Abstract
Background
Type 1 diabetes (T1DM) often coexists with other autoimmune diseases, most commonly with hypothyroidism. To date, the influence of coexisting autoimmune hypothyroidism (AHT) on the course of chronic neurovascular complications of autoimmune diabetes has not been established. The aim of the study was to assess the relationship between AHT and the occurrence of chronic T1DM complications.
Methods
The study group comprised 332 European Caucasian participants with T1DM [165 (49.7%) men]. AHT was recognized in subclinical and overt hypothyroidism and confirmed by the presence of anti-thyroid autoantibodies: anti-peroxidase (ATPO) and/or anti-thyroglobulin (ATg) and ultrasonography (hypoechogenicity, parenchymal heterogeneity, lymph nodes assessment).
Results
In the analyzed group, 48.5% of patients were diagnosed with at least one neurovascular complication. At the time of enrollment, 16.3% of participants were diagnosed with AHT. Patients with AHT, compared to those without AHT, were characterized by a higher prevalence of neurovascular complications (64.8 vs. 45.3%; P = 0.009) and retinopathy (55.6 vs. 38.9%; P = 0.02). There were significant differences between groups with and without neurovascular complications, with regard to classic risk factors for chronic diabetes complications: age, T1DM duration, SBP, DBP, HbA1c, TG, eGFR and hypertension prevalence. In the multivariate logistic regression analysis, AHT was an independent predictor of neurovascular complications after adjusting for age, DBP, HbA1c and TG (odds ratio, 2.40; 95% confidence interval, 1.17–4.92; P = 0.02).
Conclusions
AHT coexisting with T1DM was associated with a higher incidence of neurovascular complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 1 diabetes mellitus (T1DM) and autoimmune thyroid disease (AITD) are common endocrinopathies in clinical practice. The prevalence of thyroid dysfunction in T1DM is higher than in the general population and most often presents as autoimmune hypothyroidism (AHT) [1,2,3,4]. Frequent coexistence of both AITD and T1DM has its etiology in common genetic factors [5]. Undiagnosed thyroid dysfunction may influence the control of diabetes. It is known that hypothyroidism causes weight gain, impairs metabolic control and increases the risk of hypoglycemia in patients with diabetes [6]. Overt hypothyroidism leads to progression of atherosclerosis and increases the risk of cardiovascular disease (CVD) through dyslipidemia, hypertension and insulin resistance. Patients with subclinical hypothyroidism (SCH) present abnormalities such as diastolic dysfunction, higher blood pressure, arterial stiffness and endothelial dysfunction [7]. These abnormalities are of particular importance in adults with T1DM, who are at a level of risk of developing CVD that is greater than general population [8]. On the other hand, the natural course of T1DM is associated with development of microangiopathic complications, recently called neurovascular complications. It has been proved that the neurodegenerative process is involved not only in the development of diabetic neuropathy but also in retinopathy. Lesions affect retinal layers such as primary retinal ganglion cells and glial cells [9].
The influence of AHT and coexisting T1DM on the risk of developing diabetic neurovascular complications has not been researched. The aim of the study was to determine the association between coexisting AHT with T1DM and the presence of neurovascular complications.
Methods
Participants
The study was carried out in a single center in the Department of Internal Medicine and Diabetology in Poznan, Poland. 332 consecutive adult patients were admitted to the hospital with T1DM and at least 5-year history of the disease. They were recruited between years 2013–2017. Exclusion criteria are presented in Table 1. The study population comprised European Caucasian participants: 165 (49.7%) men and 167 (50.3%) women. The median age was 32 [interquartile range (IQR): 26–41] years and the T1DM duration was 14 (IQR: 9–21) years.
Diabetes was diagnosed according to WHO criteria based on classic symptoms of hyperglycaemia or hyperglycaemic crisis and a random plasma glucose concentration of ≥ 11.1 mmol/L [4]. The autoimmune etiology of diabetes was confirmed by the presence of at least one out of three examined autoantibodies [against islet cells (ICA), glutamic acid decarboxylase (GAD), insulinoma-associated tyrosine phosphatase (IA-2)] [4].
The Bioethical Committee of Poznan University of Medical Sciences approved the study protocol (reference numbers: 539/12 and 465/15) and all the participants provided written consent to take part in the study. The study was conducted according to the criteria set by the Declaration of Helsinki. All applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research. The anonymized datasets generated and analyzed during this study are available from the corresponding author on reasonable request.
Assessment of neurovascular complications
Direct ophthalmoscopy through dilated pupils was applied to assess the presence of diabetic retinopathy. The results were classified as no retinopathy, mild non-proliferative, moderate non-proliferative, severe non-proliferative and proliferative retinopathy according to the classification of the American Academy of Ophthalmology [10].
To diagnose diabetic kidney disease (DKD), either urinary albumin excretion in a 24-h collection or random albumin/creatinine ratio was assessed. An excretion rate higher than 30 mg/24 h or 30 mg/g in at least 2 out of 3 samples collected over a 3-month period was regarded as persistent albuminuria. Any conditions that could interfere with the evaluation of urinary albumin excretion were excluded. Possible secondary causes of proteinuria were taken into consideration: excessive physical activity, acute febrile illness, urinary tract infection, heart failure and hematuria. To diagnose DKD, persistent albuminuria and either at least a 10-year history of diabetes or diagnosed diabetic retinopathy were required. To assess the glomerular filtration rate, the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation was applied [11, 12]. The CKD-EPI equation includes the creatinine level in serum, age and sex. Chronic kidney disease was staged according to the National Kidney Foundation Classification [13].
The diagnosis of diabetic peripheral neuropathy (DPN) was based on the Toronto definition of probable peripheral neuropathy [14]. Symptoms of DPN were evaluated on the basis of the patient’s medical history. Protective touch sensation was examined with a 10-g monofilament; vibration with a 128-Hz tuning fork; temperature sensation with TipTherm and pain sensation with neurotips. Achilles tendon reflexes and knee reflexes were also checked. Two or more of the above were required to diagnose DPN [15].
Cardiac autonomic neuropathy (CAN) was assessed with the use of the validated ProSciCard III program (CPS MEDICAL, USA, 2010). The heart rate variability at rest, in a supine position and under standardized stimuli (deep breathing test, Valsalva maneuver, orthostatic test) was recorded. The provocative tests were performed according to standards of Task Force of the European Society of Cardiology, North American Society of Pacing and Electrophysiology. CAN was the diagnosis if two out of four tests were abnormal [16, 17].
Retinopathy, DPN, CAN and DKD were considered as neurovascular complications. If at least one of these complications was present, a patient was assigned to the neurovascular complications group.
Autoimmune hypothyroidism diagnosis
The autoimmune primary hypothyroidism group involved patients with overt or subclinical AHT which was diagnosed in the past or during present hospitalization. Patients with central secondary hypothyroidism, after iodotherapy or thyroidectomy, were excluded from the study.
Overt hypothyroidism was recognized if TSH was greater than 4.2 µIU/mL and FT4 levels were lower than normal or TSH was greater than 10 µIU/mL. Subclinical hypothyroidism (SCH) was detected if TSH was between 4.2 and 10 µIU/mL with free thyroid hormones in the normal range [18]. Moreover, the diagnosis of the autoimmune origin of hypothyroidism was based on the presence of anti-thyroid autoantibodies such as anti-thyroperoxidase autoantibodies (ATPO) or anti-thyroglobulin autoantibodies (ATg) and typical changes in the ultrasonography (hypoechogenicity, parenchymal heterogeneity, lymph nodes) [19].
Elecsys TSH, Elecsys FT4 and Elecsys FT3 on Cobas analyzers (Roche Diagnostics, Basel, Switzerland) were used to measure TSH, FT4 and FT3 concentrations. The TSH measuring range was between 0.005 and 100.0 µIU/mL. The reference interval of TSH was 0.27–4.2 µIU/mL. In the case of abnormal values of TSH, the measurement of FT3 and FT4 was performed. The FT4 measuring range was between 0.5 and 100.0 pmol/L. The reference interval of FT4 values for adults was 12–22 pmol/L. The FT3 measuring range was between 0.4 and 50.0 pmol/L. The reference interval of FT3 values for adults was 3.1–6.8 pmol/L.
Assessment of other variables
The data on age, T1DM duration, sex, medications, diagnosed hyper/hypothyroidism, and smoking status were collected by a questionnaire.
Anthropometric measurements and blood pressure checks were recorded at the time of the admission to the hospital. Blood pressure was measured in a sitting position after a 10-min rest, using the aneroid sphygmomanometer. Hypertension was diagnosed according to the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC) Guidelines. Also, it was found out if the patient had a previous diagnosis of arterial hypertension and had received appropriate treatment [20]. BMI was calculated as ratio of human body weight to squared height (expressed in kilograms and meters, respectively).
Glycated hemoglobin (HbA1c) was measured using the competitive turbidimetric inhibition immunoassay method on a Cobas analyzer (Roche Diagnostics, Basel, Switzerland). The Twin Test reaction mode allows sequential measurement of hemoglobin and HbA1c in a single cuvette (both expressed in g/dL). The mentioned assay enables detection of concentrations between 0.186 and 1.61 g/dL. The HbA1c to total hemoglobin ratio is obtained in compliance with the DCCT/NGSP description (DCCT, Diabetes Control and Complication Trial; NGSP, National Glycohemoglobin Standardisation Program).
Lipids (HDL, LDL, TG), ALT, AST were measured using commercially available enzymatic methods (Roche Diagnostics, Basel, Switzerland) and high sensitive C-reactive protein (hsCRP) using highly sensitive microparticle enzyme turbidimetric immunoassay.
Statistical analysis
Statistica V13 PL (Statsoft, Tusla, Oklahoma, United States) software was applied to perform statistical analysis. The differences were recognized as statistically significant at a P value lower than 0.05. Data were presented as median values and interquartile range (IQR) for the number and percentage of patients. Continuous variables were non-normally distributed (Shapiro–Wilk test), thus non-parametric statistical tests were applied. Association between data was collected by a questionnaire. Anthropometric and biochemical characteristics and the presence of neurovascular complications were assessed with χ2 test or Yates, χ2 test for dichotomous variables and Mann–Whitney U test for continuous variables (Tables 2, 3). Odds ratios (OR) and 95% confidence intervals (CI) for the association between AHT and neurovascular complications were estimated using logistic regression models adjusted for age, AHT, DBP, HbA1c, and TG (Table 4). Final predictors were chosen based on criteria described below Table 4.
Results
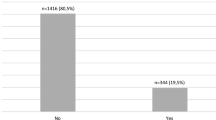
161 (48.5%) patients were diagnosed with at least one neurovascular complication. Retinopathy was diagnosed in 41.6% of patients, DKD in 13.9%, CAN in 14.3%, DPN in 20.8% (Table 2). At the time of enrollment, 16.3% of participants had been diagnosed with AHT. 82.7% of participants with AHT had been treated with l-thyroxine, of which 67.4% of them were treated effectively (TSH concentration within reference interval).
AHT prevalence was higher in the group with neurovascular complications than without (21.7 vs. 11.1%, respectively; P = 0.009). Groups differed in terms of classic risk factors for late diabetes complications: age, T1DM duration, SBP, DBP, HbA1c, HDL, TG, eGFR and hypertension prevalence. Characteristics of the study groups are presented in Table 2.
To explain the potential AHT influence on chronic diabetic complications, patients with AHT were compared to those without AHT (Table 3). Sex, T1DM duration, TSH, eGFR and hypertension prevalence were different between these groups. However, no other factor potentially influencing neurovascular complications prevalence was found. Patients with AHT were characterized by a higher prevalence of neurovascular complications (64.8 vs. 45.3%; P = 0.009) than those without AHT. Prevalence of retinopathy was significantly higher in the group with AHT (55.6 vs. 38.9%, P = 0.02). All characteristics are presented in Table 3.
In multivariate logistic regression analysis, AHT was an independent predictor of higher prevalence of neurovascular complications [OR 2.40; 95% CI 1.17–4.92; P = 0.02] after an adjustment for other potential explanatory factors such as age, DBP, HbA1c and TG (Table 4). Effective treatment with l-thyroxine in patients with AHT did not predict the occurrence of neurovascular complications in the multivariate logistic regression analysis [OR 1.87; 95% CI 0.37–9.40; P = 0.44] after adjusting for age, DBP, HbA1c and TG.
Discussion
The present study showed higher incidence of neurovascular complications among subjects with T1DM and coexisting AHT.
Oxidative stress and endothelial dysfunction are proved etiological factors causing neurovascular complications in diabetes [21]. The presence of hypothyroidism is associated with the development of oxidative stress [22]. It has also been shown that there is a continuous increase in the biochemical makers of oxidative stress in relation to the stage of hypothyroidism in subjects with autoimmune thyroiditis [23]. That could be a potential mechanism explaining the relationship between AHT and chronic diabetes complications. In this study, we showed that retinopathy is the most common neurovascular complication in patients with T1DM and AHT. Thyroid hormones are responsible for normal retinal vascular density. Lower levels of thyroid hormones could be responsible for the development of preretinal neovascularization [24, 25].
Few studies have focused on the thyroid function and microvascular complications in type 2 diabetes so far. In Asian patients with type 2 diabetes, an association between SCH and risk of retinopathy was found [24,25,26]. Yang et al. [24] found that sight-threatening diabetic retinopathy occurred more frequently in patients with higher TSH when compared with a group with lower TSH also in the euthyroid population. In patients with type 2 diabetes and prediabetes, prevalence of microalbuminuria was higher in subjects with SCH than in the euthyroid ones. It has been suggested that progression of DKD in SCH is related to changes in the renal hemodynamics, endothelial dysfunction and increasing peripheral vascular resistance [27]. Furthermore, in a cohort of almost 30,000 type 2 subjects, low thyroid function (measured as high TSH) was related to decreased eGFR [28].
Similar observations have not been noted in populations with T1DM. Only one study has been conducted on T1DM patients [29] and it showed that lower TSH levels (0.4–2.5 mU/L) were associated with a lower risk of diabetic retinopathy and renal dysfunction (eGFR lower than 60 mL/min/1.73 m2). Unfortunately, this study assessed only TSH and was based on an ethnically diverse cohort of patients. Therefore, the results cannot be extrapolated to the European population.
In the previous study of a group of T1DM patients, we have shown that the presence of thyroid autoantibodies, without previously diagnosed thyroid dysfunction, was not associated with a higher risk of chronic diabetic complications [19]. Our current results performed on AHT patients are consistent with previous observations in type 2 diabetes and prove that AHT coexisting with T1DM could be considered as a new risk factor for neurovascular complications. Therefore, we emphasize the need for early diagnosis and treatment of AHT coexisting with T1DM.
While this study resulted in some significant findings, there were some limitations to it. First, it was a single-center study. Due to the small size of the AHT group, we did not divide subjects into subclinical or overt hypothyroidism. There were no data available on the start time and course of the l-thyroxine treatment, neither the duration of AHT.
Conclusion
T1DM coexisting with AHT was associated with a higher incidence of neurovascular complications. Establishing treatment standards for concomitant AHT to reduce the risk of microangiopathy requires further research.
References
Chang CC, Huang CN, Chuang LM (1998) Autoantibodies to thyroid peroxidase in patients with type 1 diabetes in Taiwan. Eur J Endocrinol 139(1):44–48
Barker JM et al (2005) Autoantibody “subspecificity” in type 1 diabetes: risk for organ-specific autoimmunity clusters in distinct groups. Diabetes Care 28(4):850–855
Kordonouri O et al (2002) Thyroid autoimmunity in children and adolescents with type 1 diabetes: a multicenter survey. Diabetes Care 25(8):1346–1350
ADA (2018) 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care 41(Suppl 1):s13–s27
Huang W et al (1996) Although DR3-DQB1*0201 may be associated with multiple component diseases of the autoimmune polyglandular syndromes, the human leukocyte antigen DR4-DQB1*0302 haplotype is implicated only in beta-cell autoimmunity. J Clin Endocrinol Metab 81(7):2559–2563
Mohn A et al (2002) The effect of subclinical hypothyroidism on metabolic control in children and adolescents with Type 1 diabetes mellitus. Diabet Med 19(1):70–73
Biondi B, Klein I (2004) Hypothyroidism as a risk factor for cardiovascular disease. Endocrine 24(1):1–13
Soedamah-Muthu SS et al (2004) Risk factors for coronary heart disease in type 1 diabetic patients in Europe: the EURODIAB Prospective Complications Study. Diabetes Care 27(2):530–537
Araszkiewicz A, Zozulinska-Ziolkiewicz D (2016) Retinal neurodegeneration in the course of diabetes-pathogenesis and clinical perspective. Curr Neuropharmacol 14(8):805–809
Fong DS et al (2004) Retinopathy in diabetes. Diabetes Care 27(Suppl 1):S84–S87
Matsushita K et al (2012) Comparison of risk prediction using the CKD-EPI equation and the MDRD Study equation for estimated glomerular filtration rate. JAMA 307(18):1941–1951
Sonne DP, Hemmingsen B (2017) Standards of medical care in diabetes-2017. Diabetes Care 40(Suppl. 1):S1–S135
National Kidney Foundation (2012) KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis 60(5):850–886
Tesfaye S et al (2010) Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 33(10):2285–2293
Rogowicz-Frontczak A et al (2011) Carotid intima-media thickness and arterial stiffness in type 1 diabetic patients are dependent on age and mean blood pressure. Exp Clin Endocrinol Diabetes 119(5):281–285
Spallone V et al (2011) Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev 27(7):639–653
Camm AJ et al (1996) Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 93(5):1043–1065
Kitahara CM et al (2012) Body fatness and markers of thyroid function among U.S. men and women. PLoS One 7:e34979
Rogowicz-Frontczak A et al (2016) Patients with diabetes type 1 and thyroid autoimmunity have low prevalence of microangiopathic complications. Endocrine 51(1):185–188
Mancia G et al (2013) 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 34(28):2159–2219
Schalkwijk CG, Stehouwer CD (2005) Vascular complications in diabetes mellitus: the role of endothelial dysfunction. Clin Sci (Lond) 109(2):143–159
Cebeci E et al (2011) Evaluation of oxidative stress, the activities of paraoxonase and arylesterase in patients with subclinic hypothyroidism. Acta Biomed 82(3):214–222
Ates I et al (2015) The relationship between oxidative stress and autoimmunity in Hashimoto’s thyroiditis. Eur J Endocrinol 173(6):791–799
Yang JK et al (2010) An association between subclinical hypothyroidism and sight-threatening diabetic retinopathy in type 2 diabetic patients. Diabetes Care 33(5):1018–1020
Kim BY et al (2011) Association between subclinical hypothyroidism and severe diabetic retinopathy in Korean patients with type 2 diabetes. Endocr J 58(12):1065–1070
Qi Q et al (2017) Association of thyroid-stimulating hormone levels with microvascular complications in type 2 diabetes patients. Med Sci Monit 23:2715–2720
Yasuda T et al (2011) Subclinical hypothyroidism is independently associated with albuminuria in people with type 2 diabetes. Diabetes Res Clin Pract 94(3):e75–e77
Asvold BO, Bjoro T, Vatten LJ (2011) Association of thyroid function with estimated glomerular filtration rate in a population-based study: the HUNT study. Eur J Endocrinol 164(1):101–105
Rodacki M et al (2014) Should thyroid-stimulating hormone goals be reviewed in patients with type 1 diabetes mellitus? Results from the Brazilian Type 1 Diabetes Study Group. Diabet Med 31(12):1665–1672
Acknowledgements
We thank Malgorzta Grzelka and Jaroslaw Opiela for language revision of this article.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Author information
Authors and Affiliations
Contributions
ARF and BF planned and designed the study. AGW, AU, AA conducted data acquisition. BF performed the statistical analysis. ARF and BF wrote the manuscript. AGW, AU, AA, DZZ revised the manuscript. All authors approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance to the ethical standards of the institutional and/or national research committee (The Bioethical Committee of Poznan University of Medical Sciences; reference numbers: 539/12 and 465/15) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Each enrolled subject delivered a written informed consent for participation in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rogowicz-Frontczak, A., Falkowski, B., Grzelka-Wozniak, A. et al. Does autoimmune hypothyroidism increase the risk of neurovascular complications in type 1 diabetes?. J Endocrinol Invest 43, 833–839 (2020). https://doi.org/10.1007/s40618-019-01171-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-019-01171-x