Abstract
Purpose
We aimed to identify the phenotypic variability of IGF1R defects in a cohort of short children with normal GH secretion gathered through the last decade.
Patients and methods
Fifty children (25 girls) with short stature and a basal/stimulated growth hormone (GH) over 10 ng/ml having either a low birth weight or microcephaly were enrolled. MLPA and then Sanger sequence analysis were performed to detect IGF1R defects. The auxological and metabolic evaluation were carried out in index cases and their first degree family members whenever available.
Results
A total of seven (14%) IGF1R defects were detected. Two IGF1R deletions and five heterozygous variants (one frameshift, four missense) were identified. Three (likely) pathogenic, one VUS and one likely benign were classified by using ACMG. All children with IGF1R defects had a height < − 2.5SDS, birth weight < − 1.4SDS, and head circumference < − 1.36SDS. IGF-1 ranged from − 2.44 to 2.13 SDS. One child with a 15q terminal deletion had a normal phenotype and intelligence, whereas low IQ is a finding in a case with missense variant. Two parents who carried IGF1R mutations had diabetes mellitus, hypertension and hyperlipidemia, one of whom also had hypergonadotropic hypogonadism.
Conclusion
We found a deletion or variant in IGF1R in 14% of short children. Birth weight, head circumference, intelligence, dysmorphic features, IGF-1 levels and even height are not consistent among patients. Additionally, metabolic and gonadal complications may appear during adulthood, suggesting that patients should be followed into adulthood to monitor for these late complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Persistent short stature affects 10–15% of children born small for gestational age (SGA) [1]. Genetic, chromosomal and acquired disorders affecting the fetus may lead to both poor fetal growth and postnatal growth failure [2]. Genetic factors usually cause persistent short stature. Two major proteins associated with pre- and postnatal growth are IGF-1, and its receptor IGF-1 receptor (IGF-1R). IGF-1 promotes growth primarily by binding to IGF-1R and is expressed in fetal tissues as early as the formation of the zygote. Thus, defects in either IGF-1 or its receptor can result in poor pre- and postnatal growth [3, 4].
Animal studies have shown that the average birth weight of IGF1R-null mice is 45% of that in wild-type alleles, and the mutants die of respiratory failure just after birth [5]. Heterozygous mutant mice are phenotypically normal [5]. Heterozygous mutations in IGF1R in the human cause a syndrome of resistance to IGF-1 (MIM # 270450) with intrauterine and postnatal growth failure. Since the first report of patients with IGF1R defects in 2003, accumulating evidence has revealed a human growth phenotype characterized by low birth weight, failure of catch up growth, microcephaly and relatively high serum IGF-1 [6, 7].
The gene encoding IGF-1R (IGF1R) is located on the distal long arm of chromosome 15. Heterozygous terminal 15q deletions or ring chromosome 15 encompassing IGF1R may lead to clinical findings similar to IGF1R gene mutations, particularly intrauterine growth retardation and postnatal short stature. However, these patients usually have additional findings involving different organ systems, attributed to concomitant monosomy of the flanking genes in that region. Intellectual impairment, cardiac defects, genitourinary anomalies, skeletal malformation and facial dysmorphic features have been frequently reported in patients with IGF1R deletions [8,9,10].
Case selection for the analysis of IGF1R defects is still a challenge for pediatric endocrinologists. Patients with short stature and a history of low birth weight as well as a normal GH response to stimulation tests may be candidates for the investigation of IGF1R defects. Previous studies to determine the prevalence of IGF1R defects have yielded variable results. This may be related to patient selection as well as the choice of genetic methods. Patient populations are usually confined to short children with unexplained intrauterine growth retardation. Some studies have also used other phenotypic features. Moreover, the genetic analyses differ [6, 9, 11,12,13]. Studies solely screening mutations in the IGF1R gene can miss IGF1R deletions. In contrast, sole analysis of copy number changes can detect IGF1R deletions, but would omit IGF1R mutations.
IGF1R defect is not only uncommon, but its clinical and laboratory findings have also not been thoroughly defined. Restriction of inclusion criteria in study populations have led to the inclusion of patients with predefined characteristics and carry a risk of confining the clinical variability of the condition. Thus, in the current study, we aimed to identify the phenotype variability in a cohort of children presumed to be IGF1R defect gathered over the last decade.
Patients and methods
Study population
The cohort consisting of 50 children (25 girls, 25 boys) was recruited from a database of cases with short stature (height ≤ 3rd percentile) and normal GH secretion who had additionally at least one of the two major phenotypic features, either intrauterine growth retardation or microcephaly. No chronic or inflammatory disorder causing growth retardation was identified in any of the children. All children had basal/stimulated growth hormone (GH) over 10 ng/ml. Serum IGF-1 level was measured however; this was not used as an inclusion/exclusion criterion. The auxological and laboratory parameters of the cohort are shown in Table 1.
The clinical, auxological parameters as well as blood samples for IGF-1 levels and metabolic measurements were collected from the parents and siblings who were available; the pedigrees were recorded. Genetic analysis was carried out in whole cohort and the family members of the affected children.
The study protocol was approved by the local institutional review board (Decision No. GO 17/231-15), and all participants and their parents provided written informed consent.
Genetic analysis
Genomic DNA was extracted from peripheral blood leukocytes by salt precipitation. In the first step, MLPA analysis was performed in each of the patients to detect any copy number change in exons of IGF1R. MLPA analysis was performed with the SALSA MLPA® Probemix P217-B2 kit, following the manufacturer’s instructions (MRC-Holland, Amsterdam, Netherlands). In the case of any copy number change detected by MLPA, a microarray analysis was carried out to determine the exact breakpoints on DNA. Microarray analyses were performed using the Affymetrix platform (CytoScan Optima and CytoScan HD) according to the manufacturer’s instructions. In the second step, Sanger sequence analysis was performed to scan exonic or splice site mutations in the IGF1R gene in all patients with normal MLPA results (the sequences of primer pairs and reaction conditions are available upon request). Variants were classified using mainly the consensus recommendations of ACMG [14]. However, there were some shortcomings in the classification of variants according to the 2015 ACMG criteria. Thus, new Sherloc criteria developed in 2017 were also used in the classification [15]. In the new criteria, the frequency of the variant is prioritized and the positive clinic is considered to be more significant than negative functional test. In addition, 3B score (which means three points of benign characteristics) was considered sufficient for likely benign, and 4P (which means four points of pathogenic characteristics) score was required for likely pathogenic. This asymmetric condition prevents a variant to be considered as pathogenic just because it is rare. However, in such a scoring system difficulty in determining pathogenicity of the variants is inevitable when majority of the disease causing variants are missense changes in a gene, which is also true for IGF1R. Thus, missense variants are more likely to be classified as benign or VUS at best according to ACMG and Sherloc [14, 15]. What is more, short stature is not only a common trait, but it is a multifactorial (multigenic) one as well. Since IGF1R defects are a rare cause of short stature, and stature is affected by more than one gene, lack of segregation should not be considered as a strong evidence to make the case for a benign variant. Same caveat was stated in the 2015 ACMG criteria [14]. It has also been supported by the study of Giabicani et al. [16] in which two heterozygous IGF1R missense variants, classified as VUS due to non-segregation, were found to be likely pathogenic after functional studies. It is suggested that development of more focused guidance regarding the classification of variants in specific genes in specific disease groups might be necessary given that the applicability and weight assigned to certain criteria may vary by gene and disease [14]. Thus, gene-specific variant classification approach will probably be more accurate, but unfortunately unavailable for IGF1R defects or growth failure of prenatal onset yet.
In the current study, two cases had deletion and were classified as pathogenic. Classification of the identified missense variants according to ACMG and Sherloc are shown in Table 2.
Growth hormone stimulation tests
GH stimulation tests were carried out early in the morning after a 12-h fast. Blood was drawn before and 60 and 90 min after the administration of levodopa (10 mg/kg, max 500 mg) per oral. A clonidine stimulation test was performed if the peak GH response was < 10 ng/ml during the l-dopa stimulation test. Blood was drawn at 0, 30, 60, 90, 120 min after clonidine hydrochloride (150 μg/m2; max: 200 μg) administration orally. A peak GH level ≥ 10 ng/ml was considered a normal GH response to pharmacologic stimulation.
Auxological parameters and calculations
Standing height was measured in patients older than 2 years using a wall-mounted stadiometer that measured to the nearest 0.1 cm. Body mass index (BMI) was calculated by dividing the body weight in kilograms by the square meters of height. Height-SDS and BMI-SDS were calculated [17, 18]. Bone age was assessed using the Greulich–Pyle method, and puberty was assessed using Tanner staging [19, 20].
Hormone assays
GH was measured using an immunochemiluminometric assay (ICMA), which was performed on an IMMULITE 2000 System (Siemens, England). The intra- and interassay CVs were 3.7 and 5.7%, respectively, and the analytic sensitivity of the test was 0.01 ng/ml. The serum IGF-1 level was measured using Beckman Coulter trademark assay with the immunoradiometric (IRMA) method. The intra- and interassay CVs of the IGF-1 level were 2.6 and 4.5%, respectively, with an analytical sensitivity of 2 ng/ml. The serum IGF-1 and SDS were calculated using the reference tables for age and gender [21].
Results
A total of 7 (14%) cases with IGF1R defects were detected in a cohort of 50 children. The clinical, biochemical and molecular details of the patients and the affected parents are summarized in Tables 3 and 4, respectively. IGF1R deletions were identified in 2 (4%) out of 50 cases by MLPA and microarray analyses. These were de novo mutations since the parents did not carry the deletions. The two cases with a deletion in the distal part of chromosome 15q (cases 1 and 2) also had concomitant duplications in chromosomes 9p and 1p, respectively. These duplications were not considered to contribute to the clinical presentations. Sanger sequencing revealed heterozygous variants in 5 (cases 3–7) out of 48 cases with normal MLPA. A schematic presentation of these variants on IGF1R is shown in Fig. 1; four were missense, and one was a frameshift variant.
The patient in case 1 first drew clinical attention for short stature (height 62 cm, − 3.4 SDS) at 11 months of age. No intellectual disability or dysmorphic finding was noted. The echocardiographic examination and renal scan were normal. The patient’s parents were third degree cousins. GH treatment was administered at the age of 4.2 years, and her growth velocity increased from 4 cm per year to 8 cm per year.
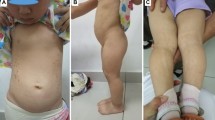
The patient in case 2 had a 15q terminal deletion as well as minor dysmorphic features, such as bilateral epicanthal folds, a high-arched palate and sandal gap toes. He had normal hearing and his IQ level was 45. Cardiac examination was normal.
Case 3 had a heterozygous missense variant (c.236C > T) that was classified as likely pathogenic in ACMG. The mutation was previously reported in a patient with short stature [22]. The patient had a twin brother with a birth weight of 1600 g. The twin brother who did not carry the variant had a normal growth rate and reached an adult height of 164.5 cm (− 1.5 SDS), whereas the patient could never catch up in growth. She had had regular menstrual bleeding since the age of 11. At the age of 19, her physical examination was normal except for short stature. Her mother, who carried the same mutation had also severe short stature and has exhibited hypertension, hyperlipidemia and diabetes mellitus since her late 30s. The mother had also experienced premature menopause at the age of 38 years with FSH 78.2 mIU/ml (normal 3.03–8.08), LH 27.1 mIU/ml (normal 1.8–11.7) and estradiol 13 pg/ml (normal 21–251). The maternal grandmother’s height was 145 cm (− 2.9 SDS); she also had diabetes mellitus and hypertension and had died of a hemorrhagic stroke at the age of 48 years. The maternal uncle also had short stature; his height was 160 cm (− 2.3 SDS). Moreover, the maternal grand-grandmother had exhibited short stature and had died of cancer at a young age.
Case 4 had a frameshift variant causing an early stop codon that was previously unreported in the GnomAd data; thus, it was classified as pathogenic. The patient was a 9.8-year-old boy with a height of 121.5 cm (− 2.5 SDS) at admission. He had a history of chronic diarrhea lasting over 2 years with an unknown etiology. Examination of the upper and lower gastrointestinal system with endoscopy and biopsies was normal. His height reached 153.5 cm (− 2.9 SDS) by the age of 16.3 years. His parents were third-degree cousins. His 47-year-old father carried the same variant, had severe short stature and had been diagnosed with hypertension and diabetes mellitus in his early 40s. He had normal gonadotropin and testosterone levels (FSH: 3.7 mIU/ml (normal 0.95–11.95), LH: 5.8 mIU/ml (normal 0.37–12) and testosterone: 235 ng/dl (normal 150–980). The patient’s paternal aunt also had short stature, with a height of 145 cm.
The patient in case 5 with a likely pathogenic variant had a height of − 3.2 SDS at the age of 13.5 years; however, he reached an adult height of − 1.5 SDS. His adult height was similar to that of his father, who had the same molecular defect.
The patient in case 6 with a heterozygous missense VUS variant had an IQ score of 80 and no significant dysmorphic features. His parents were first cousins. The patient, his mother and father all carried the same heterozygous IGF1R variant; however, only the patient and the mother had short stature. Family history revealed that one of the paternal uncles also had severe short stature (155 cm).
Case 7, who had a heterozygous missense variant classified as likely benign, had an IQ score of 46. Bilateral orchiopexy was performed at the age of 7 years. He had wide palpebral fissures and a pointed chin, bilateral clinodactyly at the 5th finger and syndactyly between the second and third toes. At the age of 13 years, serum gonadotropins were elevated [FSH: 56 mIU/ml (normal: 0.95–11.95 mIU/ml), LH: 8.57 mIU/ml (normal: 0.57–12.07 mIU/ml), T: 72.9 ng/dl (normal: 36–633). His 3.5-year-old younger brother had similar findings with low birth weight, severe microcephaly, dysmorphic features and intellectual disability. His height was at 3rd percentile. He had a wild-type IGF1R. So, the two brothers having first degree-related parents shared similar pathologic characteristics that cannot be attributed to IGF1R defect alone. Another autosomal recessive disorder may be considered in this family. However, IGF1R defect might contribute to the severity of short stature in case 7.
The pedigrees of the five children with IGF1R variants are shown in Fig. 2.
Pedigree of the five cases involving IGF1R variations. Black circles and squares represent patients with short stature + IGF1R mutation. Dotted circles and squares represent family members with short stature + no genetic analysis. Diagonal striped circles and squares represent family members with normal height + IGF1R mutation. Horizontal striped circles and squares represent family members with short stature + wild type IGF1R. The results of IGF1R gene analysis are shown below the cases
Discussion
Studies screening for IGF1R defects reported so far have shown heterogeneity in population characteristics and genetic analysis tools [6, 9, 11,12,13]. The sequential analysis of copy number using MLPA and microarrays, followed by Sanger sequencing in the current study, enabled a more effective delineation of the frequency of IGF1R defects. A total of seven (14%) molecular defects in IGF1R included pathogenic and likely pathogenic variants in 6% (3/50), deletions in 4% (2/50), VUS in 2% (1/50), and likely benign in 2% (1/50) of the patients.
Patients with IGF1R defects may have distinct clinical features regarding whether the defect is due to a deletion or a mutation. Terminal deletions of the long arm or the ring form of chromosome 15, which lead to deletion in IGF1R, are generally associated with intellectual disability, dysmorphic features and the involvement of several organs in addition to intrauterine growth retardation, short stature and microcephaly. Clinical presentation depends on the extent of the deleted genes along with the IGF1R [8,9,10]. In contrast, IGF1R mutations are generally reported to lead to auxological findings without involvement of intellectual development and other organs [23]. However, it should be kept in mind that some patients with 15q terminal deletions may have normal appearance and intelligence without organ involvement (as in our first case), most likely due to very small deletions. Consistently, additional involvement of genomic regions poor in functional genes would not cause any further clinical features, as in our first two cases with duplications at chromosome 9p, and 1p. Interestingly, some patients with IGF1R missense variants show additional features such as low intellectual capacity (case 6), gastrointestinal (case 4) and gonadal (the mother in case 3) involvement. Similar discordance has also been described in three large series of IGF1R defects [12, 16, 22].
Intrauterine growth retardation, microcephaly and short stature are hallmarks of IGF1R defects. However, in the present cohort, case 2 had a birth weight of − 1.4 SDS and cases 4 and 5 had head circumferences in the lower half of the normal range. This is in line with findings from three large cohorts [16, 22, 24] as well as other individual cases in the literature [11, 12, 25, 26]. Similarly, postnatal growth and adult height of IGF1R carriers also vary in a wide range. One of our three index cases, who attained adult height, reached a low-normal final height, similar to his father with the same molecular defect. In addition, case 6 attained an adult height of 153.5 cm (− 3.2 SDS), in contrast to his parents (with the same heterozygous variants) who were not so short. Therefore, a short-normal adult height can be a feature of IGF1R defects. There is also a wide variation in height potential and adult height among patients with IGF1R defects, ranging between − 5.5 and 1 SDS [16, 22]. The heights of the carrier parents and family members also had significant variability in adult height, ranging from very short stature to a normal height [12, 22, 27]. Thus, the scoring system proposed by Walenkamp et al. [22] for IGF1R defect should be revised to account for these clinical variabilities.
A reliable laboratory marker indicating IGF-1R deficiency is yet unavailable. IGF-1, as well as basal/peak GH levels, is far from being a marker for diagnosis, since there is a great variation in their levels as in our cases [22, 25, 28,29,30]. Patients with undernutrition tend to have low IGF-1 levels, which may be restored with weight gain [30, 31].
In the present cohort, two families with IGF1R mutations had a significant burden of early-onset cardiovascular risk factors. The mother (with a heterozygous missense mutation), grandmother and maternal uncle of case 3 all had severe short stature and relatively early onset diabetes mellitus, dyslipidemia and hypertension (in their late 30s or early 40s). Moreover, the grandmother died of stroke at the age of 48 years. Case 4 also had a father (with a heterozygous frameshift mutation) and a paternal aunt who had very short stature and were diagnosed with diabetes mellitus and hypertension in their early 40s. Animal studies suggest a possible link between IGF1R defects and glucose metabolism. Igf1r ± male mice had higher glucose levels during fasting and after a glucose load [32]. Knockout models of Igf1r on β cells show impaired glucose-dependent insulin release without a decrease in β cell mass, as well as age-dependent glucose intolerance in mice [33, 34]. A few case presentations in the literature show a propensity for impairment in glucose metabolism during adulthood [16, 27, 31]. Interestingly, diabetes mellitus at 14.5 years of age with relative insulin deficiency, as well as steroid-induced diabetes at 5 years of age, was reported in two siblings with a compound heterozygous IGF1R mutation [35]. A comparison between HbA1c and prevalence of type 2 diabetes between a limited number of adult IGF1R carriers versus adults with a history of small for gestational age using questionnaire revealed no difference [24]. Further studies in the adult carriers are required to elucidate whether there is a relationship between IGF1R defect and glucose metabolism.
The impact of IGF1R defect on gonadal function is unclear. Igf1r ± female mice have normal fertility, mating behavior and estrous cycle length compared to the corresponding features in wild-type mice [32]. Specific knockdown of IGF-1R in granulosa cells results in apoptosis, blocks folliculogenesis and ultimately leads to loss of fertility [36], suggesting IGF1R is essential for ovarian granulosa cell survival, estrogen production and formation of preovulatory follicles in female mice. Similarly, a lack of INSR and IGF1R in Sertoli cells of mouse testes gives rise to a decrease in testes size and sperm production [37]. In the current cohort, a carrier parent (case 3) experienced premature ovarian failure and early menopause. Also one patient (case 7) had hypergonadotropic hypogonadism; however, his molecular defect was classified as likely benign, and thus its association with gonadal defect is questionable. This issue requires further evaluation in adult carriers of IGF1R defect.
In conclusion, microcephaly, significant intrauterine growth retardation and severe short stature are not consistently present in IGF1R defects due to the multifactorial nature of these traits. What is more, IGF-1 and basal GH levels are not definitive diagnostic markers in most instances Adult cases with IGF1R defects should be investigated more thoroughly with respect to diabetes mellitus, cardiovascular risk factors and gonadal functions. We believe there is still a lot to learn about the characteristics and natural history of IGF1R defects.
Data availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Hokken-Koelega AC, De Ridder MA, Lemmen RJ, Den Hartog H, De Muinck-Keizer-Schrama SM, Drop SL (1995) Children born small for gestational age: do they catch up? Pediatr Res 38(2):267–271
Finken MJJ, van der Steen M, Smeets CCJ, Walenkamp MJE, de Bruin C, Hokken-Koelega ACS, Wit JM (2018) Children born small for gestational age: differential diagnosis, molecular genetic evaluation, and implications. Endocr Rev 39:851–894 (Erratum in: Endocr Rev 2019;40:96)
Klammt J, Pfäffle R, Werner H, Kiess W (2008) IGF signaling defects as causes of growth failure and IUGR. Trends Endocrinol Metab 19(6):197–205
Walenkamp MJ, Wit JM (2008) Single gene mutations causing SGA. Best Pract Res Clin Endocrinol Metab 22(3):433–446
Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A (1993) Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 75(1):59–72
Abuzzahab MJ, Schneider A, Goddard A, Grigorescu F, Lautier C, Keller E, Kiess W, Klammt J, Kratzsch J, Osgood D, Pfäffle R, Raile K, Seidel B, Smith RJ, Chernausek SD, Intrauterine Growth Retardation (IUGR) Study Group (2003) IGF-I receptor mutations resulting in intrauterine and postnatal growth retardation. N Engl J Med 349(23):2211–2222
Klammt J, Kiess W, Pfäffle R (2011) IGF1R mutations as cause of SGA. Best Pract Res Clin Endocrinol Metab 25(1):191–206
Jain S, Golde DW, Bailey R, Geffner ME (1998) Insulin-like growth factor-I resistance. Endocr Rev 19(5):625–646
Ester WA, van Duyvenvoorde HA, de Wit CC, Broekman AJ, Ruivenkamp CA, Govaerts LC, Wit JM, Hokken-Koelega AC, Losekoot M (2009) Two short children born small for gestational age with insulin-like growth factor 1 receptor haploinsufficiency illustrate the heterogeneity of its phenotype. J Clin Endocrinol Metab 94(12):4717–4727
Veenma DC, Eussen HJ, Govaerts LC, de Kort SW, Odink RJ, Wouters CH, Hokken-Koelega AC, de Klein A (2010) Phenotype-genotype correlation in a familial IGF1R microdeletion case. J Med Genet 47(7):492–498
Kawashima Y, Kanzaki S, Yang F, Kinoshita T, Hanaki K, Nagaishi J, Ohtsuka Y, Hisatome I, Ninomoya H, Nanba E, Fukushima T, Takahashi S (2005) Mutation at cleavage site of insulin-like growth factor receptor in a short-stature child born with intrauterine growth retardation. J Clin Endocrinol Metab 90(8):4679–4687
Yang L, Xu DD, Sun CJ, Wu J, Wei HY, Liu Y, Zhang MY, Luo FH (2018) IGF1R variants in patients with growth impairment: four novel variants and genotype–phenotype correlations. J Clin Endocrinol Metab 103(11):3939–3944
Leal AC, Montenegro LR, Saito RF, Ribeiro TC, Coutinho DC, Mendonca BB, Arnhold IJ, Jorge AA (2013) Analysis of the insulin-like growth factor 1 receptor gene in children born small for gestational age: in vitro characterization of a novel mutation (p.Arg511Trp). Clin Endocrinol (Oxf) 78(4):558–563
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL, ACMG Laboratory Quality Assurance Committee (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17(5):405–424
Nykamp K, Anderson M, Powers M, Garcia J, Herrera B, Ho YY, Kobayashi Y, Patil N, Thusberg J, Westbrook M, Invitae Clinical Genomics Group, Topper S (2017) Sherloc: a comprehensive refinement of the ACMG-AMP variant classification criteria. Genet Med. 19:1105–1117 (Epub 2017 May 11. Erratum in: Genet Med 2019 Jul 26)
Giabicani E, Willems M, Steunou V, Chantot-Bastaraud S, Thibaud N, Abi Habib W, Azzi S, Lam B, Bérard L, Bony-Trifunovic H, Brachet C, Brischoux-Boucher E, Caldagues E, Coutant R, Cuvelier ML, Gelwane G, Guemas I, Houang M, Isidor B, Jeandel C, Lespinasse J, Naud-Saudreau C, Jesuran-Perelroizen M, Perrin L, Piard J, Sechter C, Souchon PF, Storey C, Thomas D, Le Bouc Y, Rossignol S, Netchine I, Brioude F (2019) Increasing knowledge in IGF1R defects: lessons from 35 new patients. J Med Genet. https://doi.org/10.1136/jmedgenet-2019-106328
Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL (2002) 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11(246):1–190
Flegal KM, Cole TJ (2013) Construction of LMS parameters for the Centers for Disease Control and Prevention 2000 growth charts. Natl Health Stat Rep 11(63):1–3
Marshall WA, Tanner JM (1969) Variations in pattern of pubertal changes in girls. Arch Dis Child 44:291–303
Marshall WA, Tanner JM (1970) Variations in pattern of pubertal changes in boys. Arch Dis Child 45:13–23
Brabant G, von Mühlen A, Wüster C, Ranke MB, Kratzsch J, Kiess W, Ketelslegers JM, Wilhelmsen L, Hulthén L, Saller B, Mattsson A, Wilde J, Schemer R, Kann P, German KIMS Board (2003) Serum insulin-like growth factor I reference values for an automated chemiluminescence immunoassay system: results from a multicenter study. Horm Res 60(2):53–60
Walenkamp MJE, Robers JML, Wit JM, Zandwijken GRJ, van Duyvenvoorde HA, Oostdijk W, Hokken-Koelega ACS, Kant SG, Losekoot M (2019) Phenotypic features and response to GH treatment of patients with a molecular defect of the IGF-1 receptor. J Clin Endocrinol Metab 104(8):3157–3171
Choi JH, Kang M, Kim GH, Hong M, Jin HY, Lee BH, Park JY, Lee SM, Seo EJ, Yoo HW (2011) Clinical and functional characteristics of a novel heterozygous mutation of the IGF1R gene and IGF1R haploinsufficiency due to terminal 15q26.2-%3eqter deletion in patients with intrauterine growth retardation and postnatal catch-up growth failure. J Clin Endocrinol Metab 96(1):E130–E134
Göpel E, Rockstroh D, Pfäffle H, Schlicke M, Bechtold-Dalla Pozza S, Gannagé-Yared MH, Gucev Z, Mohn A, Harmel EM, Volkmann J, Weihrauch-Blüher S, Gausche R, Bogatsch H, Beger C, Klammt J, Pfäffle R (2019) A comprehensive cohort analysis comparing growth and GH therapy response in IGF1R mutation carriers and SGA children. J Clin Endocrinol Metab. https://doi.org/10.1210/clinem/dgz165
Fujimoto M, Kawashima Sonoyama Y, Hamajima N, Hamajima T, Kumura Y, Miyahara N, Nishimura R, Adachi K, Nanba E, Hanaki K, Kanzaki S (2015) Heterozygous nonsense mutations near the C-terminal region of IGF1R in two patients with small-for-gestational-age-related short stature. Clin Endocrinol (Oxf) 83(6):834–841
Raile K, Klammt J, Schneider A, Keller A, Laue S, Smith R, Pfäffle R, Kratzsch J, Keller E, Kiess W (2006) Clinical and functional characteristics of the human Arg59Ter insulin-like growth factor I receptor (IGF1R) mutation: implications for a gene dosage effect of the human IGF1R. J Clin Endocrinol Metab 91(6):2264–2271
Mohn A, Marcovecchio ML, de Giorgis T, Pfaeffle R, Chiarelli F, Kiess W (2011) An insulin-like growth factor-I receptor defect associated with short stature and impaired carbohydrate homeostasis in an Italian pedigree. Horm Res Paediatr 76(2):136–143
Kawashima Y, Higaki K, Fukushima T, Hakuno F, Nagaishi J, Hanaki K, Nanba E, Takahashi S, Kanzaki S (2012) Novel missense mutation in the IGF-I receptor L2 domain results in intrauterine and postnatal growth retardation. Clin Endocrinol (Oxf) 77(2):246–254
Kawashima Y, Hakuno F, Okada S, Hotsubo T, Kinoshita T, Fujimoto M, Nishimura R, Fukushima T, Hanaki K, Takahashi S, Kanzaki S (2014) Familial short stature isassociated with a novel dominant-negative heterozygous insulin-like growth factor 1 receptor (IGF1R) mutation. Clin Endocrinol (Oxf) 81(2):312–314
Labarta JI, Barrio E, Audí L, Fernández-Cancio M, Andaluz P, de Arriba A, Puga B, Calvo MT, Mayayo E, Carrascosa A, Ferrández-Longás A (2013) Familial short stature and intrauterine growth retardation associated with a novel mutation in the IGF-I receptor (IGF1R) gene. Clin Endocrinol (Oxf) 78(2):255–262
Walenkamp MJ, van der Kamp HJ, Pereira AM, Kant SG, van Duyvenvoorde HA, Kruithof MF, Breuning MH, Romijn JA, Karperien M, Wit JM (2006) A variable degree of intrauterine and postnatal growth retardation in a family with a missense mutation in the insulin-like growth factor I receptor. J Clin Endocrinol Metab 91(8):3062–3070
Holzenberger M, Dupont J, Ducos B, Leneuve P, Géloën A, Even PC, Cervera P, Le Bouc Y (2003) IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature 421(6919):182–187
Kulkarni RN, Holzenberger M, Shih DQ, Ozcan U, Stoffel M, Magnuson MA, Kahn CR (2002) Beta-cell-specific deletion of the Igf1 receptor leads to hyperinsulinemia and glucose intolerance but does not alter beta-cell mass. Nat Genet 31(1):111–115
Xuan S, Kitamura T, Nakae J, Politi K, Kido Y, Fisher PE, Morroni M, Cinti S, White MF, Herrera PL, Accili D, Efstratiadis A (2002) Defective insulin secretion in pancreatic beta cells lacking type 1 IGF receptor. J Clin Invest 110(7):1011–1019
Fang P, Cho YH, Derr MA, Rosenfeld RG, Hwa V, Cowell CT (2012) Severe short stature caused by novel compound heterozygous mutations of the insulin-like growth factor1 receptor (IGF1R). J Clin Endocrinol Metab 97(2):E243–E247
Baumgarten SC, Armouti M, Ko C, Stocco C (2017) IGF1R expression in ovarian granulosa cells is essential for steroidogenesis, follicle survival, and fertility in female mice. Endocrinology 158(7):2309–2318
Pitetti JL, Calvel P, Zimmermann C, Conne B, Papaioannou MD, Aubry F, Cederroth CR, Urner F, Fumel B, Crausaz M, Docquier M, Herrera PL, Pralong F, Germond M, Guillou F, Jégou B, Nef S (2013) An essential role for insulin and IGF1 receptors in regulating sertoli cell proliferation, testis size, and FSH action in mice. Mol Endocrinol 27(5):814–827
Acknowledgements
This study was supported by the Hacettepe University Scientific Research Unit with the project ID THD-2017-13561. We would like to thank Prof Jan Marten Wit for his excellent review of the manuscript and helpful contributions.
Funding
This study was funded by Hacettepe University Scientific Research Unit with the project ID THD-2017-13561.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study protocol was approved by the local institutional review board (Decision No. GO 17/231–15).
Informed consent
All participants and their parents provided written informed consent for participation in the study and publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gonc, E.N., Ozon, Z.A., Oguz, S. et al. Genetic IGF1R defects: new cases expand the spectrum of clinical features. J Endocrinol Invest 43, 1739–1748 (2020). https://doi.org/10.1007/s40618-020-01264-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-020-01264-y