hormone (PTH) have been discussed in several studies in relation to the onset and the maintenance of cognitive impairment, since PTH is able to cross the blood brain barrier (BBB), and several PTH receptors are additionally present in the human brain [9,10,11].
In the context of parathyroid dysfunctions, hypoparathyroidism (HPT) is commonly considered a rare endocrine condition, as suggested by the relatively low prevalence rates estimated among different clinical populations. Consistently, a large consultation of clinical databases in the United States (US) has rated a prevalence of 25 cases/100,000 subjects per year, consisting in estimated 77,000 cases in a population of nearly 300 million individuals [12]. A recent investigation throughout hospital registries in Italy has estimated a prevalence of approximatively 5 cases/100,000 individuals per year, even though this selection of cases might be too restrictive [13].
The main endocrinological features of HPT are the presence of hypocalcemia and absent or low serum PTH levels; the shared treatment is based on calcium and vitamin D supplementation [14]. This condition can present as idiopathic or secondary, such as the postsurgical form, which is not uncommon after thyroid and parathyroid surgery [15].
The clinical picture of HPT may be comprehensively characterized by a wide range of signs and symptoms including seizure, muscle cramps, paraesthesia, tetany, cardiac alterations, as well as mental and cognitive disturbances [16,17,18]. Additionally, an established neuroradiological marker of HPT has been broadly acknowledged in the presence of symmetric subcortical calcifications, which are frequently localized in the basal ganglia, as well as in other sites, such as thalamus and dentate nuclei [17].
Hypocalcemia and low PTH levels have been suggested as factors able to induce central nervous system disturbances [18, 19]; however, evidences on the occurrence of cognitive impairment are limited or underestimated.
To further address this topic, the aim of this review is to systematically summarize the available evidence concerning the occurrence of cognitive impairment among subjects suffering from idiopathic or secondary hypoparathyroidism.
Methods
Search strategy
A systematic review of the available literature was conducted in two steps. First, the studies were retrieved from the online database PubMed, Scopus and WebOf Knowledge, by matching the following keywords: “hypoparathyroidism”, "hypoparathyroid disorders", “dementia”, “cognitive”, “cognitive functions”, and “cognitive impairment”. A preliminary filter on the online search was applied by language (English) and species (Humans). Additionally, the reference lists of the included studies were examined to identify further potentially relevant studies missed during the database search. The online search was definitively completed on May 15, 2020.
Inclusion and exclusion criteria
Original, English-written research articles with an available full text, investigating the association between HPT and cognitive impairment were included in the current systematic review. Articles had to provide information on diagnostic criteria and/or clinical signs of HPT (e.g. laboratory test, neuroimaging evidence). Additionally, a clear description of the impaired cognitive domains was necessary, as well as information on the used cognitive assessment tools had to be showed. Since we expected to potentially retrieve articles published several decades ago, we also included those with at least a sufficiently reliable description of cognitive symptoms that was provided.
Retrieved articles that did not provide sufficient information on the patients’ medical condition and/or the cognitive assessment were excluded.
Eligibility screening
The eligibility of each article was assessed through a three-step procedure, by two different authors (AS, FB): the first-step screening was carried out by accounting the title, the second-step screening by accounting the abstract, and the final-step screening by accounting the full text. Conflicts regarding eligibility were resolved by consulting a senior author (AC). The review articles were not screened for eligibility; however, they were considered as a further source for potential studies not previously identified.
Data extraction
Data were extracted according to the following preliminary coding protocol shared by all the authors. From each study, general information was first extracted (i.e. author, year of publication, study design); we also collected information on the type of investigated patients, and their relative medical history. Additionally, data regarding the HPT diagnosis (laboratory and neuroradiological evidence), as well as regarding the cognitive assessment (i.e. symptoms, evaluation tools) were collected. If present, we also extracted pre–post pharmacological treatment data regarding patients’ cognitive and/or clinical status.
Quality assessment
The Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Case Reports (https://joannabriggs.org/critical_appraisal_tools) was used to evaluate the quality of the majority of included studies, which were case reports. The checklist ascertains the presence of several methodological aspects (e.g. the description of case’s demographic and anamnestic information, the validity of employed diagnostic tools, the description of procedures and results), through a four-response scoring (i.e. yes, no, unclear, not applicable).
Additionally, to evaluate the quality of the sole retrieved case-controlled study, we used the Newcastle–Ottawa Scale (NOS) (www.ohri.ca/programs/clinical_epidemiology/oxford.asp) for case-controlled studies, as previously suggested [20]. The scale rates the quality of studies by the evaluation of three aspects (i.e. selection, comparability, and outcome), and it allocates a maximum of nine stars for the highest quality.
Two independent authors (AS, FB) assessed the methodological quality of the retrieved articles to identify any potential source of bias. Disagreements were resolved by consensus with a senior author (AC). A summary of the quality assessment of included studies is provided in Table 1; explicatory models of the two quality assessment tools are provided as Supplementary Materials.
Results
Literature search results
A summary of the screening procedure is provided in the PRISMA flow diagram (Fig. 1). The initial web search strategy retrieved 103 papers; the additional independent manual search retrieved further eight articles. After removing duplicates, eighty-eight articles were screened according to title/abstract criterion. Twenty-eight full-text articles were consequently assessed for eligibility. Finally, seventeen studies were included in the current systematic review.
Included studies
The present systematic review included sixteen case report articles [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36] and one cross-sectional controlled study [37]. The main characteristics of the included studies are summarized in Table 2.
Case reports
Case reports were the most representative literature sources and involved ten women and seven men; patients’ age ranged from 57 to 77 years (mean age 68.2 ± 6.76 years) among women, and from 25 to 80 years (mean age 51.42 ± 18.85 years) among men. The presence of cognitive impairment was mostly discussed in association with idiopathic HPT (eleven case reports); five articles [25, 28,29,30, 36], involving six patients, described the occurrence of cognitive impairment following postsurgical HPT; the surgery was undergone generally due to goiter, and in one case due to thyroid adenoma.
Each study provided both laboratory and neuroradiological evidence supporting the diagnosis of HPT. Accordingly, brain calcifications were commonly detected through CT scans, involving subcortical regions, such as the basal ganglia. The majority of reports shared the presence of hypocalcemia, hyperphosphatemia and low PTH as laboratory evidence of HPT. In one case [27], the diagnosis of HPT was eventually confirmed even though normal calcium levels, and reduced PTH, 25-hydroxy-vitamin D and 1,25-dihydroxy-vitamin D levels were found in a 51-year-old man suffering from progressively worsened cognitive disturbances. A further peculiar report was described by Heckmann and colleagues that documented the case of a 74-year-old woman, who had previously undergone thyroidectomy, presenting hypercalcemia and decreased PTH levels, and complaining a progressive cognitive decline. Since the diagnosis of secondary HPT was disclosed, the unusual presence of hypercalcemia was eventually referred to a drug overdosage for previous hypocalcemia episodes [29].
In the majority of the discussed case reports of idiopathic HPT, patients referred to clinical observation suffering from cognitive disturbances, whose onset ranged from few weeks [21, 32], few years [23, 26, 31, 33], to approximately a decade before the visit [24]. Conversely, in one case, a female patient came to observation for the progression of cognitive disorders over the last year, with a diagnosis of idiopathic HPT already disclosed more than a decade before [34]. In the reported case by Terada and colleagues, the temporal relationship between the onset of cognitive symptoms and the diagnosis of idiopathic HPT was not clearly highlighted. The authors discussed the case of a 70-year-old woman referred to hospital due to stiffness in the upper extremities; contextually, a cognitive assessment as well as laboratory and radiological investigations revealed the presence of cognitive impairment and clinical signs of idiopathic HPT, respectively [35]. This temporal relationship was substantially consistent across those cases of secondary HPT (i.e. postsurgical), since the thyroidectomy was undergone from 24 to 43 years earlier, followed by the consequent occurrence of progressive cognitive disturbances.
The employment of validated test and batteries to assess cognitive functions was present in six studies [23,24,25, 29, 33, 35]; specifically, global cognitive functioning was assessed through the Mini Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MoCA); the Wechsler Adult Intelligence Scale (WAIS) was used to evaluate patient’s IQ; neuropsychological batteries allowed to measure different cognitive functions, such as memory, attention, abstract thinking, executive functions, and visuomotor coordination. In the remaining case reports, cognitive symptoms were solely described.
Cross sectional controlled study
The cross-sectional controlled study [37] involved 62 patients with idiopathic HPT (mean age 36.6 ± 15.16 years) attending the Endocrine Unit, and seventy controls (mean age 37.4 ± 15.22 years) who were unaffected attendants of admitted patients of various endocrine diseases, and who showed normal serum total calcium and phosphorus levels. The employed statistical approach consisted of computing Student’s t test and Wilcoxon test to describe differences between patients and controls; furthermore, multiple regression was performed, considering the presence of neuropsychological dysfunction among patients with idiopathic HPT as the dependent variable.
A trained psychologist carried out the neuropsychological evaluation through a battery of standard tests, assessing global cognitive functioning, orientation, visual attention, psychomotor speed, letter–number switching, verbal and visual memory, visuo-spatial abilities, response inhibition and intelligence quotient. A global cognitive dysfunction score was then calculated by combining the observed impairment in each administered test, which was scored as ‘1’ when impaired and ‘0’ if normal.
The mean serum total calcium among patients, measured at the time of the neuropsychological assessment, was 7.7 ± 1.20 mg %. CT scans were performed and to detect the presence of brain calcification (basal ganglia, thalamus, cerebellum, dentate nucleus and periventricular region).
Aggarwal and colleagues highlighted that a significant proportion of patients with idiopathic HPT, compared to controls, was more cognitively impaired [32.3% (95% CI 20.9–45.3) vs 5.7% (95% CI 1.6–14.0), p < 0.001]. Among patients, cognitive dysfunction score was significantly lower by 1.7 points in males (p = 0.02). Moreover, cognitive dysfunction score was significantly increased by 0.21 for each year increase in the duration of illness (p = 0.001) and by 5.5 for one unit increase in serum calcium–phosphorus product (p = 0.01), and eventually, the cognitive scores improved by 0.27 for every mg% increase in serum total calcium (p = 0.001).
According to the study findings, serum total calcium and its product with phosphorus were discussed as independent predictors of neuropsychological dysfunctions; conversely, intracranial calcifications resulted not significantly associated to the severity of cognitive impairment. Furthermore, Aggarwal and colleagues revealed several peculiar cognitive deficits not frequently reported, such as psychomotor retardation, reduced inhibitory control, impairment in visuo-spatial functioning, constructive apraxia and micrographic writing.
Discussion
The purpose of the present review was to systematically summarize the available evidence accounting the association between cognitive impairment and HPT. To the best of our knowledge, this contribution represents the first attempt to provide an updated state of the art on this topic.
As above mentioned, the presence of intracranial calcifications is a common finding in HPT. The calcifications are usually sited in the basal ganglia region involving lenticular and caudate nuclei, and spread to further brain areas, such as thalamus, cerebellum. Particularly, Goswami et al. [38] have quantitatively assessed the extent of calcifications within the basal ganglia obtained from 93 head computed tomography scans in patients with idiopathic HPT. The lentiform nucleus, representing putamen and globus pallidus, and caudate nuclei were the most commonly affected (69%, 56% and 55%, respectively); furthermore, the gray–white matter junction (40%), cerebellar parenchyma and dentate nucleus (32% and 24%, respectively), and thalamus (24%) were consistently associated with basal ganglia calcifications. Choroid plexus calcification was mainly observed in subjects with basal ganglia calcifications at baseline, and in those who showed progression. The occurrence and progression of calcification have been associated with the duration of disease and the calcium/phosphorus ratio [38, 39].
Noteworthy, basal ganglia calcifications are found frequently in renal replacement therapy patients with HPT, while in uremic patients with increased PTH, these calcifications are extremely rare, thus possibly implying a PTH-mediated protective mechanism against basal ganglia and intracerebral calcifications [40]. As recently suggested, in HPT patients, the lack of PTH signaling, hypocalcemia and hyperphosphatemia promote calcium–phosphate deposition, mainly in the extracellular space of basal ganglia. In fact, the sodium-dependent phosphate transporter 2 and carbonic anhydrase-II could be directly affected by the lack of PTH, leading to enhanced phosphate concentrations in the extracellular space and to inadequate degree of acidification and finally to phosphate and calcium precipitation [40].
The strict clinical meaning of the basal ganglia calcifications in HPT is still not clear. Brain calcifications have been associated with neurological dysfunctions including decreased attention, memory, information processing, executive functions and extrapyramidal symptoms. Nonetheless, nor number, volume and sites of intracranial calcifications were consistently associated with neuropsychological, extrapyramidal and cerebellar dysfunctions in the study by Aggarwal and colleagues [37] as they considered young patients while neurological dysfunction might show an association with intracranial calcification with advancing age. These neurologic dysfunctions could be due to the disruption of the corticostriatal tract carrying sensory input from cerebral cortex to striatum (caudate and putamen), finally relaying to globus pallidus, which tunes the sensory input along with dentate-thalamic tract and projects the signals back to the cortex for organized activities [41]. Besides, intracranial calcifications site may differently affect cognitive functions, since calcification occurring predominantly in the perivascular region or synaptic regions of the corticospinal tracts could result in impaired blood flow with secondary hypoxia and impaired dopamine and glutamate transmissions.
Interestingly, in the study by Aggarwal and colleagues, a multiple regression analysis identified serum total calcium and the calcium product with phosphorus as independent predictors of neuropsychological dysfunction [37]. Hypocalcemia may exacerbate the neurological dysfunction induced by cerebral calcifications; furthermore, pharmacological correction of serum calcium levels with calcium supplements and active vitamin D may lead to a partial improvement of neuromotor symptoms. On the other hand, exogenous PTH (1–84) administration in patients with HPT has been shown to improve, but not normalize, physical and mental well-being, and this may depend at least in part by the time of pharmacological intervention [16].
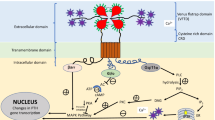
Although many of the neurological features in HPT have been considered to be linked to brain calcifications and hypocalcemia, the role of PTH receptor should be additionally taken into consideration. Particularly, the PTH 2 receptor (PTH2R) is a member of the Family B group of G protein-coupled receptors (GPCRs), distributed in both the central nervous system and different peripheral organs [42]. At central level, it is mainly expressed in the amygdala, medial preoptic area, hypothalamic paraventricular and periventricular nuclei, medial geniculate, and the pontine tegmentum. The distribution of PTH2R-immunoreactive fibers includes dense fiber network in the medial preoptic area, hypothalamic paraventricular, periventricular and infundibular (arcuate) nuclei, lateral hypothalamic area, median eminence, thalamic paraventricular nucleus, periaqueductal gray, lateral parabrachial nucleus, nucleus of the solitary tract, sensory trigeminal nuclei, medullary dorsal reticular nucleus, and dorsal horn of the spinal cord. Besides, PTH2R is also expressed by endocrine cells that include pancreatic islet somatostatin cells, thyroid parafollicular cells, and peptide secreting cells in the gastrointestinal tract, and by cells in the vasculature and heart. According to the central expression of PTH2R, it has been suggested this receptor may participate in neuroendocrine, limbic and sensory processing functions [43].
An additional element of scientific interest has widely considered the tuber infundibular peptide of 39 residues (TIP39), which has been originally purified and sequenced two decades ago, and which is acknowledged as a strong and selective natural agonist of the PTH2R [42, 44]. In this context, the brain expression of TIP39 has been recently detected in the subparafascicular region in the posterior thalamus, and the medial paralemniscal nucleus in the lateral pons [45].
Since the axon terminals of TIP39 neurons result distributed similarly as the PTH2R containing neurons, the implications of the joint TIP39-PTH2R system in nociceptive information processing in the spinal cord, in the regulation of different hypophysiotropic neurons in the hypothalamus, and in the modulation of emotional processes have been suggested. Accordingly, TIP39-KO mice demonstrated memory impairment selectively under conditions of novelty-induced arousal. Acute administration of a PTH2R antagonist in wild-type mice had a similar effect [46].
Although stimulation of PTH2R by TIP39 results in a twofold greater accumulation in cAMP than that elicited by PTH, and TIP39 is considered 100-fold more potent than PTH, it could be speculated that pathological alteration of serum PTH may lead to neuropsychological consequences also via PTH2R [44].
There is a lack of data on the ability of replacement therapy with PTH or its analogues, which are also able to restore the calcium product with phosphorus closer to normal, to significantly modify the progression of cerebral calcifications and cognitive clues in HPT [17, 47,48,49]. A further relevant point not deeply accounted among the retrieved studies is the potential impact on the onset and the maintenance of cognitive dysfunctions of the disease control by conventional therapy, since a poor control might be associated to the progression of cognitive impairment.
Despite the topic is worthy of interest, HPT is a rare disease and the overall available evidences on the association between cognitive dysfunctions and HPT are principally limited to case reports [13, 50]. Only one cross-sectional controlled study investigating cognitive profile among patients with idiopathic HPT was retrieved [37]; the overall quality of the study was fair according to the NOS score, with the main uncertainty regarding the selection criteria of the control group, which was unclear even though the control group was classified as healthy. The overall quality of case reports was evaluated through the JBI Critical Appraisal Checklist for Case Reports; the mean quality score was 4.37/8 (with scores ranging from 1/8 to 6/8). The description of patients’ sociodemographic characteristics was quite limited across the studies, since age and gender were the only factors constantly accounted in each report. No case report highlighted the occurrence of adverse effects. Anamnestic information regarding the patients’ medical history was collected and clearly described in the majority of the studies; only four studies reported unclear or vague anamnestic information. Additionally, the description of post-treatment clinical conditions was not sufficiently clear (seven studies), or it was substantially missing (three studies) in the majority of the reports. Conversely, the presentation of the patients’ current clinical condition, the description of diagnostic tests or assessment methods, and the description of the treatment procedures were more accurate. However, with regard to the description of the assessment methods, a further qualitative limitation should be additionally highlighted, since the description of the performed cognitive assessment often resulted vague, and it was not always clear whether or which assessment tool was administered.
In conclusion, the aim of the current systematic review was to provide an updated state-of-the-art concerning the association between cognitive impairment and HPT. Even though mostly based on single case reports, the presence of neuropsychological dysfunctions in the context of HPT appears to be a consistent core finding. Further longitudinal studies are strongly recommended to better understand the cognitive profile of subjects with HPT; consistently, the employment of standardized neuropsychological batteries might facilitate a better generalizability of the evidence, making them much more easily comparable. Similarly, future studies on this topic should additionally account the impact of the patients’ adherence to therapy as a potential risk factor for the progression of cognitive dysfunctions.
The presence of progressive cognitive disturbances is a core finding of aging. In this context, the investigation of potential modifiable risk factors related to cognitive impairment has gained interest increasingly over the past years [51]. Consistently, accounting the early occurrence of cognitive dysfunctions in subjects with HPT could allow physicians to more comprehensively acknowledge the wide symptomatology of HPT, as well as to better observe patients’ cognitive trajectories potentially leading to dementia. In this perspective, it could be useful, both as a challenging novel research topic and in clinical practice, to address those patients with HPT, who may exhibit early cognitive disturbances, to cognitive stimulation interventions aimed at improving cognitive reserves, following in parallel the evolution of both cognitive and disease-related symptomatology.
Abbreviations
- SCD:
-
Subjective cognitive decline
- MCI:
-
Mild cognitive impairment
- PTH:
-
Parathyroid hormone
- BBB:
-
Blood brain barrier
- HPT:
-
Hypoparathyroidism
- MMSE:
-
Mini Mental State Examination
- MoCA:
-
Montreal Cognitive Assessment
- WAIS:
-
Wechsler Adult Intelligence Scale
- PTH2R:
-
PTH 2 receptor
- GPCRs:
-
G protein-coupled receptors
- TIP39:
-
Tuber infundibular peptide of 39 residues
References
Fisher GG, Chaffee DS, Tetrick LE, Davalos DB, Potter GG (2017) Cognitive functioning, aging, and work: a review and recommendations for research and practice. J Occup Health Psychol 22(3):314–336. https://doi.org/10.1037/ocp0000086
Mungas D, Beckett L, Harvey D et al (2010) Heterogeneity of cognitive trajectories in diverse older persons. Psychol Aging 25(3):606–619. https://doi.org/10.1037/a0019502
Bezdicek O, Červenková M, Georgi H et al (2020) Long-term cognitive trajectory and activities of daily living in healthy aging. Clin Neuropsychol. https://doi.org/10.1080/13854046.2020.1745895
Hartshorne JK, Germine LT (2015) When does cognitive functioning peak? The asynchronous rise and fall of different cognitive abilities across the life span. Psychol Sci 26(4):433–443. https://doi.org/10.1177/0956797614567339
Ampadu J, Morley JE (2015) Heart failure and cognitive dysfunction. Int J Cardiol 178:12–23. https://doi.org/10.1016/j.ijcard.2014.10.087
Morley JE (2016) The complexities of diabetes in older persons. J Am Med Dir Assoc 17(10):872–874. https://doi.org/10.1016/j.jamda.2016.07.022
Lee S, Shimada H, Park H et al (2015) The association between kidney function and cognitive decline in community-dwelling, elderly Japanese people. J Am Med Dir Assoc 16(4):349.e1–349.e3495. https://doi.org/10.1016/j.jamda.2014.12.009
Catalano A, Sardella A, Bellone F, Lasco CG, Martino G, Morabito N (2019) Executive functions predict fracture risk in postmenopausal women assessed for osteoporosis. Aging Clin Exp Res. https://doi.org/10.1007/s40520-019-01426-w.10.1007/s40520-019-01426-w
Usdin TB, Gruber C, Bonner TI (1995) Identification and functional expression of a receptor selectively recognizing parathyroid hormone, the PTH2 receptor. J Biol Chem 270(26):15455–15458. https://doi.org/10.1074/jbc.270.26.15455
Bühler G, Balabanova S, Milowski S et al (1997) Detection of immunoreactive parathyroid hormone-related protein in human cerebrospinal fluid. Exp Clin Endocrinol Diabetes 105(6):336–340. https://doi.org/10.1055/s-0029-1211775
Lourida I, Thompson-Coon J, Dickens CM et al (2015) Parathyroid hormone, cognitive function and dementia: a systematic review. PLoS ONE 10(5):e0127574. https://doi.org/10.1371/journal.pone.0127574
Powers J, Joy K, Ruscio A, Lagast H (2013) Prevalence and incidence of hypoparathyroidism in the United States using a large claims database. J Bone Miner Res 28(12):2570–2576. https://doi.org/10.1002/jbmr.2004
Cianferotti L, Parri S, Gronchi G et al (2018) Prevalence of chronic hypoparathyroidism in a Mediterranean region as estimated by the analysis of anonymous healthcare database. Calcif Tissue Int 103(2):144–150. https://doi.org/10.1007/s00223-018-0405-5
Cipriani C, Pepe J, Biamonte F et al (2017) The epidemiology of hypoparathyroidism in Italy: an 8-year register-based study. Calcif Tissue Int 100(3):278–285. https://doi.org/10.1007/s00223-016-0222-7
Clarke BL, Brown EM, Collins MT et al (2016) Epidemiology and diagnosis of hypoparathyroidism. J Clin Endocrinol Metab 101(6):2284–2299. https://doi.org/10.1210/jc.2015-3908
Bilezikian JP, Khan A, Potts JT Jr et al (2011) Hypoparathyroidism in the adult: epidemiology, diagnosis, pathophysiology, target-organ involvement, treatment, and challenges for future research. J Bone Miner Res 26(10):2317–2337. https://doi.org/10.1002/jbmr.483
Bilezikian JP (2020) Hypoparathyroidism. J Clin Endocrinol Metab 105(6):1722–1736. https://doi.org/10.1210/clinem/dgaa113
Pepe J, Colangelo L, Biamonte F et al (2020) Diagnosis and management of hypocalcemia. Endocrine. https://doi.org/10.1007/s12020-020-02324-2
Kim SM, Zhao D, Schneider ALC et al (2017) Association of parathyroid hormone with 20-year cognitive decline: the ARIC study. Neurology 89(9):918–926. https://doi.org/10.1212/WNL.0000000000004290
Lenzo V, Sardella A, Martino G, Quattropani MC (2020) A systematic review of metacognitive beliefs in chronic medical conditions. Front Psychol 10:2875. https://doi.org/10.3389/fpsyg.2019.02875
Robinson KC, Kallberg MH, Crowley MF (1954) Idiopathic hypoparathyroidism presenting as dementia. Br Med J 2(4898):1203–1206. https://doi.org/10.1136/bmj.2.4898.1203
Eraut D (1974) Idiopathic hypoparathyroidism presenting as dementia. Br Med J 1(5905):429–430. https://doi.org/10.1136/bmj.1.5905.429
Mateo D, Giménez-Roldán S (1982) Dementia in Idiopathic hypoparathyroidism. Rapid efficacy of alfacalcidol. Arch Neurol 39(7):424–425. https://doi.org/10.1001/archneur.1982.00510190042013
Lorusso S, Poli V, Casmiro M (1994) Cognitive improvement following treatment in a case of idiopathic hypoparathyroidism. Eur Neurol 34(5):292–294. https://doi.org/10.1159/000117061
Nicolai A, Lazzarino LG (1994) Dementia syndrome in patients with postsurgical hypoparathyroidism and extensive brain calcifications. Eur Neurol 34(4):230–235. https://doi.org/10.1159/000117045
Roca B, Mínguez C, Sáez-Royuela A, Simón E (1995) Dementia, myopathy, and idiopathic hypoparathyroidism. Postgrad Med J 71(841):702. https://doi.org/10.1136/pgmj.71.841.702-a
Stuerenburg HJ, Hansen HC, Thie A, Kunze K (1996) Reversible dementia in idiopathic hypoparathyroidism associated with normocalcemia. Neurology 47(2):474–476. https://doi.org/10.1212/wnl.47.2.474
Gálvez-Jiménez N, Hanson MR, Cabral J (2000) Dopa-resistant parkinsonism, oculomotor disturbances, chorea, mirror movements, dyspraxia, and dementia: the expanding clinical spectrum of hypoparathyroidism. A case report. Mov Disord 15(6):1273–1276. https://doi.org/10.1002/1531-8257(200011)15:6<1273:aid-mds1038>3.0.co;2-o
Heckmann JG, Lang CJ, Neundörfer B (2000) Reversible dementia due to coexisting disease. Lancet 355(9220):2075. https://doi.org/10.1016/s0140-6736(05)73530-3
Adorni A, Lussignoli G, Geroldi C, Zanetti O (2005) Extensive brain calcification and dementia in postsurgical hypoparathyroidism. Neurology 65(9):1501. https://doi.org/10.1212/01.wnl.0000182293.34015.a9
Titlic M, Tonkic A, Jukic I, Filipovic-Grcic P, Kolic K (2008) Cognitive impairment and epilepsy seizure caused by hypoparathyroidism. Bratisl Lek Listy 109(2):79–81
Katsidzira L, Machiridza T, Ndlovu A (2010) A potentially treatable cause of dementia. Cent Afr J Med 56(5–8):41–44
Kumar G, Kaur D, Aggarwal P, Khurana T (2013) Hypoparathyroidism presenting as cognitive dysfunction. BMJ Case Rep 2013:bcr2013009220. https://doi.org/10.1136/bcr-2013-009220
Moreno Ó, García PT, Sánchez D, Sancho T, Lecumberri B (2015) Cognitive impairment and severe hypocalcemia in a patient with hypoparathyroidism and systemic sclerosis. Report of a case. Endocrinol Nutr 62(7):356–358. https://doi.org/10.1016/j.endonu.2015.04.003
Terada T, Kakimoto A, Yoshikawa E et al (2015) The possible link between GABAergic dysfunction and cognitive decline in a patient with idiopathic hypoparathyroidism. Intern Med 54(17):2245–2250. https://doi.org/10.2169/internalmedicine.54.4295
Dos Santos VM, Da Mata AM, Ribeiro KR, Calvo IC (2016) Fahr's syndrome and secondary hypoparathyroidism. Rom J Intern Med 54(1):63–65. https://doi.org/10.1515/rjim-2016-0007
Aggarwal S, Kailash S, Sagar R et al (2013) Neuropsychological dysfunction in idiopathic hypoparathyroidism and its relationship with intracranial calcification and serum total calcium. Eur J Endocrinol 168(6):895–903. https://doi.org/10.1530/EJE-12-0946
Goswami R, Sharma R, Sreenivas V, Gupta N, Ganapathy A, Das S (2012) Prevalence and progression of basal ganglia calcification and its pathogenic mechanism in patients with idiopathic hypoparathyroidism. Clin Endocrinol (Oxf) 77(2):200–206. https://doi.org/10.1111/j.1365-2265.2012.04353.x
Cusano NE, Bilezikian JP (2018) Signs and symptoms of hypoparathyroidism. Endocrinol Metab Clin N Am 47(4):759–770. https://doi.org/10.1016/j.ecl.2018.07.001
Zavatta G, Clarke BL (2020) Basal ganglia calcification in hypoparathyroidism and pseudohypoparathyroidism: local and systemic metabolic mechanisms. J Endocrinol Investig. https://doi.org/10.1007/s40618-020-01355-w
Ropper AH (2005) Abnormalities of movement and posture due to disease of the Basal Ganglia. In: Ropper AH, Brown RH (eds) Adams and Victor’s principles of neurology. Mc Graw Hill, New York, pp 55–71
Hoare SR, Bonner TI, Usdin TB (1999) Comparison of rat and human parathyroid hormone 2 (PTH2) receptor activation: PTH is a low potency partial agonist at the rat PTH2 receptor. Endocrinology 140(10):4419–4425. https://doi.org/10.1210/endo.140.10.7040
Bagó AG, Dimitrov E, Saunders R et al (2009) Parathyroid hormone 2 receptor and its endogenous ligand tuberoinfundibular peptide of 39 residues are concentrated in endocrine, viscerosensory and auditory brain regions in macaque and human. Neuroscience 162(1):128–147. https://doi.org/10.1016/j.neuroscience.2009.04.054
Usdin TB, Hoare SR, Wang T, Mezey E, Kowalak JA (1999) TIP39: a new neuropeptide and PTH2-receptor agonist from hypothalamus. Nat Neurosci 2(11):941–943. https://doi.org/10.1038/14724
Dobolyi A, Palkovits M, Usdin TB (2010) The TIP39-PTH2 receptor system: unique peptidergic cell groups in the brainstem and their interactions with central regulatory mechanisms. Prog Neurobiol 90(1):29–59. https://doi.org/10.1016/j.pneurobio.2009.10.017
Coutellier L, Logemann A, Kuo J, Rusnak M, Usdin TB (2011) TIP39 modulates effects of novelty-induced arousal on memory. Genes Brain Behav 10(1):90–99. https://doi.org/10.1111/j.1601-183X.2010.00643.x
Meola A, Vignali E, Matrone A, Cetani F, Marcocci C (2018) Efficacy and safety of long-term management of patients with chronic post-surgical hypoparathyroidism. J Endocrinol Investig 41(10):1221–1226. https://doi.org/10.1007/s40618-018-0857-5
Bollerslev J, Schalin-Jäntti C, Rejnmark L et al (2019) MANAGEMENT OF ENDOCRINE DISEASE: unmet therapeutic, educational and scientific needs in parathyroid disorders. Eur J Endocrinol 181(3):P1–P19. https://doi.org/10.1530/EJE-19-0316
Cusano NE, Rubin MR, Irani D, Sliney J Jr, Bilezikian JP (2013) Use of parathyroid hormone in hypoparathyroidism. J Endocrinol Investig 36(11):1121–1127. https://doi.org/10.1007/BF03346763
Brandi ML (2011) Genetics of hypoparathyroidism and pseudohypoparathyroidism. J Endocrinol Investig 34(7 Suppl):27–34
Sardella A, Catalano A, Lenzo V, Bellone F, Corica F, Quattropani MC, Basile G (2020) The association between cognitive reserve dimensions and frailty among elderly: a structured narrative review. Geriatr Gerontol Int. https://doi.org/10.1111/ggi.14040
Funding
None.
Author information
Authors and Affiliations
Contributions
AS: methodology, acquisition, screening and interpretation of data, drafting of the manuscript; AC: conceptualization, interpretation of data, drafting of the manuscript, final revision of the manuscript; FB, NM and GB: acquisition and screening of data; SM and FC: supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not involve any human subjects or animal work.
Informed consent.
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sardella, A., Bellone, F., Morabito, N. et al. The association between hypoparathyroidism and cognitive impairment: a systematic review. J Endocrinol Invest 44, 905–919 (2021). https://doi.org/10.1007/s40618-020-01423-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-020-01423-1