Abstract
Purpose
The aims of this study were to determining the reference value of anti-müllerian hormone (AMH) in infertile women and effect of AMH on different ovarian responses in the stratum of BMI categories.
Methods
Through a retrospective cohort study the information of 816 infertile patients referring to the referral infertility clinic of Mahdiyeh Hospital since the beginning of 2011 until the end of January 2016 were used. The normal-based method was undertaken to calculate age-specific AMH percentiles. To determine the effect of AMH on the outcomes of different ovarian responses following adjustment of associated variables, the multinomial regression model was used.
Results
Estimated reference intervals for AMH corresponding to the 2.5 and 97.5th‰ in patients with normal ovarian response are from 0.096 to 6.2 ng/mL. These values for percentiles of 5, 10, 25, 50, 75, 90, and 95% are, respectively, 0.18, 0.33, 0.77, 1.68, 3.05, 4.45, and 5.36 ng/dL. Also the reference value for the 20-year-old participants has a maximum range (0.12–7.64), while for 43-year-old ones has the lowest range (0.08–5.3). Among participants under and above 35 years old, the optimal cut-off points for predicting normal ovarian response are, respectively, 1.5 and 1.2 ng/dL. With each unit increase in the log of AMH concentration, the odds of having excessive ovarian response in patients with normal weight compared to that of having normal ovarian response is 32% higher.
Conclusions
Determining AMH reference values in IVF candidates allows specialists to measure only AMH plasma levels in IVF candidates so as to find whether or not the ovarian response is normal before applying other therapeutic measures; accordingly, they can adjust a treatment plan for each individual separately.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The population of women delaying childbearing continues to rise, and demand for a precise evaluation of ovarian reserve has grown [1]. Assessments of AMH’s predictive value in the naturally aging population is crucial for reproductive planning as well as for treatment planning for women experiencing hormone-based gynecological disorders such as endometriosis and fibroids [2]. AMH serum levels are independent of the menstrual cycle and not affected by GnRH agonists or oral contraceptives [3]. Moreover, due to the fact that AMH serum levels are related to the number of primary-antral follicles, so this hormone can be used to assess the fertility potential and the ovarian response in In Vitro Fertilization (IVF) treatment [4, 5]. Numerous studies have utilized this hormone as a marker for predicting the ovarian response before using the assisted reproductive technology (ART) [6,7,8]. As a fact, one of the most important factors in the success of ART seems to be the accurate assessment of individuals’ ovarian reserve status; hence, it is necessary to apply methods which can both predict the right time of the chance of pregnancy and live birth and determine the optimum dose of hormones chosen to stimulate the ovary [9]. Several recent studies have reported different nomograms for AMH concerning the target populations [10,11,12], of which large numbers were in fertile women [10, 11, 13]. To the best of our knowledge, studies have not yet reported the reference value of AMH in normal ovarian response women who underwent the ART.
Reference values and centile charts for AMH in women undergoing ART allow doctors to check their ovarian response in the future, by determining AMH serum levels; thus, physicians can provide better treatment for these patients. Therefore, determining both the reference value of AMH in women with a normal ovarian response and the best cut-off point to predict poor ovarian responses, the current study is an attempt to find out the better counseling and treatment strategies.
Methods
The present study is a retrospective cohort study in which all the information of all the infertile patients referring to the infertility clinic of Mahdiyeh Hospital since the beginning of 2011 until the end of January 2016 was used. The study sample included women who were eligible for ART due to fallopian tube problems, ovarian dysfunction, male factors or unexplained cases.
Patients who fulfilled all the following criteria were enrolled in the study: (a) those without any underlying endocrine disease, (b) those without hormonal drugs usage in the past 3 months, (c) those without both polycystic ovary syndrome (PCO) based on the Rotterdam criteria and azoospermia or severe oligozoospermia and (d) patients who visit for the first cycle of the ART treatment. Of the total of 876 patients referring to the infertility clinic of Mahdiyeh Hospital during 5 years, 41 patients were excluded due to lack of inclusion criteria and 19 patients were excluded due to outlier data points of AMH levels. For all the infertile patients referred to the infertility clinic of Mahdiyeh Hospital, enjoying all the inclusion criteria, and being candidate for IVF, all the levels of AMH (ELIZA, ng/mL), FSH (RIA, iu/mL), and E2 (ECL pg/mL) were measured in day 2 or 3 of the menstrual cycle. All the patients had not undergone any hormonal therapy at least 1 month prior to the study. In the next phase, patients entered a long-term standard treatment cycle with GnRH agonists (Sinafact, Sinagen group) with a daily dose of 50 iu/sQ. Later on, at the beginning of the menstrual cycle (from 1st to 3rd day), for those included in the study, the basic ultrasound was conducted to ensure the absence of any underlying pathology. In this study, the controlled ovarian stimulation was started on day 3–4 of the menstrual cycle. Moreover, the required dose of human urinary-derived HMG (Merional-IBSA-75 IU/mL Amp) was determined in accordance with the approved protocol in the infertility clinic of Mahdiyeh Hospital in Tehran and based on the patients’ age. The control ultrasound was performed every 3–4 days. Based on the ovarian response, the treatment was continued and the control ultrasound was performed again after 2–3 days. After the dominant follicle in the 16–18 mm range was observed, the final intervention was done by injecting HCG (10,000 IU, Choriomon, IBSA). 35–36 h later, oocyte retrieval was carried out and passed to the embryologists. On the condition that embryos were appropriate for transfer, embryo transfer was performed after 36–48 h. After the day of oocyte retrieval, luteal phase support was started using vaginal progesterone (Cyclogest 400 mg, Actover), and continued until the 10th week of pregnancy, in case of pregnancy. The results of all tests, ultrasounds, ovarian responses of each patient, and the dose of used medication in each cycle were recorded in the patients’ report. In the current study, patients were classified into three groups based on the number of oocytes as follows: patients with poor ovarian response (oocytes ≤ 3), those with normal ovarian response (4–12 oocytes), and those with excessive ovarian response (oocytes > 12). In the next step, to determine the reference value of AMH in patients with normal ovarian response, 114 patients due to the poor ovarian response and 199 patients due to the excessive ovarian response were excluded from the study. Finally, the information of 503 patients was applied to determine the reference value of AMH.
Anti-müllerian hormone (AMH) assay
Serum concentration of anti-müllerian hormone (AMH) at the time of recruitment was measured by the two-site enzyme immunoassay (EIA) method using Gen II kit (Beckman Coulter, Inc.CA, USA) and the Sunrise ELISA reader (Tecan Co. Salzburg, Austria). All AMH measurements were performed simultaneously at the same laboratory. AMH Gen II controls A79766 were used at two levels of concentration to monitor accuracy of assay. The intra- and inter-assay CVs were 1.9 and 2.0%, respectively.
Statistical analysis
All continuous baseline demographic and clinical data are displayed as mean ± standard deviation and categorized data are displayed as frequencies and percentages. To determine the independence of the two categorical variables, Chi-square test or Fisher’s exact test was applied. One-way ANOVA followed by Tukey’s test was used to investigate the mean differences between different ovarian responses. The correlation between anti-müllerian hormone levels and the traits of the study participants such as BMI, FSH and E2 was explored using pairwise Spearman’s correlation and partial correlation coefficients. To determine the effect of AMH on the outcomes of different ovarian responses following adjustment of associated variables, the multinomial regression model was used. This model was fitted using backward stepwise selection. The criterion to select the best model was AIC of these models. The normal-based method explained by Royston and Wright [14] and Altman and Chitty [15] was undertaken to calculate age-specific AMH percentiles. Before the percentile estimation, the data were trimmed regarding the outlier values using the mcd command in Stata software. In brief, we applied the minimum covariance determinant estimator, which is regularly used in robust statistics to approximate the location parameters and multivariate scales. These estimators could be simply used to robustify Mahalanobis distances and detect outliers’ data [16]. Fractional polynomial (FP) regression models were fitted to estimate the mean and standard deviation (SD) of the log AMH values as functions of age, respectively. Subsequently, using the scaled absolute residuals from the fitted regression model for the mean, the SD was modeled. An FP of the first degree with power 1 was selected for mean, while we did not see any relationship between the SD and age of the participants, so there is no selected model for SD. The percentiles were obtained by amalgamating these two regression models, using the assumption that the conditional distribution of log AMH values given age is normal. AMH monogram was achieved by taking antilog of the estimated curves.
The normal plot of the Z-scores from the normal model described above showed that normality does not hold (P value for the Shapiro–Wilk test < 0.001). A modulus exponential–normal (MEN) four-parameter model provided a much improved fit, with a deviance 44 lower than the normal model (P < 0.001). The normal plot of the Z-scores from the MEN model appears reasonably linear (P value for the Shapiro–Wilk test = 0.052), and 10.07% of the observations lie above the 90th‰ and 9.65% below the 10th‰. The MEN model was fitted by maximum likelihood using the STATA command xriml. The R package Optimal cut-off points was used to determine the area under Receiver Operating Characteristic (ROC) curve in addition to the cut-off values based on the Youden’s index method, along with the corresponding sensitivity, specificity, positive and negative predictive values.
Results
In this study, data were used from 816 women admitted to the infertility clinic of Mahdiyeh Hospital in Tehran. The mean age and standard deviation of participants was 32.6 ± 5.2 years, respectively. Other demographic information of the patients before omitting those with poor or excessive ovarian responses is displayed in Table 1.
The results of one-way ANOVA presented show that there were no significant differences between the concentration mean of LH and E2 hormones and the body mass index of the three groups with different ovarian responses. For other variables including AMH and FSH, the difference was significant (P < 0.001). Furthermore, the results of Chi-square test showed there were significant differences between the result of β-hCG test and different ovarian responses (P < 0.001). The results of the multi-nominal regression model using backward stepwise method to examine the relationship between AMH levels and different ovarian responses in subgroups of obese, overweight, and normal weight patients can be observed in Table 2.
The results of the above table show that with each unit increase in the log of AMH concentration, the odds ratio of having excessive ovarian response in patients with normal weight compared to that of having normal ovarian response is 32% higher. Also, in overweight patients, this value is almost 24% greater for the excessive response group compared to the normal group. Also results showed that in obese patients with each unit increase in AMH concentration the odds of having excessive response will be only 7% higher compared to normal response participants. To conclude, the likelihood of having more favorable results from ART will decrease by increasing the participants BMI.
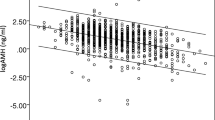
The results demonstrate that regardless of the age, the reference value of AMH (the percentiles of 2.5–97.5) in patients with normal ovarian response is from 0.096 to 6.2 ng/mL. These values for percentiles of 5, 10, 25, 50, 75, 90, and 95% are, respectively, 0.18, 0.33, 0.77, 1.68, 3.05, 4.45, and 5.36 ng/dL. Figure 1, as a result of fitting the MEN’s model, shows the calculated percentiles of 2.5–97.5 based on the age of the participants.
In the next phase, for different ages, the percentiles of 2.5, 5, 10, 25, 50, 75, 90, 95, and 97.5, as a result of fitting the MEN’s model, are reported in Table 3.
The results of the table above show that the reference value for the 20-year-old participants has a maximum range (0.12–7.64), while for 43-year-old ones have the lowest range (0.08–5.3). Given the fact that AMH changes with respect to the age are monotonous, using the 5- or 10-year age range is also expectable according to this table. For example, in the absence of the exact age of the person with the age range of 20–30 years, the reference value of those of 25 years can be used.
In the next step, the optimal cut-off points for AMH predicting the normal ovarian response in patients participated in the study and below and above 35 years are discussed. In this stage, the observed ovarian responses, more than 12 oocytes, are excluded from the study and the cut-off points for the remaining are calculated. Table 4 shows the results.
The results of the above table display that among participants under and above 35 years old, the optimal cut-off points for predicting normal ovarian response are, respectively, 1.5 and 1.2 ng/dL. This means that in participants under 35 years old, if AMH levels are reported to be less than 1.5 ng/dL, it may lead to a poor ovarian response. Moreover, as AMH is inversely related to the age, the cut-off points with sensitivity, specificity, and especially a high positive predictive value may be a suitable guide for treating women undergoing ART. Figure 2 displays the area under curve (AUC) for the cases under and above 35 years old.
The AUC of ROC and optimal cut-off values for AMH levels in participants with normal ovarian response. a ROC curve for predicting the normal ovarian response using the AMH level in participants over 35 years, showing 65% sensitivity at 93% specificity with optimal cut-off point of 1.2 ng/mL. b ROC curve for predicting the normal ovarian response using the AMH level in participants under 35 years, showing 69% sensitivity at 98% specificity with optimal cut-off point of 1.5 ng/mL
Discussion
Reporting the age-specific reference values for AMH in women with normal ovarian response (more than three oocytes) and determining the optimal cut-off points for predicting the normal ovarian response in both subgroups of under 35 years and over 35 years, the present study may greatly contribute to specialists in this field to provide better counseling and treatment strategies. Several nomograms have already been reported to provide different percentiles of AMH levels in different populations [10,11,12], but, to the best of our knowledge, no study has been done on this issue by considering infertile patients’ ovarian responses. Determining AMH reference values in normal ovarian response IVF candidates allows specialists to measure only AMH plasma levels in IVF candidates so as to find whether or not the ovarian response is normal before applying other therapeutic measures; accordingly, they can adjust a treatment plan for each individual separately. Since AMH plasma levels in infertile women are lower than the population of fertile women, enjoying reference values of this hormone is of the requirements due to its reliance on the age of participants [17, 18]. Also, because of many factors affecting ovarian functions including polycystic ovary syndrome, endometriosis, and some surgeries that result in infertility, the results of studies in which the target population is the general fertile population cannot be generalized to this specific population [19,20,21]. For example, in a recent study done by Ramezani et al. the 5 and 95th‰ of AMH are 2 and 8.9 ng/mL, respectively [11], while these values in our study are 0.18–5.36 ng/dL; hence, the results indicate that lower levels of this hormone can be seen in infertile patients compared to normal individuals in the society. In one of the studies carried out in America [22], it is shown that there is a relationship between the age of participants and the reduction in AMH levels. Therefore, up to the age of 38 years with each unit increase in the age, the reduction in AMH levels can be observed first with a slope of − 0.2 and then with a slope of − 0.1.
In this study, the body mass index of patients is considered as an important factor to investigate the relationship between AMH and ovarian responses. The results clearly show that the relationship between different ovarian responses and AMH plasma levels in patients who are overweight or obese is less severe; in other words, it can be said that using AMH as a marker to predict the ovarian behavior of overweight and obese infertile women does not seem proper. In studies conducted in different parts of the world, it has been shown there is a negative relationship between individuals’ body mass index and AMH levels [23, 24]. In a study conducted by Caillon et al. the cycle outcome was not significantly different between the normal and overweight/obese group, but they found a strong trend towards increasing transfer cancelation and miscarriage rates in overweight or obese participants [25]. It should also be noted that this relationship in obese women with polycystic ovary syndrome is more common. The difference in the approach of this study with other studies in determining AMH reference values is that the current study considers normal ovarian responses; this means that in the present study, excessive ovarian responders who often are those with PCOS and poor ovarian responders who do not respond to the treatment are excluded from the study, so a reliable estimate for other participants is provided. The reference values obtained in this study for infertile patients under the treatment and with normal ovarian response are in the range of 0.01–6.2 ng/dL with the median of 1.68 ng/dL. In a study conducted in China, it is found that the median of AMH levels in infertile women without PCOS whose age is between 20 and 31 years is 2.35 ng/dL [10]; this amount in the present study for patients aged 20–30 years is from 1.75 to 2.07 ng with the median of 1.92 ng/dL. In most studies, the median is used instead of reporting the exact amounts of AMH, so it may not seem appropriate for clinical purposes and laboratory use [26, 27]. However, in the current study, these amounts are reported for an age-specific population. Also, another issue is using different laboratory kits to measure AMH serum levels which can lead to unreliable estimates, so special attention is paid to this issue in this study. Furthermore, the undertaken method for anti-müllerian hormone (AMH) assay in the current study is comparable with fully automated AMH immunoassay such as access AMH assay regarding the precision and acceptable sample stability. In a study conducted by Pearson et al. they showed that the results from a fertile AMH reference range established using the preceding Gen II ELISA are interchangeable with the new automated Access AMH assay [28].
Another important point in this study is that the relationship between age and AMH is considered based on complex fitted models and eventually the four-parameter nonlinear MEN model as the best model to explain the relationship between age and AMH. Other studies have considered the quadratic model as the best model [18, 22].
The sustained secretion of AMH during the menstrual cycle with no significant changes during and before the cycle [29] and its plasma levels not being affected because of using external hormones [30] justify the use of this indicator for research purposes and determination of secondary causes of oligo-amenorrhoea. In this study, the optimal cut-off point (by removing the excessive ovarian responders) for predicting the optimal ovarian response of women over 35 years is estimated to be 1.2 ng/dL and that of those over 35 years is estimated to be 1.5 ng/dL. These amounts with the area under curve of 80% seem to be appropriate for predicting the normal ovarian response. In a recent study conducted in Iran, it is shown that the optimal cut-off point of AMH for predicting the poor ovarian response is 1.2 ng/mL, so it is very similar to the results obtained in the current study [31]. Besides, depending on the type of study population, several studies have reported different cut-off points the performance of which should be evaluated depending on the needs of the population studied [32, 33]. Ultimately, it should be mentioned that the goal of determining AMH reference values and its optimal cut-off points of the patients referring for fertility treatment is the assessment of ovarian reserve before exploiting the ART therapy. Considering the probability of occurrence of each of the excessive or poor ovarian responses, physicians select the final method of stimulation; thus, they can minimize the side effects such as OHSS and also cycle cancelation [34]. With a relatively high sample size, the current study using appropriate and precise statistical methods attempted to provide more accurate estimations than other studies. Also investigating the relationship between the AMH concentration and ovarian responses with regard to subgroups of BMI variable and incorporating the affecting confounders such as age and E2 into the analysis should be considered as another strength of the current study. Additionally, the age-specific range of AMH in women with the normal ovarian response allows physicians to consider patients’ age, and after the assessment of their condition, consult with them and if necessary, introduce some methods such as gamete donation or adoption.
Change history
20 July 2018
Unfortunately, the first author name was incorrectly published in the original publication. The complete correct name should read as follows.
References
Dewailly D et al (2014) The physiology and clinical utility of anti-müllerian hormone in women. Hum Reprod Update 20(3):370–385
Meczekalski B et al (2016) Fertility in women of late reproductive age: the role of serum anti-müllerian hormone (AMH) levels in its assessment. J Endocrinol Invest 39(11):1259–1265
Chen Y et al (2014) The impact of endometrioma and laparoscopic cystectomy on ovarian reserve and the exploration of related factors assessed by serum anti-müllerian hormone: a prospective cohort study. J Ovarian Res 7(1):1–8
Kotanidis L et al (2016) The use of serum anti-müllerian hormone (AMH) levels and antral follicle count (AFC) to predict the number of oocytes collected and availability of embryos for cryopreservation in IVF. J Endocrinol Invest 39(12):1459–1464
Chang M-Y et al (1998) Use of the antral follicle count to predict the outcome of assisted reproductive technologies. Fertil Steril 69(3):505–510
Broer S et al (2011) Anti-müllerian hormone predicts menopause: a long-term follow-up study in normoovulatory women. J Clin Endocrinol Metab 96(8):2532–2539
Eldar-Geva T et al (2005) Dynamic assays of inhibin B, anti-müllerian hormone and estradiol following FSH stimulation and ovarian ultrasonography as predictors of IVF outcome. Hum Reprod 20(11):3178–3183
Freeman EW et al (2012) Anti-müllerian hormone as a predictor of time to menopause in late reproductive age women. J Clin Endocrinol Metab 97(5):1673–1680
Maheshwari A, Fowler P, Bhattacharya S (2006) Assessment of ovarian reserve—should we perform tests of ovarian reserve routinely? Hum Reprod 21(11):2729–2735
Cui Y et al (2014) Age-specific serum antimüllerian hormone levels in women with and without polycystic ovary syndrome. Fertil Steril 102(1):230–236
Tehrani F et al (2014) Age-specific serum anti-müllerian hormone levels: estimates from a large population-based sample. Climacteric 17(5):591–597
Naasan M et al (2014) Anti-müllerian hormone normogram in an Irish subfertile population. Irish J Med Sci (1971) 184(1):213–218
Du X et al (2016) Age-specific normal reference range for serum anti-müllerian hormone in healthy Chinese Han women: a nationwide population-based study. Reprod Sci 23(8):1019–1027
Royston P, Wright E (1998) How to construct ‘normal ranges’ for fetal variables. Ultrasound Obstet Gynecol 11(1):30–38
Altman D, Chitty L (1993) Design and analysis of studies to derive charts of fetal size. Ultrasound Obstet Gynecol 3(6):378–384
Verardi V, Dehon C (2010) Multivariate outlier detection in Stata. Stata J 10(2):259–266
Goodman C et al (2014) Age-specific serum antimüllerian hormone concentrations are lower in infertile women. Fertil Steril 2(101):e12
Lee JY et al (2012) Age-related distributions of anti-müllerian hormone level and anti-müllerian hormone models. Acta Obstet Gynecol Scand 91(8):970–975
Tehrani FR et al (2010) Is polycystic ovary syndrome an exception for reproductive aging? Hum Reprod 25(7):1775–1781
Lemos NA et al (2008) Decreased anti-müllerian hormone and altered ovarian follicular cohort in infertile patients with mild/minimal endometriosis. Fertil Steril 89(5):1064–1068
Uncu G et al (2013) Prospective assessment of the impact of endometriomas and their removal on ovarian reserve and determinants of the rate of decline in ovarian reserve. Hum Reprod 28(8):2140–2145
Seifer DB, Baker VL, Leader B (2011) Age-specific serum anti-müllerian hormone values for 17,120 women presenting to fertility centers within the United States. Fertil Steril 95(2):747–750
Kriseman M et al (2015) Antimüllerian hormone levels are inversely associated with body mass index (BMI) in women with polycystic ovary syndrome. J Assist Reprod Genet 32(9):1313–1316
Moy V et al (2015) Obesity adversely affects serum anti-müllerian hormone (AMH) levels in Caucasian women. J Assist Reprod Genet 32(9):1305–1311
Caillon H et al (2015) Effects of female increased body mass index on in vitro fertilization cycles outcome. Obes Res Clin Pract 9(4):382–388
Yoo JH et al (2011) Age specific serum anti-müllerian hormone levels in 1,298 Korean women with regular menstruation. Clin Exp Reprod Med 38(2):93–97
La Marca A et al (2012) Normal serum anti-müllerian hormone levels in the general female population and the relationship with reproductive history. Eur J Obstet Gynecol Reprod Biol 163(2):180–184
Pearson K et al (2016) Assessment of the Access AMH assay as an automated, high-performance replacement for the AMH Generation II manual ELISA. Reprod Biol Endocrinol 14(1):8
La Marca A et al (2006) Serum anti-müllerian hormone throughout the human menstrual cycle. Hum Reprod 21(12):3103–3107
Li HWR et al (2011) Serum anti-müllerian hormone level is not altered in women using hormonal contraceptives. Contraception 83(6):582–585
Heidar Z et al (2015) Prediction of different ovarian responses using anti-müllerian hormone following a long agonist treatment protocol for IVF. J Endocrinol Investig 38:1–9
Ficicioglu C et al (2014) Which cut-off value of serum anti-müllerian hormone level can predict poor ovarian reserve, poor ovarian response to stimulation and in vitro fertilization success? A prospective data analysis. Gynecol Endocrinol 30(5):372–376
Yassin MM, Sharif FA, Laqqan MM (2013) Anti-müllerian hormone as a predictor of ovarian reserve and ovarian response in IVF women from Gaza strip. Iran J Reprod Med 11(4):261
Broekmans F et al (2006) A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update 12(6):685–718
Author information
Authors and Affiliations
Contributions
ZH suggested the initial concept and provided administrative and technical support for conducting the study. MB, FF contributed to the study design and did critical revision of the manuscript for important intellectual content. ZH and FF contributed to the study design by preparing the final report and drafting the manuscript. MB performed the statistical analysis and interpretation of data. MM and ZH provided the technical and material support for the study and supervised the data collection. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Hiedar, Z., Bakhtiyari, M., Foroozanfard, F. et al. Age-specific reference values and cut-off points for anti-müllerian hormone in infertile women following a long agonist treatment protocol for IVF. J Endocrinol Invest 41, 773–780 (2018). https://doi.org/10.1007/s40618-017-0802-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-017-0802-z